Abstract
Background. Aluminum phosphide poisoning has high mortality resulting from cardiac impairment and hemodynamic disorders. We report two cases of aluminum phosphide associated with reversible myocardial injury. Cases Reports. A 19-year-old woman and a 28-year-old man were admitted to hospital following ingestion of aluminum phosphide. The clinical course was characterized by the development of a shock syndrome requiring the use of vasoactive amines in the woman. However, the arterial hypotension in the man was improved by fluid filling and vasoactive drugs. The myocardial injury was objectively documented in both cases. The electrocardiogram showed ST-segment elevations and diffusely abnormal repolarization. The plasma concentrations of cardiac enzymes were elevated. In the second case, echocardiography showed similar myocardial involvement with left ventricular hypokinesis (left ventricle ejection fraction 30%). In both cases, there was progressive improvement in hemodynamic status, cardiac traces, and biochemical values. A simultaneous improvement was observed in echocardiogram of the second case (left ventricle ejection fraction increased to 50%). Conclusion. Reversible myocardial injury following aluminum phosphide poisoning has been described in few cases. We objectively documented progressive clinical and electrical improvement in two cases.
Introduction
Phostoxin®, a rodenticide fumigant, is used for storage and protection of grains. It is available in tablets of 3 g consisting of 56% aluminum phosphide and 44% aluminium carbonate, and when it is in contact with humidity it discharges 1 g of phosphine (PH3) (Citation1). In Morocco, aluminum phosphide (AP) poisoning is a significant healthcare problem (Citation2) with a high mortality despite the progress of critical care. Most poisoned patients die from refractory cardiac shock (Citation1). Few cases of reversible cardiac injury have been described in the literature. We report two cases of AP poisoning with reversible myocardial toxicity.
Case reports
Case 1
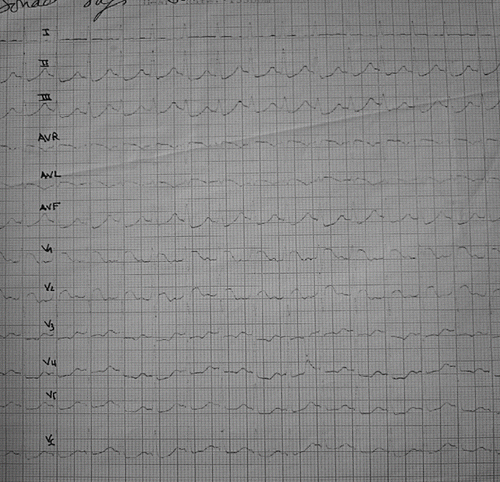
A 19-year-old woman unintentinally ingested three tablets of Phostoxin®. The substance was initially mixed with food for rodent control. At first, the patient complained of diffuse cramping abdominal pain. One hour later, she started vomiting. She was admitted to our critical care unit two hours after ingestion. Physical examination revealed profuse sweating, a heart rate of 110 beats/minute, and blood pressure of 120/80 mmHg. Chest radiography was normal. The admission electrocardiogram (ECG) showed ST-segment elevations in leads V1 and V2 (). Serum concentrations of aspartateaminotransferase (AST) and alanineaminotransferase (ALT) were 209 IU/L (N = 45 IU/L) and 280 IU/L (N = 50 IU/L), respectively. The blood concentration of creatine kinase (CK) was 1200 IU/L (N = 100 IU/L). Toxicological blood screening was positive for AP. Gastric lavage was performed on admission. One hour later, the patient became hypotensive (BP 70/40 mm/Hg) and was given 1 liter of crystalloid (normal saline) without improvement. A central venous pressure of 18 cmH2O was recorded. Dobutamine was administrated in progressive doses (20 μgs/kg/min), which stabilized the hemodynamic state. The electrocardiogram showed increase of ST-segment in anteroseptal territory (). Echocardiography revealed diffuse myocardial injury in both ventricles with severe hypokinesia in the left ventricle (LV). The left ventricle ejection fraction (LVEF) was ≤20%. The right ventricle was also dilated and hypokinetic. Over the ensuing days there was progressive improvement in the hemodynamic state that permitted the weaning of dobutamine on day 8 after admission. A simultaneous improvement was observed in electrocardiographic and biochemical values. The echocardiography done on day 8 revealed an LVEF of 30%. The patient was discharged after 10 days of hospitalization.
Case 2
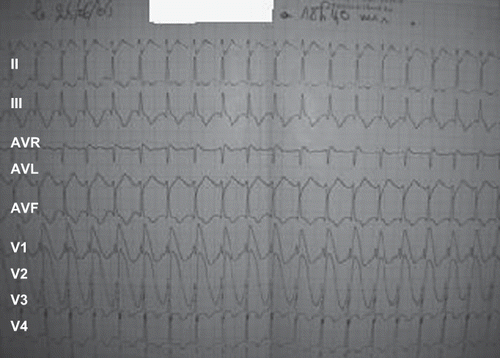
A 28-year-old man was being monitored for depressive syndrome for six months prior to admission. He intentionally ingested one 3 g tablet of Phostoxin® dissolved in water. Two hours later, he presented at the emergency department with profuse vomiting, intense epigastric pain, and dizziness. He received gastric lavage and was immediately transferred to the intensive care unit. Physical examination upon admission revealed a normal level of consciousness, blood pressure of 110/60 mmHg, and heat rate of 108 beats/minute. The chest x-ray was normal. The electrocardiogram on admission showed elevated ST-segments in leads V2, V3, and V4 (). Laboratory examinations revealed an international normalized ratio of 2.02, troponin 37.5 ng/ml, and CK 1110 IU/L. The toxicological blood screening was positive for AP. Two hours after admission, hypotension developed (BP 90/50) and then improved by vascular replenishment (1 liter of normal saline) and vasoactive drugs administration (dobutamine 5 μgs/kg/min). On day 2 of hospitalization, echocardiography demonstrated a homogeneous global hypokinesia of the LV with LVEF at 30% (). At four days post-ingestion, the serum concentrations of AST and the ALT were 137 IU/L and 134 IU/L, respectively. Both biologic and echocardiographic parameters later improved. The LVEF increased from 30% to 50% () on day 8 post-ingestion and the troponin concentration decreased to 3.5 ng/ml on day 7 after admission. The hepatic enzymes normalized and the patient was discharged after 10 days.
Fig. 3. Electrocardiogram in admission of the second patient showing ST-segment elevated in leads V1, V2, and V3.
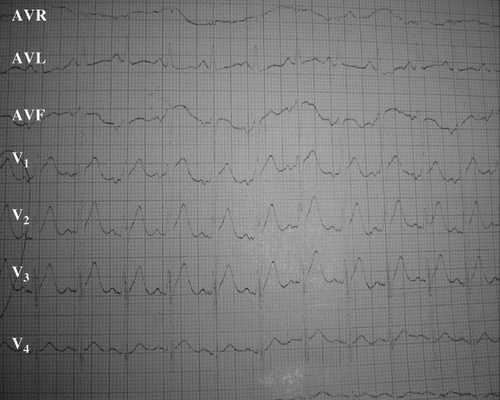
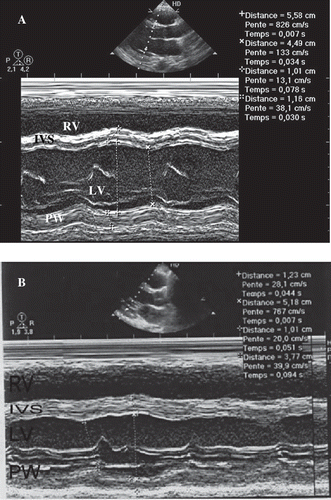
Fig. 4. Echocardiographically parasternal long axis depicted improvement of ventricle function in the second case. A) Echocardiogram obtained on the second day after admission depicts global hypokinesis with LVEF of 30% (calculated by Simpson method) and dilatation of LV. B) Echocardiogram taken eight days after admission showed improvement of ventricle function LVEF of 30%. RV: right ventricle; IVS: interventricular septum; LV: left ventricle; PW: posterior wall.
Discussion
Myocardial injury following AP poisoning has been documented on ECGs in several studies (Citation3–6), but the use of echocardiography is rarely described in the literature. AP-induced cardiotoxicity was responsible for a high level of mortality, about 59%, in the Sing series (Citation7). The pathogenesis of AP toxicity is metabolic. Indeed, immediate release of phosphine gas from AP when mixed with aqueous solutions leads to rapid absorption thorough the lungs. This is responsible for systemic poisoning with cellular hypoxia by oxidative phosphorylation inhibition (Citation1,Citation8). Cardiac toxicity due to AP and phosphine exposure is represented by a depression in myocardial cellular metabolism, as well as myocardial necrosis due to the release of reactive oxygen intermediates (Citation9). Indeed, significant decreases in glutathione concentrations were shown in different tissues during AP poisoning (Citation10). Glutathione is known to be an important factor protecting against oxidation by catalyzing the reduction of the oxygen peroxide in O2 and H20 (Citation10). We think that the ingestion of 3–9 g of AP was not lethal in our two cases and this can be explained by early provocated vomiting 15–30 minutes after ingestion. In fact, absence of vomiting has been found in some studies to be a severity factor (Citation2).
Hypermagnesemia (Citation11,Citation12) and hypomagnesemia (Citation13,Citation14) following acute phosphine poisoning was described in the literature but the pathogenesis of magnesium level abnormalities was not clear. In our cases, the serum concentration of magnesium was not checked because of the lack of resources in our hospital. The toxicity of AP is systemic and can affect all organs, but particularly cardiac and vascular tissues. In one study, 64% of 29 patients presented with obvious cardiac dysfunction of which 40% died in spite of adequate treatment (Citation15). In another study concerning 32 patients, 28 presented cardiac dysrhythmias and 15 had shock (Citation4). The mechanism of this shock is not clear but clinical, biological, and electrical observations suggest that myocardial involvement is responsible for the acute circulatory insufficiency (Citation16). However, a hypovolemia induced by several episodes of vomiting could explain the shock since the hypotension was corriged partially by fluid filling in the second case. A reversible dysfunction of the left ventricle at the end of five days has been described (Citation3,Citation17). This is similar to our patients who developed a state of shock with ST-segment elevations and diffusely abnormal repolarization. Chugh et al. reported that 50% of cases presented electrocardiographic abnormalities (Citation5) and Gupta et al. found ECG abnormalities in 80% of cases, including 40% with abnormalities of the ST-segment and the T wave (Citation3). Echocardiography of our two patients found a global hypokinesia of the LV. This result is similar to that the study of Bhasin et al. who objectively showed a generalized hypokinesis of LV wall and interventricular septum in 80% of their cases. Indeed, this study revealed akinesis and pericarditis in 3% and 35% of the cases, respectively (Citation18). Thus, the study of Bajaj et al. showed a global hypokinesis of the LV in the three patients that underwent serial venticulography (Citation19). However, other authors have described a focal myocardial necrosis induced by acute phosphine poisoning (Citation11,Citation20). The improvement of myocardial necrosis was progressive on day 8 of hospitalization. Both of our cases recovered following the correction of cardiovascular shock. In our first case, clinical improvement permitted the discontinuation of the vasoactive drugs; hemodynamic stabilization in the second case required essentially vascular replenishment by crystalloids and low doses of vasoactive amines. In addition, the cardiac enzymes decreased, ST-segment abnormalities resolved, and echocardiography demonstrated in the second case improved myocardial contractility.
Despite the severity of the poisoning, as judged by the cardiac failure and the unavailability of an antidote, we noted a favorable outcome in our patients. This may, in part, be explained by the vomiting occurring after ingestion and the emergency care.
Conclusion
Myocardial injury following AP poisoning is responsible for significant mortality, primarily due to hemodynamic failure. The reversibility of this toxic myocarditis depends on optimal support of the cardiovascular system.
Acknowledgements
We wish to acknowledge the contribution of Dr. Ibtissam Khoudri and Dr. Rachid Benzidia for assistance in the preparation of this article.
References
- Gupta S, Ahlawat SK. Aluminium posphide poisoning. A review. J Toxicol Clin Toxicol 1995; 33: 19–24
- Hajouji Idrissi M, Oualili L, Abidi K, Abouqal R, Kerkeb O, Zeggwagh AA. Severity factors of aluminum phosphide poisoning (phostoxin). Ann Fr Anesth Reanim 2006; 25: 382–385
- Gupta MS, Malik A, Shama VK. Cardiovascular manifestaions in aluminium phosphide poisoning with special reference to echocardiographic changes. J Assoc Physicians India 1995; 43: 773–774; 779–780
- Singh RB, Rastogi SS, Singh DS. Cardiovascular manifestations of aluminium phosphide intoxication. J Assoc Physicians India 1989; 37: 590–592
- Chugh SN, Chung K, Ram S, Malhotra KC. Electrocardiographic abnormalities in aluminium phosphide poisoning with special reference to its incidence, pathogenesis, mortality and histopathology. J Indian Med Assoc 1991; 89: 32–35
- Siwach SB, Singh H, Sharma D, Katyal VK, Bhardwaj G. Cardiac arrhythmias in aluminium phosphide poisoning studied by on continuous holter and cadioscopic monitoring. J Assoc Physicians India 1998; 46: 598–601
- Singh S, Singh D, Wig N, Jit I, Sharma BK. Aluminum phosphide ingestion – a clinico-pathologic study. J Toxicol Clin Toxicol 1996; 34: 703–706
- Abder-Rahman H. Effect of aluminium phosphide on blood glucose level. Vet Human Toxicol 1999; 41: 31–32
- Chugh SN, Kolley T, Kakkar R, Chugh K, Sharma A. A critical evaluation of anti-peroxydant effect of intravenous magnesium in acute aluminium phosphide poisoning. Magnes Res 1997; 10: 225–230
- Hsu C-H, Chi B-C, Liu M-Y, Li J-H, Chen C-J, Chen R-Y. Phosphine-induced oxidative damage in rats: Role of glutathione. Toxicology 2002; 179: 1–8
- Singh RB, Singh RG, Singh U. Hypermagnesemia following aluminium phosphide poisoning. Int J Clin Pharmacol Ther Toxicol 1991; 29: 82–85
- Singh RB, Saharia RB, Sharma VK. Can aluminium phosphide poisoning cause hypermagnesemia? A study of 121 patients. Magnes Trace Elem 1990; 9: 212–218
- Siwach SB, Singh P, Ahlawat S, Dua A, Sharam D. Serum and tissue magnesium content in patients of aluminium phosphide poisoning and critical evaluation of high dose magnesium sulphate therapy in reducing mortality. J Assoc Physicians India 1994; 42: 107–110
- Chugh SN, Jaggal KN, Sharma A, Arora B, Malhotra KC. Magnesium levels in acute cardiotoxicity due to aluminium phosphide poisoning. Indian J Med Res 1991; 94: 437–439
- Khosla SN, Nand N, Kumar P. Cardiovascular complications of aluminium phosphide poisoning. Angiology 1988; 39: 355–359
- Lall SB, Sinha K, Mittra S, Seth SD. An experimental study on cardiotoxicity of aluminum phosphide. Indian J Exp Biol 1997; 35: 1060–1064
- Misra UK, Tripathi AK, Pandey R, Bhargwa B. Acute phosphing poisoning following ingestion of aluminium phosphide. Human Toxicol 1988; 7: 343–345
- Bhasin P, Mital HS, Mitra A. An echocardiographic study in aluminium phosphide poisoning (Abstract). J Assoc Physicians India 1991; 39: 851
- Bajaj R, Wasir HS, Agarwal R, Malhotra A, Chopra P, Bhatia ML. Alumnium phosphide poisoning. Clinical toxicity and outcome in eleven intensively monitored patients. Natl Med J India 1988; 1: 270–274
- Wilson R, Lovejoy FH, Jaeger RJ, Landrigan PL. Acute phosphine poisoning aboard a grain freighter. Epidemilogic, clinical and pathological findings. JAMA 1980; 244: 215–218