Abstract
Introduction. Prometryn is a triazine herbicide, which is one of the most extensively used groups of herbicides. The mechanism of acute triazine herbicide toxicity in humans is not known. We report a first case of acute prometryn poisoning. Case report. A 62-year-old male ingested 50 g of prometryn and ethanol in a suicide attempt. On arrival two hours after ingestion, he was somnolent and vomited. Seven hours after ingestion laboratory tests showed metabolic acidosis with a calculated anion gap of 47.5 mmol/L and lactate of 23.4 mmol/L. Gas chromatography/mass spectrometry revealed serum prometryn concentrations of 48.1 mg/L. Hemodialysis corrected metabolic acidosis, but the serum prometryn concentration increased to 67.7 mg/L. The lactate level after hemodialysis was 11.7 mmol/L and returned within normal limits 47 hours after ingestion. The patient was discharged without any sequelae after psychiatric evaluation. Conclusion. In high anion gap metabolic acidosis we should consider poisoning with prometryn and other triazine herbicides. Hemodialysis corrects metabolic derangements, but it does not lower serum prometryn concentration.
Introduction
Prometryn (2,4-bis(isopropylamino)-6-(methylthio)-s-triazine, CAS 7287–19–6, see ) is a triazine herbicide (Citation1). Triazine herbicides are one of the most extensively used groups of herbicide in the United States, particularly due to atrazine (2-chloro-4-ethylamino-6-isopropylamine-s-triazine), which is the second most commonly applied pesticide in the United States (Citation2). Prometryn and other triazine herbicides were prohibited in the European Union by the European Commission three years ago. Nevertheless, they can still be legally purchased in some member states of the European Union such as the United Kingdom, where triazine herbicides represented around 5% of all applied herbicides in 2004 (Citation3). Triazine herbicides are also registered for use in Africa, Asia, and the Pacific region.
Triazine herbicides inhibit photosynthetic electron transfer and oxidative phosphorylation in plants (Citation4). Acute animal triazine herbicide poisoning results in weakness, ataxia, increased body temperature, severe diarrhea, respiratory distress, hepatic and renal injury, and finally death (Citation5). The metabolism of prometryn by rats primarily occurs by N-dealkylation, conjugation through the sulfur group, sulfur oxidation and disulfide formation, and 90–98% of [14C] prometryn residues is eliminated in the urine and feces within seven days (Citation6). Surprisingly little is known about the acute toxicity of triazine herbicides in humans and there is only one case report of acute self-poisoning with the herbicide atrazine (Citation7). In the above-mentioned patient concomitant ingestion of atrazine, aminotriazole, ethylene glycol and formaldehyde resulted in coma, shock, metabolic acidosis, gastrointestinal bleeding, hepatic necrosis, renal failure, disseminated intravascular coagulation, and death after three days (Citation7). Additionally there is a report of five patients who accidentally ingested triazine explosive RDX (hexahydro-1,3,5-trinitro-1,3,5-triazine) (Citation8). Observed signs and symptoms in those patients included nausea, vomiting, abdominal tenderness, generalized tonic-clonic convulsions, postictal coma, generalized muscle spasms, myoclonus, sinus tachycardia, and ventricular premature beats (Citation8). Their laboratory results revealed leucocytosis, methemoglobinemia, hyperglycemia, hypokaliemia, elevated levels of serum aspartate transaminase, alanine transaminase, lactic dehydrogenase, creatine phosphokinase, and high anion gap metabolic acidosis (Citation8). The toxicokinetic parameters of prometryn in humans such as rate of absorption, distribution, metabolism, and elimination are not known. The toxic dose of triazine herbicides in their various formulations is also unknown, but may be high, as the oral lethal dose of prometryn in rats is 3150 mg/kg (Citation9). The treatment of acute poisoning is only supportive. Hemodialysis is unlikely to be useful since only 120 mg of atrazine was removed by hemodialysis in a man who had ingested a total of 100 g of atrazine (Citation7).
We report a first case of acute prometryn poisoning.
Case report
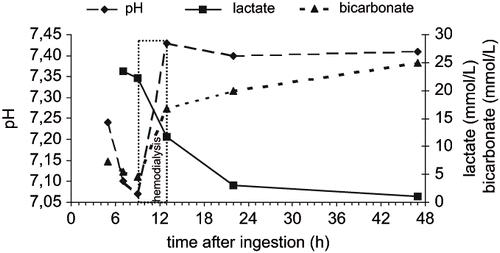
A 62-year-old male, weighing 68 kg, with no previous medical history ingested approximately 100 ml of Prohelan T (suspension concentrate of prometryn, 500 g/L) and an unknown quantity of wine in a suicide attempt two hours before arrival at the Emergency Department (ED). On arrival, he was somnolent and vomited white gastric content, which had a similar color to Prohelan T. His vital signs were tympanic temperature 36.3°C, pulse 90 beats per minute and blood pressure supine 150/80 mmHg, respiratory rate 24 counts/min, and SpO2 96% on room air. His remaining physical examination was otherwise unremarkable and no focal neurological signs were detected. His initial laboratory test results, obtained two hours after ingestion, were white blood cells 10.4 × 109/L, hemoglobin 14.1 g/dL, hematocrit 0. 42, platelets 355 × 106/L, serum sodium 147 mmol/L (147 mEq/L), potassium 3.2 mmol/L (3.2 mEq/L), chloride 101 mmol/L (101 mEq/L), glucose 9.4 mmol/L (168 mg/dL), BUN 4.3 mmol/L (12.1 mg/dL), creatinine 107 μmol/L (1.21 mg/dL), and myoglobine 6.1 nmol/L (107 μg/L). Serum hepatocellular enzyme levels were normal. Ethanol in serum was 43 mmol/L (195 mg/dL). An ECG revealed a normal sinus rhythm and the chest x-ray was normal. Gastric lavage was performed and 40g of activated charcoal was given on arrival. Afterwards, a continuous infusion of 5% glucose and 0.9% sodium chloride was started at a rate of 100 ml/h. During the subsequent three hours his condition gradually deteriorated. He became uncooperative and his pulse increased to 155 beats per minute, blood pressure supine to 190/100 mmHg and respiratory rate to 40 counts/min. The Kussmaul pattern of breathing indicated metabolic acidosis and arterial blood gas analysis five hours after ingestion indeed revealed pH 7.24, bicarbonate 7.3 mmol/L, base excess −17.5, pCO2 2.3 kPa (17.5 mmHg) and pO2 14.1 kPa (108.5 mmHg) while breathing ambient air (). Seven hours after ingestion laboratory tests showed serum sodium 150 mmol/L, potassium 3.9 mmol/L, chloride 97 mmol/L, glucose 6.0 mmol/L, BUN 3.9 mmol/L, creatinine 111 μmol/L, lactate 23.4 mmol/L, pH 7.10, bicarbonate 5.5 mmol/L, base excess −22.1, pCO2 2.4 kPa, and pO2 17.6 kPa. The calculated anion gap ([sodium] – [chloride] – [bicarbonate]) was 47.5 mmol/L (normal value: 8–12 mmol/L). Plasma osmolarity was not measured. The plasma butyrylcholinesterase level was 135 μkat/L (normal value: 88–215 μkat/L). Acetoactetate and beta-hydroxybutyrate in blood were negative. Oxalate crystals in urine were not present and urine toxicological screening with immunological tests did not reveal the presence of any drug of abuse (methamphetamine, cocaine, heroin, methadone, tetrahydrocannabinoids, benzodiazepines). Toxicological analysis with gas chromatography/mass spectrometry revealed prometryn in the gastric content and blood. Methanol, ethylene glycol and medications including acetaminophen, salicylates, isoniazid, iron, metformin, and theophylline as possible causes of metabolic acidosis, were not found. During subsequent hours diuresis gradually decreased. Nine hours after ingestion creatinine was increased to 130 μmol/L and arterial blood gas analysis revealed a further decrease in pH 7.07, bicarbonate 4.5 mmol/L, base excess −23.4 and pCO2 2.1 kPa. Afterwards a 4-hour-long bicarbonate hemodialysis was performed. The patient became orientated and cooperative, and blood pressure and respiratory rate normalized during hemodialysis. Laboratory results after hemodialysis, 13 hours after ingestion, showed white blood cells 15.7 × 109/L, hemoglobin 11.2 g/dL, hematocrit 0.32, platelets 183 × 106/L, serum sodium 141 mmol/L, potassium 4.1 mmol/L, chloride 102 mmol/L, creatinine 107 μmol/L, lactate 11.7 mmol/L, pH 7.43, bicarbonate 16.7 mmol/L, and pCO2 3.4 kPa. The calculated anion gap was 22.3 mmol/L. Ethanol in serum was 0.24 mmol/L. 18 hours after ingestion (five hours after hemodialysis) pneumonia was diagnosed and treatment with amoxycillin and clavulanic acid was started. 22 hours after ingestion (nine hours after hemodialysis) lactate level was 3.0 mmol/L and 47 hours after ingestion (34 hours after hemodialysis) lactate level was within normal limits: 0.6–2.4 mmol/L. Renal function did not deteriorate again and creatinine was normal on discharge. The liver function was normal all the time during hospitalization. The patient was discharged without any sequelae on the sixth day after psychiatric evaluation and pneumonia improvement. Three months later all laboratory results were within normal limits.
Fig. 2. pH, bicartbonate, and lactate levels after prometryn ingestion and treatment with hemodialysis.
Subsequent quantitative gas chromatography/mass spectrometry analysis of blood samples taken on arrival in hospital (two hours after ingestion) and after hemodialysis (13 hours after ingestion) revealed serum concentrations of prometryn 48.1 mg/L and 67.7 mg/L, respectively.
Serum prometryn concentration determination procedure
An aliquot of the patient's serum was extracted with butyl chloride at pH 9.6 (phosphate buffer). The organic solvent was evaporated under nitrogen and the residue dissolved in a small volume of methanol before analysis. 1 μL of the methanol solution was injected into the chromatographic system. An Agilent 6890 gas chromatograph (GC) and 5973N mass selective detector (MSD) in EI scan mode were used for analysis. The compounds were separated on a 30m HP-5MS ((5%-phenyl)-methylpolysiloxane) fused-silica capillary column (0.25 mm i.d., 0.25 μm film thickness) at a constant flow of 1 mL He/min and identified according to their mass spectra and retention times (PMW_TOX3.L). The injector temperature was 275°C, the oven temperature was initially 60°C (for 2 min) and then rose to 300°C at 20°C/min, and the detector temperature was 300°C. The total run time was 30 min. After identification the GC-MS instrument was calibrated with prometryn standards prepared in the same way as the patient's samples. The ion m/z 241 was used for quantification and the ion m/z 184 as a qualifier. The calibration curve was linear in the range of concentrations from blank to 70 mg/L, and extraction efficiency was more than 70%.
Discussion
This is the first case of acute prometryn poisoning in a human according to Medline database. Furthermore, it is also the first case report of acute poisoning with any triazine herbicide without concomitant toxins, except ethanol.
In this patient, depressed consciousness level and gastrointestinal symptoms with vomiting could be due to prometryn and ethanol poisoning. Hypokalemia was most probably the result of vomiting. Yet the most prominent feature in this patient was high anion gap metabolic acidosis determined using the following equation: anion gap = [sodium] – [chloride] – [bicarbonate] (Citation10). Interestingly, similar metabolic acidosis was also observed in patients poisoned with other triazines such as RDX and atrazine (Citation7,Citation8). The mechanism of metabolic acidosis in triazine herbicide poisoning in humans is not known, but tirapazamine (3-amino-1,2,4-benzotriazine-1,4-dioxide), which is a triazine anti-cancer drug, acts as an uncoupler of obligatory linkage between the respiratory chain and the oxidative phosphorylation in human cell mitochondria (Citation11), while simazine (6-chloro-N2,N4-diethyl-1,3,5-triazine-2,4-diamine), which is one of the triazine herbicides, is known to decrease parameters of oxidative phosphorylation in animals (Citation12). Inhibition of oxidative phosphorylation results in the accumulation of anionic intermediates of the Krebs cycle (citrate, isocitrate, alpha ketoglutarate, succinate, and malate), pyruvate and lactate. Accordingly the high anion gap in triazine herbicide poisoning could be the result of increased levels of intermediates of the Krebs cycle, pyruvate and lactate levels, which was indeed elevated in this patient. In future it would also be interesting to measure circulating anions associated with the Krebs cycle and pyruvate in triazine herbicide poisoned patients and to evaluate the possible inhibition of oxidative phosphorylation by prometryn. Uncoupling of the linkage between the respiratory chain and the oxidative phosphorylation by prometryn is questionable, since it does not have an acid dissociable group to carry the proton and a bulky lipophilic group to cross a membrane that are typical features of uncoupling agents () (Citation13).
Other causes of metabolic acidosis in this patient, such as hypotension and alcoholic ketoacidosis, were unlikely since the patient was not hypotensive, acetoacetate, and beta-hydroxybutyrate were negative, and alcohol withdrawal syndrome was not present. However, prometryn metabolites could contribute to unmeasured anions since the metabolism of prometryn by rats results in the formation of more than 30 free polar metabolites, but this should still be evaluated (Citation6). In the future, prometryn and probably other triazine poisoning should be considered in the differential diagnosis of high anion gap metabolic acidosis of unknown origin, what has not been the case in the articles dealing with metabolic acidosis as yet (Citation10).
Another interesting feature in this prometryn-poisoned patient was the improvement of clinical symptoms by hemodialysis and concomitant increase of serum prometryn concentration after hemodialysis compared to its concentration before hemodialysis. This observation indicates that serum prometryn concentration does not correlate with the clinical picture of poisoning and that hemodialysis cannot remove prometryn effectively, probably due to its low solubility in water (Kow logP = 3.1) and, consequently, high distribution volume (Vd) (Citation4). Nevertheless, hemodialysis effectively corrected high anion gap metabolic acidosis in prometryn poisoning and perhaps also removed its hydrophilic and possible toxic metabolites, what could contribute to the patient's clinical improvement during hemodialysis (Citation6). Interestingly, a similar correction of high anion gap metabolic acidosis resistant to sodium bicarbonate by hemodialysis was also observed in the above-mentioned patients who ingested triazine explosive RDX (Citation8). The administration of sodium bicarbonate in triazine poisoned patients for correction of acidosis is questionable since bicarbonate has only been shown to improve the outcome in patients poisoned with agents whose elimination may be increased through alkalinization and patients poisoned with drugs that cause a blockade of cardiac sodium channels (Citation10).
The delayed increase of serum prometryn concentration might indicate its slow gastrointestinal absorption with hemodialysis performed in the early phase of absorption while prometryn was still being absorbed, its redistributive kinetic pattern from fat stores being due to its high lipid solubility or just hemoconcentration during hemodialysis. However, this is unlikely since hematocrit was found to be decreased after hemodialysis.
In the future, gastric lavage and activated charcoal should be considered to reduce prometryn absorption, and hemodialysis only to correct high anion gap metabolic acidosis.
Conclusion
In high anion gap metabolic acidosis we should consider prometryn and other triazine herbicide poisoning. Hemodialysis corrects metabolic derangements, but it does not lower serum prometryn concentration.
References
- http://www.epa.gov/oppsrrd1/REDs/0467.pdf, Prometryn.
- http://www.epa.gov/oppbead1/pestsales/01pestsales/market_estimates2001.pdf, Most commonly used conventional Pesticide Active Ingredients in the U.S. Agricultural Market Sector.
- http://pusstats.csl.gov.uk, Pesticide usage statistics.
- Prometryn (666). The e-pesticide manual13th. BCPC Publications, Hampshire 2003
- Kobel W, Sumner DD, Campbell JB, Hudson DB, Johnson JL. Protective effect of activated charcoal in cattle poisoned with atrazine. Vet Hum Toxicol 1985; 27: 185–188
- Maynard MS, Brumback D, Itterly W, Capps T, Rose R. Metabolism of [(1)(4)C]prometryn in rats. J Agric Food Chem 1999; 47: 3858–3865
- Pommery J, Mathieu M, Mathieu D, Lhermitte M. Atrazine in plasma and tissue following atrazine-aminotriazole-ethylene glycol-formaldehyde poisoning. J Toxicol Clin Toxicol 1993; 31: 323–31
- Kucukardali Y, Acar HV, Ozkan S, Nalbant S, Yazgan Y, Atasoyu EM, Keskin O, Naz A, Akyatan N, Gokben M, Danaci M. Accidental oral poisoning caused by RDX (cyclonite): A report of 5 cases. J Intensive Care Med 2003; 18: 42–46
- http://www.inchem.org/documents/pds/pdsother/class.pdf, The WHO recommended classification of pesticides by hazard and guidelines to classification 2004.
- Judge BS. Metabolic acidosis: differentiating the causes in the poisoned patient. Med Clin North Am 2005; 89: 1107–1124
- Ara G, Coleman CN, Teicher BA. SR-4233 (Tirapazamine) acts as an uncoupler of oxidative phosphorylation in human MCF-7 breast carcinoma cells. Cancer Lett 1994; 85: 195–203
- Vancova O, Ulicna O, Horecky J, Zeljenkova D, Wimmerova S, Trnovec T. Liver steatosis and disorders of mitochondrial oxidative phosphorylation after experimental administration of simazine. Bratisl Lek Listy 2000; 101: 423–428
- Wallace K. Mitochondrial targets of drug toxicity. Annu Rev Pharmacol Toxicol 2000; 40: 353–388