Abstract
Introduction. Reports of acute levodopa-carbidopa overdose are rare and no case of an acute overdose with a controlled-release formulation has been described. We describe such a case in which serial concentrations of catecholamines were measured. Case Report. A 55-year-old man ingested 89 tablets of Sinemet® 50/200 (17.8 g of levodopa, 4.45 g of carbidopa). Clinical effects and plasma concentrations of dopamine, noradrenalin and adrenalin were assessed over 66 hours. On admission 2.5 hours after the ingestion, his physical examination was normal except for mydriasis and urine retention. Five hours post-ingestion he had psychomotor agitation, delirium with logorrhea, joviality, visual hallucinations, regular sinus tachycardia and xerostomia. The clinical course included two episodes hypotension and four of transient tachycardia. Treatment was symptomatic and supportive. Clinical toxicity reappeared 48 hours after the intoxication. The patient was discharged at the end of the fourth day with amnesia for the event. Discussion. Dopamine showed an initial plasma concentration peak 14 hours after the toxic ingestion, followed by a second peak 38 hours after the ingestion. The initial peak of noradrenalin occurred 20 hours post-ingestion with a second lower peak at 38 hours. There were no elevations in adrenalin concentrations. Conclusion. There appeared to be no correlation between the intensity of the clinical signs and the blood concentrations of dopamine and noradrenalin, although the resolution of the clinical signs did correspond to these catecholamines return to normal values. Patients who ingest controlled-release formulations need to be observed until after the second catecholamine peak.
Introduction
Few cases of acute overdose with levodopa or levodopa conjugated with carbidopa have been reported (Citation1–3), though levodopa has been used for more than 35 years in the treatment of Parkinson's disease (Citation4–6). This is the first report of an acute overdose of a controlled-release formulation of levodopa with serial measurements of dopamine, adrenalin and noradrenalin plasma concentrations.
Case report
A 55-year-old man, treated for three years for the Parkinson's disease, ingested before witnesses, 89 tablets of Sinemet® 50/200 (17.8 g of levodopa, 4.45 g of carbidopa), as well as a small quantity of ethanol.
On admission 2.5 hours after the ingestion, his physical examination was normal except for mydriasis and urine retention. The results of the electrocardiogram and the biochemical screen were normal. Five hours after ingestion, he had psychomotor agitation, delirium with logorrhea, joviality, visual hallucinations, regular sinus tachycardia, and xerostomia. The clinical examination, vital signs and urine output were measured serially (). Blood samples were taken at intervals to follow the toxicokinetic evolution of the degradation of the levodopa-carbidopa association.
Table 1. Evolution of clinical signs after an acute overdose with controlled-release levodopa-carbidopa
Clinical signs of overdose with levodopa and its metabolites were observed for nearly 66 hours following the ingestion. Two episodes of hypotension (blood pressure <110/60 mmHg) and four of spontaneously resolving tachycardia (heart rate >90 beats/minute) were observed. An electrocardiogram made during a phase of tachycardia showed a normal QRS and no repolarisation abnormalities. Rhabdomyolysis reached its peak on the second day with a creatine kinase of 34,846 UI/L (reference <165 IU/L) and aspartate aminotransferase of 1113 UI/L (reference <40 IU/L).
The patient was treated in a general ward attached to the emergency unit. Treatment was exclusively symptomatic and consisted of intravenous hydration with three liters of isotonic solution every 24 hours, sedation with midazolam, and transient oxygen therapy. The clinical course was favorable and followed by amnesia of the event. The patient was discharged by the end of the fourth day after a psychiatric examination.
Analytical results
Laboratory analyses of dopamine, adrenalin, and noradrenalin were performed by the Merieux Laboratory in Lyon (BP 7322 – 69357 LYON, France) using high performance liquid chromatography (HPLC). These metabolites were chosen because the associated clinical signs are well known. The plasma concentrations of these compounds are shown in .
Fig. 1. Kinetic evolution of the catecholamins after an acute overdose with controlled-release levodopa-carbidopa.
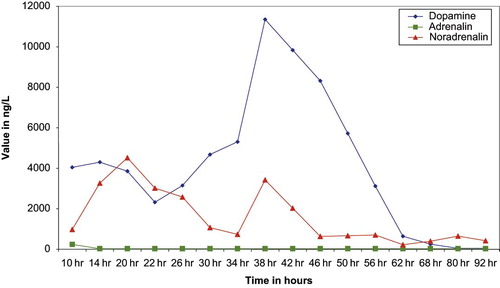
Ten hours after the ingestion, the first concentrations of dopamine, noradrenalin and adrenalin were well above reference values (dopamine <229 ng/L, adrenalin <164 ng/L, noradrenaline <507 ng/L). The first peak of dopamine, which is the main metabolite of the degradation of levodopa with 3-O-methyldopa, was observed 14 hours after the ingestion, followed by a progressive decrease and then by a second peak 24 hours after the first. Adrenalin concentrations reached a peak of 240 ng/L ten hours after ingestion and quickly declined to the reference range. Noradrenalin, after an initial peak at 20 hours after ingestion, progressively declined until hour 34. This first peak occurred six hours later than that of dopamine; a short second shorter peak occurred 38 hours after the ingestion (at the same time as the second peak of dopamine), which was followed by a rapid decline. This second peak, contrary to that observed with dopamine, was smaller to the first. The normalization of noradrenalin concentrations was quicker than that of dopamine.
Discussion
Levodopa, a metabolic precursor of dopamine, crosses the blood-brain barrier is an effective agent for the treatment of Parkinson's disease (Citation7–9). Carbidopa is a peripheral decarboxylase inhibitor that does not cross the blood brain barrier. The concomitant administration of levodopa and carbidopa, as in Sinemet®, enhances the clinical benefit of levodopa by decreasing its peripheral metabolism, thus providing more drug for delivery into the brain to be converted into dopamine (Citation10).
The controlled-release formulation is a slow-eroding matrix that gradually releases its contents. This formulation is designed to permit a more prolonged absorption of both ingredients, resulting in the elimination of the sharp peaks in plasma levodopa associated with conventional Sinemet® while yielding higher end-of-dose plasma levels during chronic therapy (Citation10). The intestinal absorption of both molecules is rapid.
In this case report, the period between the ingestion of the toxic agents and the first clinical signs, as well as the evolution of the toxic effects, are consistent with an overdose of a controlled-release formulation (Citation10,Citation11). Few cases of acute overdose of levodopa on its own (Citation3) or in association with a decarboxylase inhibitor (Citation1,Citation2) have been published. Contrary to the other case reports, which were overdoses of standard formulations of levodopa, the main clinical signs in our case were observed five hours after the ingestion of the toxic agents.
In our case we observed a second peak of dopamine that was 2.5 times higher than the first and that may have been due to the controlled-release formulation. No second peaks of dopamine or its metabolites were reported in an intentional ingestion of levodopa 6 g and carbidopa 1.5 g (Citation1) or in an ingestion of levodopa 1.5 g and carbidopa 150 mg (Citation2). In these cases, the peak concentrations of levodopa and its metabolites were reached in a few hours and were followed by quick returns to normal.
In another report, a 61-year-old man who had been treated with a daily dose of levodopa 7.5 g ingested levodopa 100 g over 12 hours (Citation3). Contrary to our case, the peak urine concentrations of levodopa, dopamine, dihydroxyphenylacetic acid, homovanillic acid, and noradrenalin were reached on the day of the ingestion, with values 10 to 24 times higher than the reference range; the homovanillic acid peak concentration was only five times above the normal. A return to normal was observed as early as the second day after the overdose, without a second peak of dopamine.
In our patient, signs of clinical toxicity reappeared 48 hours after the intoxication and 10 hours after the second peaks of dopamine and noradrenalin. There appears to be no correlation between the intensity of the clinical signs and the blood concentrations of dopamine and noradrenalin, although the resolution of the clinical signs did correspond to these catecholamines return to normal values. Other reports have noted similar findings (Citation1–3); the clinical courses in these case reports turned favorable within one or two days, which differ from our case. Like other authors (Citation1–3), we observed rhabdomyolysis, which was most likely due to our patient's agitation.
Conclusion
Following an acute overdose of 89 tablets of Sinemet® 50/200 (17.8 g of levodopa, 4.45 g of carbidopa), there appeared to be no correlation between the intensity of the clinical signs and the plasma concentrations of dopamine and noradrenalin, although the resolution of the clinical signs did correspond to these catecholamines return to normal values. The presence of second plasma peak concentrations of dopamine and noradrenalin, distant from the ingestion phase may be attributed to the controlled release formulation. Because of the delay in onset of clinical effects and also because of the second phase of clinical effects, it seems prudent to observe a patient who has ingested a controlled-release formulation of levodopa-carbidopa for an extended period of time.
Notes
*The research is original and the information has not been reported to any large extent in a previously published article or contained in another article that has been submitted or accepted for publication in print or electronic media.
References
- Stuerenburg HJ, Schoser BG. Acute overdosage and intoxication with carbidopa/levodopa can be detected in the subacute stage by measurement of 3-o-methyldopa. J Neurol Neurosurg Psychiatry 1999; 67: 122–123
- Sporer KA. Carbidopa-levodopa overdose. Am J Emerg Med 1991; 9: 47–48
- Hoehn MM, Rutledge CO. Acute overdose with levodopa. Clinical and biochemical consequences. Neurology 1975; 25: 792–794
- Cotzias GC, Papavasiliou PS, Gellene R. Modification of parkinsonism-chronic treatment with L-dopa. N Engl J Med 1969; 280: 337–45
- Barbeau A. L-dopa therapy in Parkinson's disease: a critical review of a nine years’ experience. Can Med Assoc J 1969; 101: 59–68
- Yahr MD, Duvoisin RC, Schear MJ, et al. Treatment of parkinsonism with levodopa. Arch Neurol 1969; 21: 343–354
- Nutt JG, Fellman JH. Pharmacokinetics of levodopa. Clin Neuropharmacol 1984; 7: 35–49
- Muenter MD, Tyce GM. L-dopa therapy of Parkinson's disease: plasma L-dopa concentration, therapeutic response, and side effects. Mayo Clin Proc 1971; 46: 231–239
- Tolosa E, Marti MJ, Valldeoriola F, Molinuevo JL. History of levodopa and dopamine agonists in Parkinson's disease treatment. Neurology 1998; 50(suppl 6)2–10
- Yeh KC, August TF, Bush DF, Lasseter KC, et al. Pharmacokinetics and bioavailability of Sinemet CR: A summary of human studies. Neurology 1989; 39(suppl 2)25–37
- Cedarbaum JM, Kutt H, McDowell FH. A pharmacokinetic and pharmacodynamic comparison of Sinemet CR (50/200) and standard Sinemet (25/100). Neurology 1989; 39(suppl 2)38–44