Abstract
Introduction. Acute gastroscopy is seldom advocated in cases of drug overdose. However, this intervention is sometimes recommended in cases where a pharmacobezoar of toxic tablets has formed. Case Reports. We describe two patients who were admitted after major ingestion of slow release clomipramine. In one case an abdominal x-ray was highly suspicious of a large pharmacobezoar in the stomach and in the other case a tablet conglomerate totally obstructed the oesophagus. Both conditions were successfully managed by acute gastroscopy. Discussion. There are limited and inconclusive recommendations in the literature concerning the optimal treatment of pharmacobezoars. Conclusion. This article provides further evidence that slow release clomipramine may be capable of forming a radio-opaque pharmacobezoar. The clinical courses in these two cases suggest that tablet removal by gastroscopy should be considered in selected cases of drug poisoning. Suspicion of a pharmacobezoar may warrant diagnostic investigations such as imaging studies and endoscopy.
Introduction
Endoscopic retrieval of tablets or capsules from the gastrointestinal (GI) tract after drug overdose is seldom advocated (Citation1). Some reports on this intervention, with variable clinical consequences, have been published (Citation2–4). In one of these case reports a pharmacobezoar of slow release theophylline tablets was successfully removed by gastroscopy after gastric lavage had failed (Citation2). In another case of overdose with slow release theophylline, a computed tomography was performed to investigate the cause of abdominal pain. The CT scan showed several tablet-shaped shadows in the stomach. Gastric lavage was ineffective, but endoscopic removal of a large number of tablets was feasible (Citation3). In a report of four cases of poisoning with slow release clomipramine, a pharmacobezoar in the gastric area was suspected on plain x-ray in each case. Three of these four patients underwent unsuccessful endoscopy. Moreover, the procedures were suspected of having caused GI haemorrhages (Citation4). It is difficult to know whether a pharmacobezoar has formed. Such formation should be suspected if, despite apparently effective GI decontamination, there is evidence of continuing absorption. If the case history or clinical findings give reason to suspect the presence of a bezoar, or if an aggregate of radio-opaque tablets is evident on x-ray, acute gastroscopy may be considered. In this situation, endoscopy is of diagnostic value (Citation2–8) and might also provide a treatment option (Citation2,Citation3,Citation6). However, the presence of a pharmacobezoar does not always necessitate its removal and endoscopy may in some cases carry an unacceptable risk/benefit ratio (Citation4). We describe two patients who were successfully managed by acute gastroscopy after major suicidal ingestion of slow release clomipramine.
Case reports
Case 1
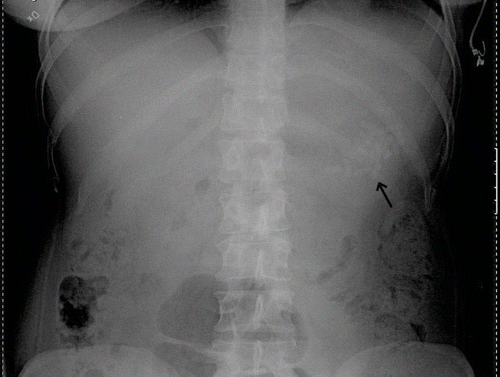
A 28-year-old woman with a borderline personality disorder phoned the hospital and stated that she had swallowed 60 slow release tablets of clomipramine 75 mg, in total 4.5 grams, but no other drugs. The ambulance arrived 30 minutes after the ingestion and the patient was given 25 g of activated charcoal during transport to hospital. She was asymptomatic on admission and displayed normal vital signs. An ECG was normal. Gastric lavage with 7 liters of fluid was performed using an orogastric tube (size 30 Fr), but there were no obvious signs of tablet retrieval. An additional 50 g of activated charcoal was administered and the patient was transferred to the intensive care unit (ICU) for further observation. The poisons information centre recommended an immediate x-ray investigation, since slow release clomipramine has been shown to be radio-opaque (Citation4). An abdominal x-ray 4 hours after the ingestion suggested the presence of a large pharmacobezoar in the stomach (). It was decided to anesthetize and intubate the patient in order to try to remove the suspected concretion endoscopically. An experienced surgeon performed the gastroscopy at the ICU. When the bezoar had been confirmed visually in the fundus of the stomach, a gastric tube intended for gastric lavage (30 Fr) was taped side to side to the gastroscope. Additional side orifices were added by a pair of scissors. The pharmacobezoar was crushed with pliers and the debris was sucked out through the gastric tube. Large lumps were grasped with a wire basket or pliers and extracted by removing the whole instrument. The presence of black charcoal in the stomach impaired the visibility. The gastroscope was inserted and removed 12 times altogether and it was estimated that 40–50 tablets in the form of whole and partial together-melted and crunched lumps were extracted in this way. No signs of bleeding were noted. After the endoscopic procedure, the patient was wakened and remained asymptomatic during 24 hours of observation in the ICU. Repeated ECG recordings and routine laboratory tests were normal and there were no complications attributable to the endoscopic intervention.
Case 2
A 25-year-old woman was taken to hospital approximately 4 hours after a witnessed intake of at least 6 grams of clomipramine in a slow release preparation (Anafranil-Retard 75 mg, 80 depot tablets) and 120 mg oxazepam (Sobril 10 mg, 12 tablets). She had vomited once before arrival. On admission her level of consciousness was distinctly reduced but she was arousable on strong painful stimulation. The heart rate was 120 beats/minute and the blood pressure 150/90 mmHg. The ECG showed sinus tachycardia with non-specific ST-T changes but normal QRS complexes. The patient was prophylacticly intubated prior to a planned gastric lavage and intended administration of charcoal. However, after successful orotracheal intubation, it proved impossible to pass the gastric tube through the oesophagus, despite several attempts by a senior physician. It was therefore decided to perform a gastroscopy to clarify if there was any anatomical malformation or a tablet obstruction. This intervention was undertaken 5.5 hours after the overdose at the surgical operation unit by a skilled otolaryngologist. The endoscope easily entered the upper esophagus, but approximately 10–15 cm below the larynx he noted large amounts of tablet residues and granular material, which were extracted through the endoscope. In the distal part of the oesophagus an initially impenetrable, cement-like obstruction was observed. With great efforts including the use of pliers, flushing and suction, it was finally possible to remove this conglomeration and enter the stomach. Some tablet residues were noted in the stomach also, and these could easily be sucked out through the endoscope. A nasogastric tube was inserted and 50 g of activated charcoal was instilled. The patient was thereafter admitted to the ICU for mechanical ventilation and further observation, but never showed any significant signs of cardiovascular disturbances. Extubation was performed 20 hours post-ingestion, after which the patient gradually woke up. After another 24 hours of observation in a medical ward she had returned to her normal status. All routine laboratory tests were normal, including repeated check-ups of arterial blood gases, electrolytes, and haemoglobin.
Discussion
Serum levels of clomipramine were not measured in the two cases described above. However, a massive intake of clomipramine was evident in the first patient from herown history and was witnessed in the second case. Moreover, endoscopically retrieved pieces of tablets from the concretions more or less verified the exposure in both cases, even though no clomipramine analysis was performed. Neither of the two patients developed severe symptoms, despite their intake of large and highly toxic doses. It seems reasonable to assume that the favorable clinical courses were due to the removal of the pharmacobezoars together with the administration of activated charcoal. The expected clinical consequences after overdoses with slow release clomipramine in the dose range in question are severe and include CNS depression, seizures, pathological ECG changes, dysrythmias and cardiovascular instability (Citation4,Citation9).
Slow release clomipramine in overdose has previously been implicated in causing pharmacobezoars (Citation4). Other medications reported to occasionally form bezoars include amitriptyline, potassium chloride in sustained release (SR) preparations, SR procainamide, SR nifedipine, SR carbamazepine, SR meprobamate, SR iron, and also verapamil and theophylline with or without SR, and enteric-coated aspirin (Citation2,Citation5–8,Citation10). Besides overdose, risk factors for the formation of pharmacobezoars include dehydration, alterations in the GI anatomy, diseases or disorders causing dysmotility of the GI tract, and certain co-ingested medications, e.g., anticholinergic drugs and opiates (Citation5).
In case 1, a pharmacobezoar was suspected at the x-ray examination. Slow release clomipramine preparations in overdose have previously been associated with radio-opaque pharmacobezoar formation (Citation4). This is probably due to the plastic skeleton that is part of the slow release mechanism. This knowledge, in conjunction with the history, led us to recommend the x-ray investigation. Other medications reported to be visible on abdominal x-ray or computed tomography include sustained release preparations of chlorpromazine, lithium, potassium chloride and salicylates, as well as iron with or without SR (Citation5,Citation6). Conglomerations of medications other than these may also be visible on x-ray (Citation10). A plain abdominal x-ray or CT scan can only indicate the presence of a pharmacobezoar, however, and can not exclude it (Citation6). In case 2, the presence of large quantities of depot tablets or even a bezoar in the upper GI tract was suspected because of the witnessed massive intake and the obvious obstruction of the oesophagus. Suspicion of a pharmacobezoar should also be raised in cases of repeated vomiting, bleeding or abdominal pain as signs of GI obstruction or perforation (Citation5), in cases of a protracted clinical course without improvement, or of continuing drug absorption despite presumably effective GI decontamination (Citation6).
The recommendations in the literature concerning the optimal treatment of patients with pharmacobezoars are limited, variable, and inconclusive (Citation1–6,Citation8,Citation10). As these cases are rare, there are no clinical studies to rely on. Factors to consider are the toxicity of the ingested pharmaceutical, the size and location of the pharmacobezoar and the symptoms and condition of the patient. Suggested management options include commonly applied measures such as gastric lavage, repeated administration of activated charcoal, close observation, and monitoring of the plasma drug concentrations. However, less consistently used methods such as whole bowel irrigation or endoscopic retrieval followed by GI decontamination are also recommended in selected cases. Surgical removal by gastrotomy or laparotomy has also been advocated (Citation5), especially in patients with bowel obstruction caused by iron bezoars (Citation6,Citation11).
In the two cases presented here it was decided to use the endoscopic option and the pharmacobezoars were removed without complications. This may have shortened the ICU stay and reduced the need for repeated x-rays and laboratory monitoring. In addition it may have prevented the development of severe symptoms. The endoscopists involved suggested that better designed endoscopic tools for pharmacobezoar extraction would help facilitate these procedures. Lapostolle et al. (Citation4) reported a case series with very similar clinical circumstances. Three of the four patients in their report underwent endoscopy, but in those cases no significant amounts of the clomipramine conglomerations were retrieved. Furthermore, the endoscopic procedures were suspected of having caused significant haemorrhages in all three cases. We have no obvious explanation for the discrepancy in the clinical consequences of the endoscopic interventions between their cases and ours.
In conclusion, the present report provides further evidence that slow release clomipramine may be capable of forming a pharmacobezoar which may be radio-opaque. Moreover, the clinical courses of our two cases suggest that tablet removal by gastroscopy should be considered in selected cases of drug poisoning. Suspicion of a pharmacobezoar may warrant diagnostic investigations such as imaging studies and endoscopy. However, gastroscopic fragmentation and extraction of a pharmacobezoar is not an easy task and may cause complications, hence, the procedure is recommended only if a suitably experienced endoscopist is available.
References
- Nelson L. As if there weren't enough controversies in gastrointestinal decontamination. J Toxicol Clin Toxicol 2000; 38: 483–4
- Cereda JM, Scott J, Quigley EMM. Endoscopic removal of pharmacobezoar of slow release theophylline. BMJ 1986; 293: 1143
- Saeki S, Shimoda T, Sakai H, Soejima Y, Matuse H, Kohno S. Successful treatment of theophylline toxicity by upper gastrointestinal endoscopy. Respir Med 2003; 97: 734–5
- Lapostolle F, Finot MA, Adnet F, Borron SW, Baud FJ, Bismuth C. Radiopacity of clomipramine conglomerations and unsuccessful endoscopy: Report of 4 cases. J Toxicol Clin Toxicol 2000; 38: 477–82
- Taylor JR, Streetman DS, Castle SS. Medication bezoars: A literature review and report of a case. Ann Pharmacother 1998; 32: 940–6
- Buckley NA, Dawson AH, Reith DA. Controlled release drugs in overdose. Clinical considerations. Drug Safety 1995; 12: 73–84
- Peeters JWPM, van der Werf SDJ. Gastric stenosis after potassium chloride ingestion. Endoscopy 1998; 30: 110
- Schwartz HS. Acute meprobamate poisoning with gastrotomy and removal of a drug-containing mass. N Engl J Med 1976; 295: 1177–8
- Liebelt EL. Cyclic antidepressants. Goldfrank's Toxicologic Emergencies8th, Flomenbaum, Goldfrank, Hoffman, Howland, Lewin, Nelson. McGraw-Hill, New York 2006; 1083–97
- Robert R, Frat J-P, Veinstein A, Rouffineau J. Protective effect of medication bezoar after a massive beta-blocker, digoxin, and amitriptyline poisoning. J Toxicol Clin Toxicol 2005; 43: 381–2
- Landsman I, Bricker JT, Reid BS, Bloss RS. Emergency gastrotomy: Treatment of choice for iron bezoar. J Pediatr Surg 1987; 22: 184–5