Abstract
Introduction. The use of hand warmers in Hong Kong during cold weather has become increasingly popular. Iron powder is one of the ingredients. We report the first cases of ingestion of hand warmer contents. Care reports. Four elderly patients ingested the contents of hand warmers. All had no or mild symptoms, one had radiopaque particles in the stomach, two showed transiently increased serum iron concentrations (within the reference range), and all recovered with only supportive care. Discussion. These hand warmers contain a mixture of iron powder, activated charcoal, vermiculite, sodium chloride, and water. Iron powder accounts for about 50% of the weight (range 95–120 g). There are no reports of elemental iron or iron oxides ingestion causing iron toxicity and no published data on the absorption, elimination, adverse effects, or toxicities in humans after unintentional ingestion of hand warmer contents. A single oral dose toxicity test of hand warmer contents (2 g/kg) resulted in no toxicity or deaths in ten rats. Ingestions of one hand warmer packet or less can be treated with observation and supportive care as needed. Conclusions. Although this case series is small, the lack of toxicity is consistent with animal studies. It appears unlikely that significant toxicity will occur after the ingestion of one hand warmer packet. The ingestion of larger amounts might lead to iron-related toxicity and may justify more aggressive management. Proper labeling by local distributors may prevent further unintentional ingestions of these non-food products.
Keywords:
Introduction
Hand warmers are popular items in cold weather. There are different types of warmers with different mechanisms. One of the most commonly used hand warmers works through a simple exothermic chemical reaction by exposing its contents (iron powder, activated charcoal, salt, and vermiculite) to air and moisture after the cellophane bag is ripped open. Although it is used worldwide, especially in Japan, Canada, and United States, during outdoor winter sports time, there are no reports on toxicity related to these iron-containing hand warmers in literature. We present four cases of elderly patients who mistakenly ingested the contents of these hand warmers and briefly discuss the possible adverse effects and how to manage exposure to these warmers.
Case reports
Case 1
In December 2003, an 82-year-old man with a history of diabetes, pulmonary tuberculosis, and cancer of esophagus with stenting was brought to the Emergency Department (ED) by the community nurse 2h after ingesting one packet of a hand warmer (iron powder, charcoal, vermiculite, and salt) which he had mistaken for chocolate powder. The patient mixed the contents in warm water for drinking in the morning. He vomited a few times soon after ingestion. In the ED, his blood pressure was 146/72 mmHg, pulse rate 70 beats/min, and respiratory rate 16 breaths/min. His bedside glucose was 190 mg/dL (10.5 mmol/L). His electrocardiogram showed sinus rhythm. An abdominal radiograph done 3h post-ingestion showed no radiopacities. Initial blood investigation ordered 3h post-ingestion showed venous blood pH 7.38, bicarbonate 29 mmol/L, and glucose 158.5 mg/dL (8.8 mmol/L). He had a normchromic, normocytic anemia (hemoglobin 10.5 g/dL) and a white blood cell count of 14.5 x 109/L. His blood electrolytes, renal, and liver function tests were unremarkable. A normal saline infusion was begun and whole bowel irrigation with polyethylene glycol electrolyte (PGE) at a rate of 2 L/h was started at 6 h post-ingestion. However, the patient refused to drink any more PGE after having taken 1 L without any vomiting. Nasogastric tube insertion was attempted for administration of PGE but failed, probably due to the pre-existing malignant esophageal stricture. He was kept for observation, showing stable vital signs all along. Further blood tests revealed his serum iron level to be 95 μg/dL (17.1 μmol/L) (Reference: 78–179 μg/dL or 14–32 μmol/L) at 7 h post ingestion. He passed dark iron powder-stained stool at 15 h post-ingestion. The patient remained asymptomatic during overnight observation and tolerated an oral diet well. The second serum iron concentration 24 h after ingestion was 20.7 μg/dL (3.7 μmol/L). He was discharged from the ED after being observed for 26 h.
Case 2
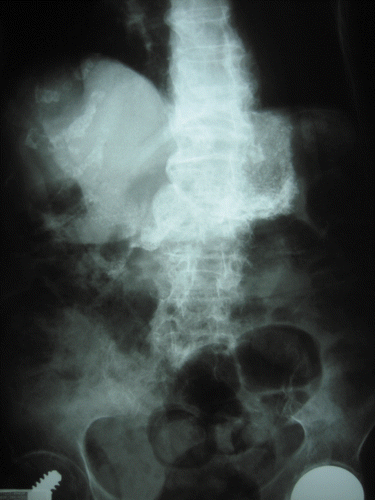
In January 2004, a 93-year-old woman with a medical history of ischemic heart disease and hypertension attended the ED after she mistakenly ingested one packet of hand warmer (iron powder, charcoal, vermiculite, and salt) at home 2.5 h before (). She was asymptomatic on presentation. Her blood pressure was 141/64 mm Hg and pulse rate was 65 beats/min. A bedside finger stick glucose was 130 mg/dL (7.2 mmol/L). Serum iron concentration was not measured. Arterial blood gas taken 3 h post-ingestion showed pH 7.37 and bicarbonate 25 mmol/L. Her electrocardiogram showed sinus rhythm. Radiographs were taken 3 h post-ingestion. The chest radiograph was clear but the abdomen radiograph showed radiopaque particles in the stomach (). No gastrointestinal decontamination was done and no further laboratory tests were ordered. She remained asymptomatic with normal vital signs during observation and tolerated oral diet well. She was discharged 6 h later from ED without follow up.
Case 3
In April 2006, an 88-year-old man with a medical history of hypertension, cataract, benign prostatic hypertrophy, and stroke presented to the ED after he had mistakenly ingested less than one pack of hand warmer (iron powder, charcoal, vermiculite, and salt) 2 h before. He was asymptomatic on presentation. His blood pressure was 133/67 mmHg, and pulse rate 77 beats/min. His bedside glucose was 97 mg/dL (5.4 mmol/L). His complete blood picture showed hypochromic (MCH 20.3 pg) microcytic (MCV 63.5 fL) anemia (hemoglobin 8.6 g/dL). His serum urea concentration was 32 mg/dL (11.4 mmol/L) and creatinine 1.55 mg/dL (124 μmol/L). He was given 50 g of activated charcoal 4 h post-ingestion. A serum iron concentration taken 6 h post-ingestion was 201 μg/dL (36 μmol/L) and the repeat serum iron level 20 h post-ingestion was 28 μg/dL (5 μmol/L). He also had a urinary tract infection and was admitted to the medical ward for further management. He did not have any clinical features of iron poisoning and was discharged 6 days later.
Case 4
In November 2006, a 90-year-old woman with a history of dementia, depression, gouty arthritis, and hypertension was seen in the ED because she had mistakenly ingested the powder of one pack of hand warmer about 30 min ago at home. The hand warmer was found to have Chinese description on the package concerning its proper usage and warning of possible thermal injury as the product may reach 87°C (188.6°F) (). Her blood pressure was 142/68 mmHg and pulse rate 65 beats/min. No gastrointestinal decontamination or laboratory investigations were ordered. She remained asymptomatic for 6 h post-ingestion and was discharged home.
Discussion
Iron-containing warmers were previously used by soldiers during the Korean War. After some refinement and product design, Japan became a mass producer of hand warmers. Our patients mistook the contents of these warmers for food or drink.
The hand warmer content is a mixture of metallic iron powder, activated charcoal, vermiculite, and salt. The exact proportions of content are not specified on the package. According to a Japanese manufacturer of one brand of the hand warmers, about 50% of the weight was iron powder; their hand warmers come in sizes ranging from 95 to 120 g. One of the local distributors reported that their hand warmer used by the patient in case 4 weighed 26 g and contained iron powder (50%), purified water (25%), activated carbon (20%), vermiculite (3%), and sodium chloride (2%).
When the powdered iron in the warmer is exposed to oxygen and moisture in the air, it oxidizes through a simple chemical reaction similar to rusting, which slowly releases heat. The salt acts as a catalyst and the charcoal helps disperse the heat inside the warmer. The vermiculite keeps the heat from dissipating rapidly so that the heat can last for hours, until all the iron powder is converted to iron oxides.
There are no reports of elemental iron or iron oxides ingestion causing iron toxicity and no published data on the absorption, elimination, adverse effects, or toxicities in humans after unintentional ingestion of the warmer contents. Activated charcoal, vermiculite, and salts are considered non-toxic substances. Product information given by one of the major manufacturers stated that their single oral dose toxicity tests (administration of 2 g/kg of the warmer contents to ten rats) did not result in any deaths or abnormalities. The manufacturer estimated that the LD50 for a single oral dose is much greater than 2 g/kg in rats. These data are consistent with previous animal studies demonstrating less than 1% of ingested reduced iron is absorbed (Citation1).
Be that as it may, there is a theoretical risk of iron poisoning caused by the fine iron powder and iron oxides that react with hydrochloric acid in stomach to form iron chloride which can be absorbed (Citation2). Significant ferric chloride poisoning can be serious (Citation3). This concern is partially supported by the initial transiently increased serum iron concentrations in cases 1 and 3, even though they stayed within the reference range. Thermal injury to gut is another potential risk as the warmer content may reach a temperature of 87°C under normal use, according to the hand warmer package in case 4.
The charcoal may cause vomiting as seen in therapeutic administration of activated charcoal in poisoned patients (Citation4). The transient repeated vomiting in case 1 may have been related to the charcoal and his esophageal carcinoma.
There was no evidence of thermal injury, pulmonary aspiration, or iron toxicity in our cases. One explanation may be reduced gastric acid due to the presence of food in the stomach or the age of the patients. The formation of iron chloride from reduced iron and iron oxides may be slow and inefficient, even in empty stomach, and once the powder passed into the small intestine, there would be no gastric acid.
The limited number of our patients may not reflect the whole picture or suicidal patients ingesting massive amounts of the hand warmer contents.
The management of unintentional ingestion of hand warmers contents was inconsistent, probably due to lack of previous reported cases. Although the unintentional ingestion of a single packet may not cause significant mortality or morbidity, it is advisable to seek medical assessment in view of the theoretical risks of iron toxicity (Citation3,Citation5,Citation6,Citation7).
Induced vomiting is unnecessary because it may cause charcoal aspiration, and repeated vomiting may be confused with symptoms of iron toxicity. Gastric lavage is generally ineffective. Theoretically, gastric dilution by drinking water or milk might decrease iron chloride formation. Oral deferoxamine is ineffective (Citation8). Activated charcoal is not useful as iron will not bind significantly to activated charcoal. The use of whole bowel irrigation needs individual consideration on its benefit–risk assessment in cases of massive intentional ingestions. Generally, for the unintentional ingestion of one packet of powder, conservative management without gastrointestinal decontamination and 6 h of observation is adequate.
An abdomen radiograph may show radioopaque particles although such findings do not seem to carry any clinical significance, in contrast to the typical iron poisoning cases (Citation13–18). Serum iron concentrations 4 h post-ingestion is recommended for massive intentional ingestions (Citation5,Citation19,Citation20). Patients who have no or mild symptoms, or who have ingested less than one packet of the powder probably do not require serum iron concentrations. An electrocardiogram and baseline blood tests such as complete blood picture with white blood cell count, blood glucose, electrolytes, renal function, and acid–base status should be ordered as clinically indicated (Citation15). Patients who remain asymptomatic 6 h post-ingestion can be discharged from the ED.
Conclusion
We presented four cases of unintentional ingestion of hand warmer contents. All cases had no or mild clinical effects, two had transiently increased serum iron concentration, and all recovered with only supportive care as needed. Although this case series is small, the lack of toxicity is consistent with animal studies. It appears unlikely that significant toxicity will occur after the ingestion of one hand warmer packet. The ingestion of larger amounts might lead to iron-related toxicity and may justify more aggressive management, including appropriate gastrointestinal decontamination. Thermal injury due to exothermic reaction may be theoretically possible. After unintentional ingestion of one hand warmer packet, a 6 h observation in an ED is prudent, until the picture of clinical toxicity is clarified. Since the hand warmer packets often only had labels in Japanese, unintentional ingestions may happen from time to time. Proper labeling by local distributors may prevent further unintentional ingestions of these non-food products.
References
- EM Boyd, and MN Shanas. Studies on the low toxicity of reduced iron, B.P. 1932. Can. Med Assoc J. 1967; 96:1141–1146.
- M Tenenbein. Toxicokinetics and toxicodynamics of iron poisoning. Toxicol Lett 1998; 102–103:653–656.
- ML Wu, CC Yang, J Ger, and JF Deng. A fatal case of acute ferric chloride poisoning. Vet Hum Toxicol 1998; 40:31–34.
- KC Osterhoudt, D Durbin, ER Alpern, and FM Henreting. Risk factors for emesis after therapeutic use of activated charcoal in acutely poisoned children. Pediatrics 2004; 113:806–810.
- w Banner, and TG Tong. Iron poisoning. Pediatr Clin North Am 1986; 33:393–409.
- J Jacobs, H Greene, and BR Gendel. Acute iron intoxication. N Engl J Med 1965; 273:1124–1127.
- AT Proudfoot, D Simpson, and EH Dyson. Management of acute iron poisoning. Med Toxicol 1986; 1:83–100.
- TW Jackson, LJ Ling, and V Washington. The effect of oral deferoxamine on iron absorption in humans. J Toxicol Clin Toxicol 1995; 33:325–329.
- M Tenenbein. Whole-bowel irrigation in iron poisoning. J Pediatr 1987; 111:142–145.
- JM Kaczorowski, and PM Wax. Five days of whole-bowel irrigation in a case of pediatric iron ingestion. Ann Emerg Med 1996; 27:258–263.
- GW Everson, EJ Bertaccini, and JO O'Leary. Use of whole-bowel irrigation in an infant following iron overdose. Am J Emerg Med 1991; 9:366–369.
- J Tur, S Aks, F Ampuero, and DO Hryhorczuk. Successful therapy of iron intoxication in pregnancy with intravenous deferoxamine and whole-bowel irrigation. Vet Hum Toxicol 1993; 35:441–444.
- RCW Ng, K Perry, and DJ Martin. Iron poisoning: assessment of radiography in diagnosis and management. Clin Pediatr 1979; 18:614–616.
- TW Staple, and WH McAlister. Roentgenographic visualization of iron preparations in the gastrointestinal tract. Radiology 1964; 83:1051–1056.
- W Palatnick, and M Tenenbein. Leukocytosis, hyperglycemia, vomiting, and positive x-rays are not indicators of severity of iron overdose in adults. Am J Emerg Med 1996; 14:454–455.
- R Foxford, and L Goldfrank. Gastrotomy: a surgical approach to iron overdose. Ann Emerg Med. 1985; 14:1223–1226.
- CD Peterson, and GC Fifield. Emergency gastrotomy for acute iron poisoning. Med toxicol 1986; 1:83–100.
- J Venturelli, Y Kwee, and N Morris. Gastrotomy in the management of acute iron poisoning. J Pediatr 1982; 100:768–769.
- LJ Ling, CS Hornfeldt, and JP Winter. Absorption of iron after experimental overdose of chewable vitamins. Am J Emerg Med 1991; 9:24–26.
- KK Burkhart, KW Kulig, and KB Hammond. The rise in the total iron-binding capacity after iron overdose. Ann Emerg Med 1991; 20:532–535.