To the Editor:
We read the letter to the Editor, “Laboratory Diagnosis of 1,4-BD and GHB Overdose by Routine Urine Organic Acid Analysis” by Quang et al. (Citation1) with great interest and applaud their findings, which describe the utility of a urine organic acids method for the diagnosis of GHB overdose. We agree with the authors that routine laboratory detection of gamma-hydroxybutyric acid (GHB) and 1,4-butanediol (1,4-BD) is not readily available in the hospital-based toxicology laboratories. Generally, the hospital laboratories rely on one of only a few reference laboratories in the country for GHB testing. Due to its popularity as a date rape drug and frequently observed cases of overdose, it is desirable to have a GHB assay available in the clinical laboratories (Citation2–6). In some series, GHB was the second most common drug (cocaine being the most common) detected in young people presenting with drug-induced coma (Citation7). It is recommended that GHB intoxication should be considered in any patient, particularly any young person, who presents with rapid onset of coma of unknown cause when head trauma, metabolic disorders, central nervous system infection, and increased intracranial pressure have been ruled out (Citation8). In hospital laboratories, urine organic acids assays are more readily available than GHB assays and, as shown by Quang et al. (Citation1), can be used for detection of GHB. In our present study, we provide additional data to extend the utility of organic acids method for the diagnosis of GHB overdose.
In Quang et al.'s study (Citation1), the GHB dose administered to the rats is much higher as compared to the usual doses consumed by humans. In their study, mice were given 600 mg/kg of 1,4-butanediol. In humans, oral or intravenous doses of 10 mg/kg cause amnesia and hypotonia; doses of 20–30 mg/kg are sleep-inducing; and doses of 50 mg/kg or higher produce anesthesia. Illicit use of GHB often involves oral doses of one teaspoon, which is equivalent to approximately 2.5 g or 35 mg/kg in a 70 kg adult (Citation9). As their method was qualitative, it is not known what concentrations of GHB were present in rat urines, or if they were comparable to the GHB concentrations reported in human urines in overdose situations. Oral doses of 1 and 2 g given to a 100 kg man resulted in urine GHB levels of 17 and 29 mg/L, respectively, at 1.5–2.0 hours (Citation10). A peak urine GHB concentration of 1100 mg/L was observed within the first 4 hours after a 100 mg/kg oral dose (Citation11). A woman who ingested GHB and became a victim of sexual assault had a urine GHB concentration of 27 mg/L (Citation12). A man who ingested 3 g of 1,4-butanediol and developed coma and seizures for approximately 3 hours had a reported urine GHB concentration of 925 mg/L (Citation13). Based on the reported GHB levels in human urines, we investigated our urine organic acids method for GHB concentrations of 10 and 100 mg/L.
It is well known that in the common gas chromatographic mass spectrometric methods of urine organic acids analysis, silylated urea and GHB co-elute or elute very closely and produce derivatives with similar chromatographic and mass spectral characteristics (Citation14). As GHB is a drug of abuse it is very important that the drug be identified and quantified with high confidence and urea interference be therefore eliminated. In Quang et al. (Citation1), solid phase extraction was used and it is assumed to be the methodological step performed to eliminate urea. This was followed by liquid phase extraction of organic acids from urine. Generally, urine organic acids methods involve either solid or liquid phase extraction, but not both. In a recent American College of Medical Genetics and College of American Pathologists Biochemical Genetics Laboratory Survey, only five laboratories used solid phase extraction and 40 laboratories used liquid phase extraction for the analysis of organic acids (Citation15). We employed the commonly used liquid phase extraction method to investigate GHB's detection with our method.
We performed a preliminary study with eight urine samples to investigate the ability of a liquid phase extraction method to extract GHB. We also tested the effect of urease in reducing urea interference. We then chose two urine samples with creatinine values of 43 and 110 mg/dL (representing typical urine samples) for more detailed studies. We spiked these samples with GHB to obtain final concentrations of 10 and 100 mg/L. These concentrations were chosen based on reported urine levels found in GHB overdoses. To 1 mL of urine sample 0.1 mL of tropic acid (final concentration 50 μM) was added as an internal standard. To investigate the affect of urease in reducing urea interference, the samples were treated with water or urease (200 units; from Sigma Chemical Co., St. Louis), and incubated at 37°C for 30 minutes. The samples were acidified by addition of 0.5 mL of 6N HCl and then double extracted with 3 mL ethyl acetate. The ethyl acetate layers were combined and dried in a water bath at 32°C. The residue containing organic acids and GHB was derivatized using 0.1 mL of BSTFA (Bis(trimethylsily)trifluoroacetamide) with 1% TMCS (trimethylchlorosilane)/pyridine (2:1) at 60°C for 30 minutes. The samples were analyzed on GC 5890/MS 5972 (Agilent Technologies, Wilmington, DE). Data acquisition and analysis were done using Chemstation software. The column was 30 meters ZB-1 (Phenomenex, Torrance, CA). The chromatographic conditions were: 1 μL splitless injection, helium flow rate of 1 mL/minute, injector temperature of 250°C, starting temperature 60°C held for 1 minute and then increased, 7°C/minute, to 280°C and held for 3 minutes.
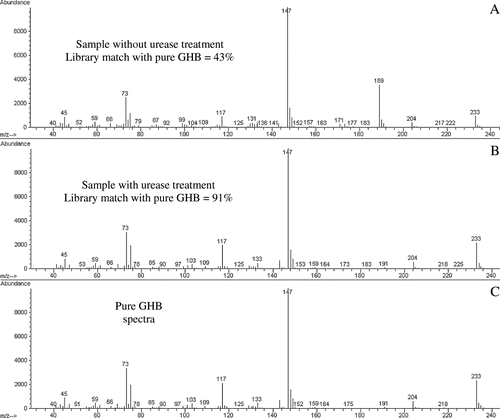
shows the total ion chromatograms of organic acids including GHB from a sample with and without urease treatment. The chromatogram A shows two high abundance urea peaks, one of which co-elutes with GHB. The chromatogram B represents the same sample treated with urease. The urease treatment completely eliminated the urea interference. The mass spectra library match in the absence (A) or presence (B) of urease as compared to the pure GHB (C) spectrum were 43% and 91%, respectively (). The data demonstrates with urease treatment, GHB can be identified with high confidence and urea interference eliminated.
Fig. 1. Total ion chromatograms from a urine sample without (A) or with (B) urea treatment. Note the disappearance of urea with urease treatment. One mL of sample, with GHB concentration of 100 mg/L, was used for analysis.
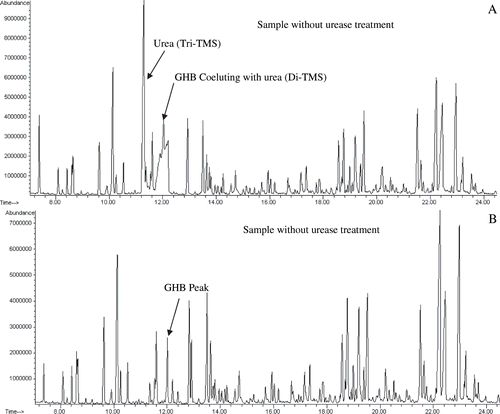
Fig. 2. Mass spectra of a urine sample without (A) or with (B) urea treatment. Sample without urease treatment shows only 43% match whereas the sample treated with urease shows 91% match to pure GHB (C).
In conclusion, we demonstrate that a liquid phase extraction method of urine organic acids, with the addition of a urease treatment step, can be used for the identification of GHB in urine at clinically relevant concentrations. The additional urease step is easy to accomplish and adds a significant value to the assay. The availability of a GHB assay in hospital laboratories is important for medical management, particularly as potential GHB antidotes are developed (Citation16–18).
References
- Quang LS, Levy HL, Law T, Desai MC, Maher TJ, Boyer EW, Shannon MW, Woolf AD. Laboratory diagnosis of 1,4-BD and GHB overdose by routine urine organic acid analysis. Clin Toxicol 2005; 43: 321–323
- Brown TC. Epidemic of gamma-hydroxybutyrate (GHB) ingestion. Med J Aust 2004; 181: 343
- Caldicott DG, Chow FY, Burns BJ, Felgate PD, Byard RW. Fatalities associated with the use of gamma-hydroxybutyrate and its analogues in Australasia. Med J Aust 2004; 181: 310–313
- Eckstein M, Henderson SO, DelaCruz P, Newton E. Gamma hydroxybutyrate (GHB): report of a mass intoxication and review of the literature. Prehosp Emerg Care 1999; 3: 357–361
- 5. Liechti ME, Kunz I, Greminger P, Speich R, Kupferschmidt H. Clinical features of gamma-hydroxybutyrate and gamma-butyrolactone toxicity and concomitant drug and alcohol use. Drug Alcohol Depend 2005 (electronic publication ahead of print).
- Mason PE, Kerns WP, 2nd. Gamma hydroxybutyric acid (GHB) intoxication. Acad Emerg Med 2002; 9: 730–739
- Miro O, Nogue S, Espinosa G, To-Figueras J, Sanchez M. Trends in illicit drug emergencies: the emerging role of gamma-hydroxybutyrate. J Toxicol Clin Toxicol 2002; 40: 129–135
- Snead OCI, Gibson KM. Gamma-hydroxybutyric acid. N Engl J Med 2005; 352: 2721–2732
- Baselt R. Disposition of toxic drugs and chemicals in man 7th. Biomedical Publications – California, Foster City 2004
- Kavanagh PV, Kenny P, Feely J. The urinary excretion of gamma-hydroxybutyric acid in man. J Pharm Pharmacol 2001; 53: 399–402
- Hoes MJ, Vree TB, Guelen PJ. Gamma-hydroxybutyric acid as hypnotic. Clinical and pharmacokinetic evaluation of gamma-hydroxybutyric acid as hypnotic in man. Encephale 1980; 6: 93–99
- Stillwell ME. Drug-facilitated sexual assault involving gamma-hydroxybutyric acid. J Forensic Sci 2002; 47: 1133–1134
- Megarbane B, Fompeydie D, Garnier R, Baud FJ. Treatment of a 1,4-butanediol poisoning with fomepizole. J Toxicol Clin Toxicol 2002; 40: 77–80
- Couper F, Marinetti L. Gamma-hydroxybutyrate (GHB) – Effects on human performance and behavior. Forensic Sci Rev 2002; 14: 101–121
- 15. Participant Summary. American College of Medical Genetics/ College of American Pathologists Biochemical Genetics Laboratory Survey A, 2005:5.
- Quang LS, Desai MC, Shannon MW, Woolf AD, Maher TJ. 4-methylpyrazole decreases 1,4-butanediol toxicity by blocking its in vivo biotransformation to gamma-hydroxybutyric acid. Ann N Y Acad Sci 2004; 1025: 528–537
- Quang LS, Desai MC, Kraner JC, Shannon MW, Woolf AD, Maher TJ. Enzyme and receptor antagonists for preventing toxicity from the gamma-hydroxybutyric acid precursor 1,4-butanediol in CD-1 mice. Ann N Y Acad Sci 2002; 965: 461–472
- Quang LS, Shannon MW, Woolf AD, Desai MC, Maher TJ. Pretreatment of CD-1 mice with 4-methylpyrazole blocks toxicity from the gamma-hydroxybutyrate precursor, 1,4-butanediol. Life Sci 2002; 71: 771–778
