Abstract
Introduction. Cassia angustifolia (Senna), used as a laxative, is a plant from the Fabaceae family. It includes hydroxyanthracene glycosides, also known as Senna Sennoside. These glycosides stimulate the peristalsis of the colon and alter colonic absorption and secretion resulting in fluid accumulation and expulsion. In the literature, there are repots illustrating the hepatotoxic effects of Cassia angustifolia but there is no report of portal vein thrombosis caused by Cassia Angustifolia. Case Report. A 42-year-old woman was admitted to the emergency department with a five-day history of worsening epigastric pain, anorexia, episodic vomiting, and intermittent fever. She reported that she had boiled dried senna leaves she had bought from herbalists and drank approximately 200 mL daily for two years. Color Doppler screening found an echogen thrombus obliterating portal vein bifurcation and the right branch. The lumen was obstructed at this level and there was no blood flow through it. Treatment with thrombolytics was unsuccessful. Discussion. Severe hepatotoxicity senna use is unusual. The cause of senna-related hepatotoxicity is unclear but could be explained by the exposure of the liver to unusual amounts of toxic metabolites of anthraquinone glycosides. Conclusion. Chronic use of Cassia angustifolia may rarely be associated with portal vein thrombosis.
Keywords:
Introduction
Herbal drugs are being used by a great number of people. Senna fruit from Cassia angustifolia is one of the most important natural drugs used worldwide for the treatment of constipation (Citation1). It is one of the most popular laxatives, especially in the elderly (Citation2). Its main components are the sennosides which pass through the small intestine as prodrugs and are then metabolized by large intestine bacteria to the active metabolite rheinanthrone (Citation3,Citation4). Senna contains anthraquinones including dianthrone glycosides (1.5% to 3%), sennosides A and B (rhein dianthrones), sennosides C and D (rhein aloe-emodin heterodianthrones). Numerous minor sennosides have been identified, and all appear to contribute to the laxative effect. The plant also contains free anthroquinones in small amounts including rhein, aloe-emodin, chrysophanol and their glycosides (Citation5,Citation6).
Sennosides act by two independent mechanisms: changes in colonic motility and alterations in colonic absorption and secretion resulting in fluid accumulation. Colonic motilitiy changes are caused indirectly by epithelial cell damage (release of cytokines) (Citation7). The effect of anthranoids on secretion and absorption is principally induced by a direct interaction between the laxative and the epithelial cells (Citation8). Direct inhibition of the Na+/K+ ATPase system prevents the absorption of sodium and water from the bowel lumen into the circulation. Disruption of tight junctions between colonic epithelial cells results in an enhanced permeability of the epithelium and secretion of fluid and electrolytes (Citation9).
Senna leaves or pods have been used as a cathartic laxative at doses of 0.6 to 2 g/day, with a daily dose of sennoside B from 20 to 30 mg. Senna should not be used at higher doses or for extended periods of time (Citation10). Whole senna usage can also cause loss of fluids, hypokalemia and diarrhea. Other side effects include abdominal pain, excessive bowel activity, diarrhea, and diaper rash (Citation11). The safety of senna during pregnancy and breast-feeding is controversial although senna would appear to be the stimulant laxative of choice during pregnancy and lactation (Citation12). None of the breast-fed infants experienced abnormal stool consistency from their mothers' ingestion of senna laxatives (Citation13,Citation14). However, most publications suggest avoiding senna during the pregnancy (Citation5,Citation15,Citation16). The United States Food and Drug Administration assigned a pregnancy category C for senna products (Citation17). Senna is not recommended for children under the age of two years (Citation18). People with Crohn's disease, ulcerative colitis, appendicitis, intestinal obstructions, and abdominal pain should not be given senna (Citation19).
Acute or chronic liver damage occurred after ingestion of senna alkaloids (Citation20CitationCitation22). Severe hepatotoxicity is unusual, but could be explained by the exposure of the liver to unusual amounts of toxic metabolites of anthraquinone glycosides.
Portal vein thrombosis (PVT) may occur in the presence or absence of an accompanying liver disease and a combination of local and systemic factors are increasingly recognized to be important in its development. Both local and systemic factors may contribute to the development of PVT ().
Table 1. Etiology of PVT (Citation23)
We report the first case of portal vein thrombosis associated with the chronic use of Cassia angustifolia.
Case report
A 42-year-old woman was admitted to the emergency department with a five-day history of worsening epigastric pain, anorexia, episodic vomiting, and intermittent fever. There was no history of precipitant trauma, abdominal operation or illness. She reported that she had boiled the dried senna leaves that she had bought from herbalists and drank approximately 200 mL daily for two years. She denied any medication usage including oral contraceptive, acetaminophen and non-steroidal anti-inflammatory drugs, drinking any alcohol or smoking. Her vital signs were: blood pressure 122/71 mm Hg, pulse rate 90beats/minute, respiratory rate 16 breaths/minute, temperature 37°C, and SPO2 97% by pulse-oximeter in room air.
Abdominal examination revealed generalized tenderness, but no rebound tenderness or guarding. The liver was not palpable or tender. The spleen was not palpable and there was no evidence of ascites.
There were mild abnormalities of liver transaminases [alanine aminotransferase (ALT) 81 U/L (0–31 U/L), aspartate aminotransferase (AST) 48 U/L (0–32 U/L)] but serum amylase level was within normal limits. Thrombophilic screening (antithrombin, protein C, protein S, activated protein C resistance, Factor V Leiden level, G20210A prothrombin gene mutation, Methylenetetrahydrofolate reductase, D-dimer) did not reveal any abnormalities. Serological studies for hepatitis A (IgM type antihepatitis A virus antibody), hepatitis B (HBs antigen and anti-HBc and HBe antibodies), hepatitis C (anti-hepatitis C virus antibody and hepatitis C RNA), and HIV were negative. The hemoglobin count was found 8.9 g/dl. Ferritin, serum iron and total iron binding capacity test results and hemoglobin electrophoresis were compatible with iron deficiency anemia. Ultrasound screening showed normal size of liver and spleen and there was no ascites in the abdominal area. Color Doppler screening found an echogen thrombus obliterating portal vein bifurcation and the right branch. The lumen was obstructed at this level and there was no blood flow through it. These findings were compatible with acute phase portal vein thrombosis ().
Fig. 1. (A) Portal vein ultrasonographic image and (B) color Doppler demonstrating the presence of PVT.
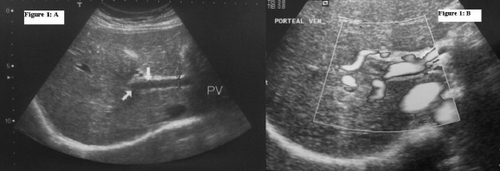
The patient was admitted to gastroenterology service for the treatment of portal vein thrombosis. The patient's abdominal pain settled considerably in the 48 hours following the infusion of Tissue Plasminogen Activator (50 mg total), despite a repeat Doppler ultrasound demonstrating persistent occlusion of the portal vein. Blood flow was seen in the superior mesenteric vein. The endoscopic examination of the upper gastrointestinal system was found normal; there were no varicose veins at esophagus. Warfarin (target INR 2.5) was commenced after two weeks of enoksaparin sodium 4000 Anti-XA IU/0.4 ml therapy. There were no bleeding complications. Warfarin 7.5 mg/day was suggested to the patient and she was discharged from the hospital. The patient was controlled routinely for two months after the discharge, and no complications or other adverse events were found in this period. At the end of the two-month-therapy, there was no improvement in the obstruction of the portal vein.
Discussion
PVT is a rare condition in non-cirrhotic patients. There are case reports documenting PVT in association with prothrombotic tendency (Antithrombin, protein C, protein S, activated protein C resistance, Factor V Leiden level, G20210A prothrombin gene mutation, homocysteinaemia) (Citation24CitationCitationCitationCitationCitationCitation30). Causes of PVT are summarized in . Etiological factors in non-cirrhotic PVT patients are prothrombotic states in 40 to 70% and local factors in 10 to 50% (Citation31,Citation32). This case was a non-cirrhotic patient, and the examinations revealed no prothrombotic states or local factors.
Chronic abuse of Cassia Angustifolia may be associated with chronic diarrhea with fluid and electrolyte loss. Senna laxative abuse may be related to hepatotoxicity (Citation20CitationCitation22,Citation32). There is one report of a woman who developed liver failure after ingesting a large amount of senna (1 liter of a tea containing 70 grams of dried herb) daily for more than three years (Citation33).
In Turkey, there are three available commercial forms of Sennoside B. These are Senokot 7.5mg®, Pursennid 12 mg®, Roha-Lax 20 mg®. Senna leaves include Sennosides A, B, C, and D subgroups. However, commercial forms only include Sennoside B subgroup. So, side effects of noncommercial form may be seen frequently. There are two case reports in which the commercial form of Sennoside B was associated with liver toxicity (Citation21,Citation34). However, there are no reports of PVT being associated with the use of Sennosides.
In recent years, several in vitro experiments have shown mutagenic effects of senna itself and of several anthranoid ingredients (Citation35,Citation36). The suggestion that the chronic use of anthranoid laxatives might cause colorectal cancer in humans (Citation35,Citation37,Citation38) has never been confirmed either in laboratory or clinical studies (Citation39CitationCitation41). No malignancy was found in our patient.
The fluid and electrolyte loss during chronic senna use may lead to dehydration and potentially negative effects on the coagulation. Dehydration is a possible risk factor for venous thromboembolism (Citation42,Citation43) but there are no reports in the literature that examine the relation between PVT and dehydration. No kidney function or electrolyte abnormality was found in our patient.
PVT is diagnosed by imaging methods including ultrasound, Doppler ultrasound (to distinguish between a benign and malignant thrombus), magnetic resonance angiography or contrast-enhanced computed tomography, and digital subtraction angiography (Citation44,Citation45). Doppler ultrasonography was used for our patient's diagnosis and further treatment.
PVT reatment options include supportive care, local or systemic thrombolysis, anticoagulant therapy and balloon or surgical thrombectomy. Spontaneous resolution of the thrombus is possible.
Resolution of PVT with systemic thrombolysis has also been reported (Citation46). Several adult case series have documented the utility of anticoagulant therapy in the management of PVT (Citation47,Citation48). Systemic thrombolysis and oral anticoagulant therapy were used for our patient.
Conclusion
Herbal medicine has become very popular in recent years. However, it should not be forgotten that this medicine can cause adverse effects. Cassia angustifolia is a plant used as a laxative not only in Turkey but also in many other countries around the world. It is known to cause hepatotoxicity if a large amount of it is used chronically; however, this case suggests that long term use may be associated with PVT.
Acknowledgment
This study was supported by Akdeniz University Research Projects Unit.
Notes
*This case report has been accepted for poster presentation at The Fourth Mediterranean Emergency Medicine Congress (MEMC IV) Sorrento, Italy September 15–19, 2007.
References
- E Leng-Peschlow. (1992). The importance of senna products. Pharmacol 44 (suppl. 1):1–2.
- KW Heaton, and HA Cripps. (1993). Straining at stool and laxative taking in an English population. Dig Dis Sci 38 (6):1004–8.
- J Lemli. (1988). Metabolism of sennosides—an overview. Pharmacol 36 (suppl. 1):126–128.
- A Chevallier. Encyclopedia of Medicinal Plants. DK Publishing, New York, (1996), 72.
- C Newall, LA Anderson, and JD Phillipson. Herbal Medicines. Pharmaceutical Press, London, (1996), 243–44.
- N Bisset. Herbal Drugs and Phytopharmaceuticals. CRC Press Inc, StuttgartGermany, (1994), 463–66.
- MR Van der Ohe, M Camilleri, LK Kvols, and GM Thomforde. (1993). Motor dysfunction of the small bowel and colon in patients with the carcinoid syndrome and diarrhea. N Engl J Med 329:1073–8.
- A Van Hoestenberghe, P De Witte, K Geboes, H Eyssen, G Nijs, and J Lemli. (1992). The effect of rhein and rhein anthrone on intestinal fluid transport and on large intestine transit in germ free rats. Eur J Pharmacol 212:121–3.
- BA Van Gorkom, EG De Vries, A Karrenbeld, and JH Kleibeuker. (1999r). Review article: Anthranoid laxatives and their potential carcinogenic effects. Aliment Pharmacol Ther. 13 (4):443–52.
- . Herbal Medicine: Expanded Commission E MonographsM Blumenthal, J Brinckmann, and A Goldberg. Integrative Medicine Communications, Newton, MA, (2000).
- HA Spiller, ML Winter, JA Weber, EP Krenzelok, DL Anderson, and ML Ryan. (2003). Skin breakdown and blisters from senna-containing laxatives in young children. Ann Pharmacother. 37 (5):636–9.
- JM Gattuso, and MA Kamm. (1994). Adverse effects of drugs used in the management of constipation and diarrhoea. Drug Safety 10 (1):47–65.
- P Faber, and A Strenge-Hesse. (1989). Senna-containing laxatives: Excretion in the breast milk?. Geburtshilfe Frauenheilkd 49 (11):958–62.
- http://aappolicy.aappublications.org/cgi/content/full/pediatrics;108/3/776#T6,2005 American Academy of Pediatrics. The transfer of drugs and other chemicals in to human milk..
- U Mengs. (1986). Reproductive toxicological investigations with sennosides. Arzneimittelforschung. 36:1355–8.
- P Faber, and A Strenge-Hesse. (1988). Relevance of rhein excretion into breast milk. Pharmacol 36 (suppl. 1):212–20.
- NL Roberts. (2005). CMT Pharmacology 101. Journal of the American Association for Medical Transcription 24 (1):31–33.
- http://www.fda.gov/OHRMS/DOCKETS/dailys/03/Aug03/081503/78n-0036l-bkg0004-08-tab10-vol1.pdf U.S. Food and Drug Administration..
- . The Complete Commission E Monographs: Therapeutic Guide to Herbal MedicinesM Blumenthal, WR Busse, A Goldberg, J Gruenwald, T Hall, CW Riggins, and RS Rister. Integrative Medicine Communications, Boston, MA, (1998), 204–8.
- F Stickel, and D Schuppan. (2007). Herbal medicine in the treatment of liver diseases. Dig Liver Dis. 39 (4):293–304.
- A Sonmez, MI Yilmaz, R Mas, A Ozcan, B Celasun, T Dogru, A Taslipinar, and IH Kocar. (2005). Subacute cholestatic hepatitis likely related to the use of senna for chronic constipation. Acta Gastroenterol Belg 68 (3):385–7.
- F Stickel, HK Seitz, EG Hahn, and D Schuppan. (2001). Liver toxicity of drugs of plant origin. Z Gastroenterol 39 (3):225–32.234–7.
- GJ Webster, AK Burroughs, and SM Riordan. (2005). Review article: portal vein thrombosis-new insights into aetiology and management. Aliment Pharmacol Ther 21 (1):1–9.
- J Pati, M Srivastava, and P Sahni. (2003). Extra hepatic portal vein thrombosis in a child associated with lupus anticoagulant. J Trop Pediatr 49:191–2.
- Y Hirohato, A Murata, S Abe, and M Otsuki. (2001). Portal vein thrombosis associated with antiphospholipid syndrome. J Gastroenterol 36:574–8.
- L Brady, D Magilavy, and D Black. (1996). Portal vein thrombosis associated with antiphospholipid antibodies in a child. J Pediatr Gastroenterol Nutr 23:470–3.
- R Arav-Boger, S Reif, and Y Bujanover. (1995). Portal vein thrombosis caused by protein C and protein S deficiency associated with cytomegalovirus infection. J Pediatr 126:586–8.
- C Seixas, G Hessel, C Ribeiro, V Arruda, and J Annichino-Bizzacchi. (1997). Factor V Leiden is not common in children with portal vein thrombosis. Thromb Haemost 77:258–61.
- C Seixas, G Hessel, L Siqueira, T Machada, A Gallisoni, and J Annichino-Bizzacchi. (1998). Study of hemostasis in pediatric patients with portal vein thrombosis. Haematologica 83:946–60.
- V Ahuja, N Marwaha, Y Chawla, and J Dilawari. (1999). Coagulation abnormalities in idiopathic portal vein thrombosis. J Gastroenterol Hepatol 14:1210–1.
- HLA Janssen, A Wijnhoud, EB Haagsma, M van Uum, CMJ van Nieuwkerk, RP Adang, RAFM Chamuleau, J Van Hattum, FP Vleggaar, BE Hansen, FR Rosendaal, and B Van Hoek. (2001). Extrahepatic portal vein thrombosis: aetiology and determinants of survival. Gut 49:720–4.
- MH Denninger, Y Chait, N Casadevall, S Hillaire, MC Guillin, A Bezeaud, S Erlinger, J Briere, and D Valla. (2000). Cause of portal or hepatic venous thrombosis in adults: The role of multiple concurrent factors. Hepatology 31:587–91.
- B Vanderperren, M Rizzo, L Angenot, V Haufroid, M Jadoul, and P Hantson. (2005). Acute liver failure with renal impairment related to the abuse of senna anthraquinone glycosides. Ann Pharmacother 39 (7–8):1353–7.
- U Beuers, U Spengler, and G Pape. (1991). Hepatitis after chronic abuse of senna. Lancet 337:372–3.
- J Westendorf, B Marquardt, M Poginski, J Dominiak, H Schmidt, and H Marquardt. (1990). Genotoxicity of naturally occurring hydroxyanthraquinones. Mutat Res 240:1–12.
- A Heidemann, HG Miltenburger, and U Mengs. (1993). The genotoxicity status of senna. Pharmacol 47 (Suppl 1):178–186.
- CP Siegers. (1992). Anthranoid laxatives and colorectal cancer. Trends Pharmacol Sci 13:229–231.
- J Westendorf. (1993). Pharmakologische und toxikologische Bewertung von Anthranoiden. Pharm Z 138:3891–3902.
- GA Kune. (1993). Laxative use not a risk for colorectal cancer: data from the melbourne colorectal cancer study. Z Gastroenterol 31:140–143.
- G Nusko, B Schneider, G Müller, J Kusche, and EG Hahn. (1993). Retrospective study on laxative use and melanosis coli as risk factors for colorectal neoplasma. Pharamcol 47 (suppl. 1):234–241.
- A Lyden-Sokolowski, A Nilsson, and P Sjoberg. (1993). Two-year carcinogenicity study with sennosides in the rat: Emphasis on gastro-intestinal alterations. Pharmacol 47 (suppl. 1):209–215.
- P Prandoni. (2006). Acquired risk factors of venous thromboembolism in medical patients. Pathophysiol Haemost Thromb 35 (1–2):128–32.
- EF Mammen. (1992). Pathogenesis of venous thrombosis. Chest 102 (6 suppl):640S–644S.
- N Hidajat, H Stobbe, V Griesshaber, R Felix, and RJ Schroder. (2005). Imaging and radiological interventions of portal vein thrombosis. Acta Radiol 46 (4):336–43.
- N Hidajat, H Stobbe, V Griesshaber, RJ Schroder, and R Felix. (2005). Portal vein thrombosis: Etiology, diagnostic strategy, therapy and management. Vasa 34 (2):81–92.
- A Tateishi, H Mitsui, T Oki, J Morishita, H Maekawa, N Yahagi, T Maruyama, M Ichinose, S Ohnishi, Y Shiratori, M Minami, S Kontetsu, N Hori, T Watanabe, H Nagawa, and M Omata. (2001). Extensive mesenteric vein and portal vein thrombosis successfully treated by thrombolysis and anticoagulation. J Gastroenterol Hepatol 16:1429–33.
- B Condat, F Pessione, M Denninger, S Hillaire, and D Valla. (2000). Recent portal or mesenteric venous thrombosis: Increased recognition and frequent recanalisation on anticoagulant therapy. Hepatology 32:466–70.
- B Condat, F Pessione, S Hillaire, M Denninger, M Guillin, M Poliquin, A Hadengue, S Erlinger, and D Valla. (2001). Current outcome of portal vein thrombosis in adults: Risk and benefit of anticoagulant therapy. Gastroenterology. 120:490–7.