Abstract
Introduction. The severity of ethylene glycol toxicity is related to the metabolic acidosis resulting from the biotransformation of ethylene glycol into toxic metabolites. Glycolic acid causes severe acidosis and oxalate precipitates as calcium oxalate in the kidneys and other tissues. Case Report. An adult male was taken to the local hospital by the team rescue and was apparently unconscious; severe metabolic acidosis and renal failure led to death a few hours after the arrival. Confocal laser scanning microscopy demonstrated oxalate crystals deposition within the tubular epithelial cells and widespread necrosis of the tubular epithelium in the proximal tubules. Toxicological examinations revealed ethylene glycol; the blood level was 250 mg/L and in urine the concentration was 0.3%. Discussion. In cases of ethylene glycol poisoning, calcium oxalate may be excreted not only as dihydrate crystals, but also as monohydrate crystals. Direct toxicity, cortical edema, and inhibition of mitochondrial activity, as evidenced by decreased succinate dehydrogenase activity, are possible mechanisms of crystal damage. Since calcium oxalate monohydrate crystals are transported intracellularly by kidney cells, the renal toxicity of ethylene glycol may result from inhibition of mitochondrial respiratory function in proximal tubular cells by calcium oxalate monohydrate crystals. Conclusions. The histologic diagnosis of acute renal failure secondary to ethylene glycol poisoning depends on the recognitions of the changes of acute tubular damage in association with calcium oxalate crystals deposition within the tubular epithelial cells and the widespread necrosis of the tubular epithelium in the proximal tubules.
Introduction
Ethylene glycol (EG) is a common ingredient in products such as antifreeze, hydraulic brake fluids, household cleaners, and cosmetics. EG toxicity is related to its metabolites which cause metabolic acidosis and lead to cardiopulmonary depression, acute renal failure and central nervous system deficits (Citation1). The presence of calcium oxalate crystals in the urine and the development of renal failure are characteristics of EG poisoning. We present a fatal case of EG poisoning in which microscopic examinations of urine and kidney specimens established a post-mortem diagnosis of EG poisoning and identified the precise location of calcium oxalate crystals in the proximal tubules of the kidneys.
Case report
A 33-year-old man was found unconscious in a goods wagon he used for shelter and taken to the local hospital by the rescue team. The results of an arterial blood gas analysis were pH 7.094, pCO2 18.7 mmHg, pO2 89.8 mmHg, HCO3- 5.8 mmol/L, BE -21.3 mmol/L. Chemistry results were creatinine 3.83 mg/dL, urea 58.3 mg/dL, glucose 106 mg/dL, potassium 5.37 mEq/L, sodium 141 mEq/L and calcium of 7.0 mg/dL. The anion gap was 46 mmol/L. Hematology results were WBC 15,900/mm3, RBC 5,410,000/mm3, platelets 291,000/mm3, hemoglobin 15.2 g/dL, and hematocrit 46%. An electrocardiogram showed sinus rhythm at a rate of 120 beats/minute with a partial right bundle branch block but no evidence of acute ischemia. Twenty minutes after arrival, the patient exhibited fixed pupils and had cardiac arrest; prompt cardiopulmonary resuscitation attempts were unsuccessful. Witnesses reported that he had a history of alcohol abuse and, in the very last days before his death, he had been adding ethylene glycol to his wine.
A complete autopsy was performed 30 hours after death. External examination revealed no signs of injury. Internal examination was unremarkable except for edema of lungs and brain, and intense hyperemia. Microscopic analysis of the urine showed numerous calcium oxalate monohydrate crystals and many erythrocytes. Routine microscopic histopatological studies were performed using formalin fixed paraffin-embedded tissue sectioned at 4 μm and stained with hematoxylin-eosin. The specimens were examined under transmitted bright field illumination and phase contrast mode using a light microscope (DM 4000 B Leica, Cambridge, UK) connected to a photocamera computer system (DC 480 Leica). A three-dimensional reconstruction of tissues by means of a confocal microscope (True Confocal Scanner, Leica TCS SP2) was performed.
Histological examination revealed massive pulmonary and cerebral edema. Examination of the renal tubules and leptomeningeal vessels showed brownish and slightly metachromatic deposits with the typical morphology of calcium oxalate crystals. Three-dimensional images of histological sections on confocal laser scanning microscopy were recorded (). Birefringence of crystals was evident on polarized microscopy and a 3-D reconstruction was made by a confocal laser scanning microscopy. Oxalate crystals deposition within the tubular epithelial cells and widespread necrosis of the tubular epithelium in the proximal tubules were evident (). Monohydrate and dihydrate crystals in urine were visualized with the typical needle-like appearance and the symmetrical mussel-shaped appearance, respectively ().
Fig. 1. Oxalate crystals deposit within the renal tubules. (A) These deposits are laminated (in black) and (B) birefringent under polarized light (H&E x40); (C) visualization of the oxalate crystals deposits with confocal laser scanning microscope; (D) tubular epithelial cells with cytoplasmic swelling and acute necrosis; (E) birefringent crystals in the renal tubules; (F) scan for interstitial connective and (G) definitive evidence of calcium oxalate crystals within the renal tubules (in white); interstitial connective in green, erythrocytes in yellow and epithelial nuclei in red.
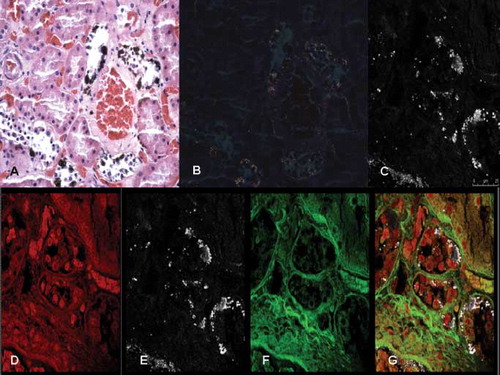
Fig. 2. Confocal laser scanning microscopy study of the kidneys. (A) Oxalate crystals deposition within the tubular epithelial cells: widespread necrosis of the tubular epithelium in the proximal tubules; (B) and (C) high magnification (inserts) of the oxalate crystals deposition in a 3-D view; and (D) 3-D reconstruction of oxalate crystals (in pink) and the same image (insert) obtained from the z-lines cut surface.
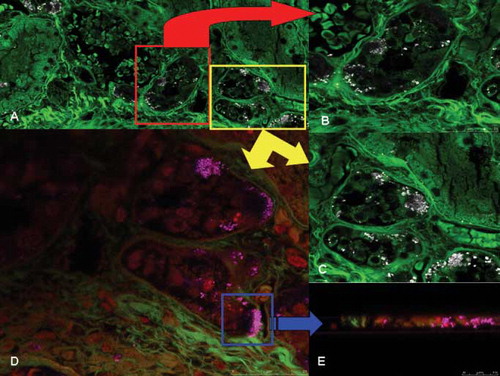
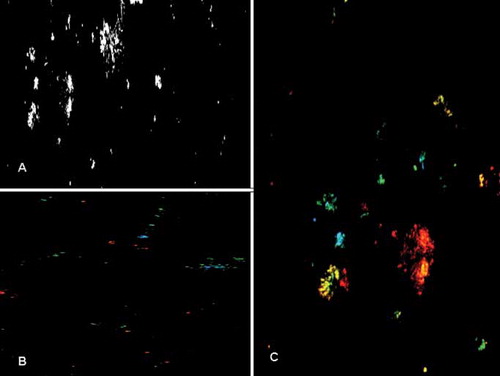
Fig. 3. Calcium oxalate crystals in urine observed under confocal laser scanning microscope. (A) Needle-like appearance of calcium oxalate crystals. Monohydrate crystals are visualized (B) in green and blue colors under different laser beams; and (C) dihydrate crystals with the characteristic symmetrical mussel-shaped appearance are visualized in red and yellow.
Toxicological examinations found ethylene glycol: 250 mg/L in the blood and 0.3% in the urine. There was no ethanol in the blood or urine and a toxicological screen of the urine identified no heroin, cocaine, amphetamine or methamphetamine. The man's bottle and the wine glass contained ethylene glycol/ethanol in a concentration of 76%mL/mL.
Discussion
In the present case, post-mortem findings and toxicological analysis supported the conclusion that this was an unintentional fatal case of EG acute oral intoxication. The toxicity of EG is due to a combination of the severe metabolic acidosis caused by glycolic acid and the precipitation of calcium oxalate crystals resulting in impaired organ function, especially in the kidneys (Citation2). Deposition of calcium oxalate crystals, primarily in proximal tubule epithelium, contributes to the renal parenchymal toxicity. Renal toxicity is ascribed to the terminal metabolite oxalic acid, which precipitates in the kidney in the form of calcium oxalate crystals and is believed to cause physical damage to the renal tubules. The human relevance of the renal toxicity of EG is indicated by the similarity between animals and humans of metabolic pathways, the observation of renal oxalate crystals in toxicity studies in experimental animals and human poisonings, and cases of human kidney and bladder stones related to dietary oxalates and oxalate precursors (Citation3). A recent working hypothesis is that the ethylene glycol metabolite oxalate accumulates in the renal proximal tubule in sufficient amounts to form calcium oxalate monohydrate crystals, which are internalized by the proximal tubular cell, where they produce mitochondrial damage that results in cell death, tubular necrosis and the resulting renal failure (Citation4). In this study has been demonstrate that calcium oxalate monohydrate crystals, and not the oxalate ion, damages rat kidney mitochondria and induces the mitochondrial permeability transition, which may then lead to renal cell death (Citation4). Since calcium oxalate monohydrate crystals are transported intracellularly by kidney cells, the renal toxicity of ethylene glycol may result from inhibition of mitochondrial respiratory function in proximal tubular cells by calcium oxalate monohydrate crystals. Production of the mitochondrial permeability transition is known to inhibit ATP synthesis by mitochondria and is a major cause of necrotic cell death. Hence, interference with mitochondrial energy production may be an important factor in the renal injury induced by accumulation of calcium oxalate monohydrate crystals in kidney tissue during acute and subchronic ethylene glycol exposure (Citation4).
Conclusions
The histologic diagnosis of acute renal failure secondary to EG poisoning depends on the recognitions of the changes of acute tubular damage in association with tissue oxalate deposition within the tubular epithelial cells and the widespread necrosis of the tubular epithelium in the proximal tubules (Citation5). Calcium oxalate monohydrate crystals, and not the oxalate ion, are responsible for the membrane damage and cell death observed in normal human and rat PT cells and suggest that calcium oxalate monohydrate crystals accumulation in the kidney is responsible for the renal toxicity associated with ethylene glycol exposure (Citation6). The presence in vivo of forms of oxalate other than calcium oxalate monohydrate crystals may eventually be demonstrated to have little impact on the overall toxicity associated with ethylene glycol poisoning (Citation7). Either and/or both calcium oxalate monohydrate and calcium dihydrate crystals might form and interact with kidney epithelium (Citation8,Citation9).
In our fatal case, the specimens were examined by confocal laser scanning microscopy for calcium crystals within the tubular lumen, attached to the tubular walls or internalized into the tubular cells. We observed crystals attached to the tubular cell epithelium and some of them were seen inside the tubular cells, demonstrating that crystal-cell interaction resulted in movement of crystals from the tubular lumen into the cells as the results in kidney tissue of crystal adhesion and/or endocytosis (Citation10). These findings may be of interest to better understand the genesis of renal damage and for future therapeutic applications (Citation11,Citation12).
References
- Froberg K, Dorion RP, McMartin KE. The role of calcium oxalate crystal deposition in cerebral vessels during ethylene glycol poisoning. Clin Toxicol 2006; 44: 315–318
- Jacobsen D. Ethylene Glycol and other Glycols. Critical Care toxicology. Diagnosis and management of the critically poisoned patient, J Brent, KL Wallace, KK Burkhart, SD Phillips, JW Donovan. Elsevier Mosby, PhiladelphiaUSA 2005; 777–790
- Corley RA, Meek ME, Carney EW. Mode of action: oxalate crystal-induced renal tubule degeneration and glycolic acid-induced dysmorphogenesis-renal and developmental effects of ethylene glycol. Crit Rev Toxicol 2005; 35: 691–702
- McMartin KE, Wallace KB. Calcium oxalate monohydrate, a metabolite of ethylene glycol, is toxic for rat renal mitochondrial function. Toxicol Sci 2005; 84: 195–200
- Eder AF, McGrath CM, Dowdy YG, Tomaszewski JE, Rosenberg FM, Wilson RB, Wolf BA, Shaw LM. Ethylene glycol poisoning: Toxicokinetic and analytical factors affecting laboratory diagnosis. Clin Chem 1998; 44: 168–177
- Guo C, McMartin KE. The cytotoxicity of oxalate, metabolite of ethylene glycol, is due to calcium oxalate monohydrate formation. Toxicol 2005; 208: 347–355
- Lewin-Smith MR, Kalasinsky VF, Mullick FG. Correspondence Re: C. Guo, K.E. McMartin, The cytotoxicity of oxalate, metabolite of ethylene glycol, is due to calcium oxalate monohydrate formation. Toxicology 2006; 222: 160–161
- Yamaguchi S, Wiessner JH, Hasegawa AT, Hung LY, Mandel GS, Mandel NS. Study of a rat model for calcium oxalate crystal formation without severe renal damage in selected conditions. Int J Urol 2005; 12: 290–298
- Khan SR, Thamilselvan S. Nephrolithiasis: A consequence of renal epithelial cell exposure to oxalate and calcium oxalate crystals. Mol Urol 2000; 4: 305–312
- Ebisuno S, Kohjimoto Y, Tamura M, Inagaki T, Ohkawa T. Histological observations of the adhesion and endocytosis of calcium oxalate crystals in MDCK cells and in rat and human kidney. Urol Int 1997; 58: 227–231
- Khan SR. Hyperoxaluria-induced oxidative stress and antioxidants for renal protection. Urol Res 2005; 33: 349–357
- Pizon AF, Brooks DE. Hyperosmolality: another indication for hemodialysis following acute ethylene glycol poisoning. Clin Toxicol 2006; 44: 181–183