Abstract
Introduction. Although several cases of IV injection of metallic mercury have been reported, it still remains an uncommon event. Case report. A 34-year-old male came to hospital because complaining of pleuritic chest pain. X-ray showed radio dense punctate lesions in both lung fields, as well as around both elbows. Mercury concentration in blood (140 μg/L) and urine (320 μg/L) from the patient were significantly elevated, compared with the reference concentrations of ≤ 2.0 μg/L mercury in blood and urine. The course of renal elimination of mercury and the mercury concentration in whole blood during 5 months of chelation therapy with sodium 2,3-dimercapto-1-propanesulfonate (Dimaval®) were monitored. Furthermore, the time-course of mercury in scalp hair from the patient was determined. Conclusion. We report a case of probable consecutive IV administration of metallic mercury.
Introduction
In its elemental (metallic) form, mercury is the only metal that exists in the liquid state at room temperature. Mercury is a complex element with clinical manifestations defined by the chemical form, route, and dose of its exposure (Citation1). Different compounds of mercury can be readily found in a variety of medical, industrial, and environmental sources. Determination of mercury concentration in hair, blood, and urine can be essential in separating mercury induced symptoms from those caused by diseases or pharmaceuticals. Furthermore, to access potential clinical and toxicological aspects of mercury intoxication, knowledge of the mercury speciation actually present in these samples is beneficial. Over time, mercury is gradually eliminated from the body by normal physiological processes. This alone may be insufficient to recover from mercury intoxication, and therefore chelation therapy is often considered. Chelation consists of the introduction of a charged molecule (typically containing sulfhydryl groups) into the body for the purpose of binding specific the Hg2+ ion, and facilitating the elimination of the formed mercury complex through urine (Citation2).
Self-administration of metallic mercury through the intravenous route is understandably uncommon, but it is not an unheard-of event with almost 40 cases of intravenous injection of metallic mercury published within in the last 30 years (Footnote3). Deliberate intravenous administration of metallic mercury has been usually reported in conjunction with psychiatric disorders and in suicide attempts (Citation4).
We describe a case of intravenous injection of metallic mercury with mild acute toxicity. Furthermore, we report changes of the mercury concentration in whole blood, and the excretion of mercury in urine during chelation therapy with Dimaval® (DMPS). Additionally, we present the mercury time-course in scalp hair from the patient, corresponding to hair growth over one year before admission to hospital.
Case report
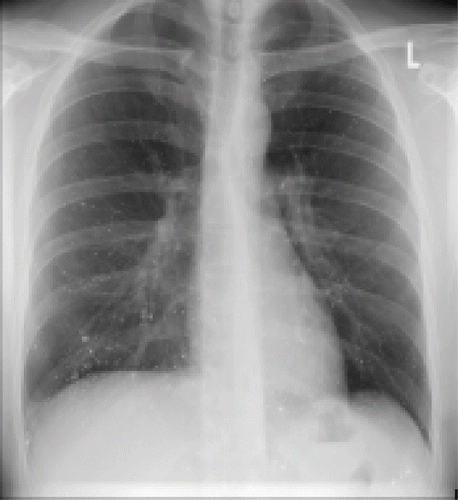
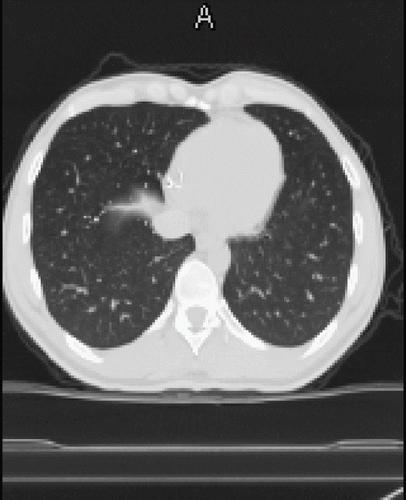
A 34-year-old male came to hospital complaining of pleuritic chest pain. According to his prior medical history, the patient had no previous history of lung disease. At admission, chest X-ray revealed radio dense punctate lesions in both lung fields (). He had a normal chest radiograph one year prior to admission. A CT scan of the thorax () and the abdomen (not shown) confirmed diffuse particles in capillaries of the lungs, liver lobes, midabdomen, and iliac wings. After an endoscopic withdrawal of pulmonary samples, pure metallic mercury inclusions could be identified visibly and mass spectrometrically, as well as multiple pulmonary infarctions and embolism. When questioned, the patient denied self-administration of metallic mercury by intravenous injection. The patient could provide no information on the amount of mercury injected, nor the number of times injected. At admission, the patient was in good general condition, except for apparent venous inflammation at both elbows and both distal forearms. An ultrasonography of both arms showed mercury depots related to the arm vein, predominant at both elbows and at the right dorsal and distal forearm. To reduce the source of continuous mercury exposure, subcutaneous deposits of mercury on both forearms and elbows were surgical removed, three months after admission. On neurological examination, a passive tremor was diagnosed, but computed tomography scans of the head excluded mercury depots. The diagnosis for IV intoxication with elemental mercury was further confirmed by monitoring the elevated mercury levels in whole blood and urine. On admission, mercury concentrations in blood and urine were 140 μg/L, and 320 μg/L; reference values for urine and whole blood of non-exposed individuals are ≤ 2.0 μg/L mercury (Footnote5). Chelation therapy with oral DMPS (sodium 2,3-dimercapto-1-propanesulfonate) Dimaval® was started one month after admission. On the first day of chelation, 1200 mg (6 × 200 mg) DMPS were given. The patient reported taking DMPS every 12 hours from the second day on.
Methods
Total mercury concentrations in blood, urine, and scalp hair from the patient were determined with inductively coupled plasma mass spectrometer (ICPMS) after mineralisation with microwave-assisted acid digestion. Hair was washed in ethanol for 15 minutes in an Elma Transsonic ultrasonic bath T700/H (Singen, Germany). Then, it was washed three times in Milli-Q water (18.2 Mω*cm resistivity) and afterwards again in ethanol (each for 15 minutes in an ultrasonic bath). After washing, the hair sample was dried overnight at 60˚C. To obtain a time profile, the 14 cm long hair was cut every 0.7 cm. Thereafter, portions (∼50–100 mg) of hair were weighed to 0.1 mg into 12 mL quartz vials. After the addition of 2 mL of nitric acid and 3 mL Milli-Q water, the samples were mineralised in an autoclave digestion system (UltraCLAVE 3, EMLS Leutkirch, Germany). After digestion, the samples were transferred into 15 mL polypropylene test tubes and diluted to a final volume of 10 mL with Milli-Q water. Portions of the urine and blood samples (∼1 g) were weighed to 0.1 mg into 12 mL quartz vials. After the addition of 2 mL of nitric acid and 2 mL Milli-Q water, the samples were mineralised. After digestion the samples were transferred into 15 mL polypropylene test tubes and diluted to a final volume of 10 mL with Milli-Q water.
Mercury speciation analysis in diluted urine and blood extracts was performed with an Agilent 1100 series HPLC system (Agilent, Waldbronn, Germany) with a Hamilton PRP-X200 (10 μm, 4.1 id × 250 mm length, Hamilton Company, Bonaduz, Switzerland) analytical column and ICPMS detection, as described elsewhere (Citation6). Urine samples were diluted, according to their previously determined total mercury concentrations, in the range of 1 + 9 and 1 + 99 in mobile phase (50 mmol l−1 pyridine, 5% v/w MeOH, 0.5% w/w L-cysteine), and afterwards filtered through 0.2 μm Nylon® filters (LaPhaPack, Langerwehe, Germany). For HPLC-ICPMS measurements, Seronorm™ (Trace Elements Urine, Sero, Billingstad, Norway) was used as a reference. After mercury speciation analysis, we obtained an inorganic mercury concentration of 49 ± 1 μg/L (n = 3) in Seronorm™, in good agreement with the recommended concentration of 48 μg/L. Blood samples (∼250 mg) were extracted with 1 mL of mobile phase, sonicated for 15 minutes, centrifuged for 20 minutes at 4500 rpm in a Jouan C4–22 centrifuge (Saint Mazaire, France), and the filtered through 0.2 μm Nylon® filters (LaPhaPack, Langerwehe, Germany) supernatant was injected for chromatographic investigations.
Discussion
In comparison with inhalation of mercury vapours, the short-term toxicity of mercury from intravenous administration of metallic mercury can be considered relatively harmless. After injection, metallic mercury is very slowly removed from the body, mostly by urinary and gastrointestinal excretion, and by exhalation to a lesser extent. Multiple punctures from intravenous mercury injections were observed on both elbows and proximal wrists, but mercury was found in many organs and tissues throughout the patient. When mercury is injected intravenously it embolises mainly to the lungs (Citation7). Through pre-capillary or capillary pulmonary shunts it can pass to the blood stream and could therefore be transported towards the liver. The extents of mercury deposits in the pulmonary capillary beds are typical of an intravenous entry point (Citation4). Although no information on the date and the amount of mercury injected was available, apparent injection sites on both elbows and proximal wrists show that repeated doses of metallic mercury have been administered. Computed tomography scans of the head showed no mercury depots in vessels of the cerebrum, although the passive tremor had increased over time. Stability of elemental mercury depots in tissues and organs permitted an evaluation of the course of mercury in whole blood during treatment with DMPS, and the excretion of mercury through urine during chelation therapy (Citation8). One month after admission, total mercury concentrations in whole blood and urine were 140 μg/L, and 320 ± μg/L. The time-course of total mercury in whole blood, and the excretion of mercury in urine spot samples (24 h urine was not obtainable) during chelation therapy with DMPS (concentration normalised to specific gravity) are shown in .
Fig. 3. Course of mercury in whole blood (a) and excretion in urine, normalised to specific gravity (b), during chelation therapy with DMPS.
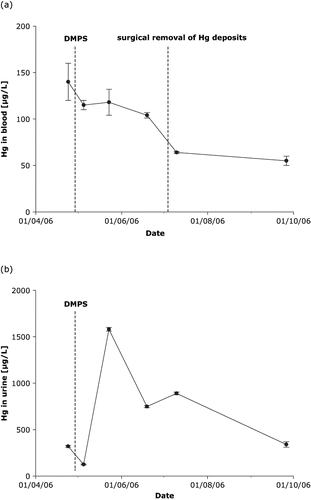
During 2 months of chelation therapy, mercury blood concentration slowly decreased from initially 140 μg/L to 104 μg/L. After surgical removal of mercury deposits at the forearms and elbows and continued chelation therapy with DMPS, the mercury level in blood decreased markedly to 64 μg/L. Renal elimination of mercury rapidly increased a few days after therapy with DMPS was started (from 320 μg/L to 1580 μg/L mercury). During DMPS therapy, mercury concentration in urine slowly decreased to almost initial values, as demonstrated in . Based on an excretion rate of 2 L/day at a concentration of 500 μg/L mercury during DMPS therapy, and the assumption that about 10 g of elemental mercury (content of one clinical thermometer) had been administered intravenously, we estimate that it would take almost 30 years to eliminate the injected mercury through the urine. It has been postulated that, after absorption into tissues, elemental mercury is slowly oxidised to form divalent inorganic Hg2+. Thereby, elemental mercury becomes activated in the body and causes toxic effects through binding to sulfhydryl groups in proteins. (10). In clinical studies, the mercury speciation is rarely determined; instead, conclusions are often based on total mercury measurements (11). Although most clinical reports describe only total mercury concentrations, we used HPLC-ICPMS and found only inorganic mercury (Hg2+) in the blood and urine samples with no detectable organic mercury (MDL 0.1 μg Hg/L).
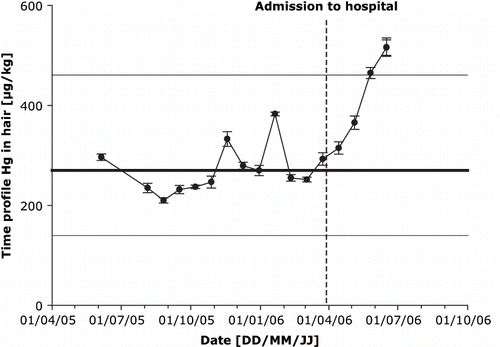
Although hair is not a good biomarker for monitoring the acute intoxication with elemental mercury, scalp hair from the patient was analysed to obtain a time-course of mercury exposure. In previous work, scalp hair from Austrian students was analysed, for a range of elements, including mercury. The median concentration of mercury obtained in hair samples from 400 Austrian students was 270 μg/kg (370 ± 350 mean ± σ; Goessler W., personal communication, 2006). It is known that human scalp hair grows approximately 1 cm per month (12). Hair from the patient was received at a length of 14 cm, to obtain a mercury profile over time the hair was cut every 0.7 cm. The mercury profile started approximately one year before admission to hospital (potential date of IV mercury administration) (). One year before admission to hospital, the mercury levels in scalp hair of our patient (250 μg/kg) were similar to the median value of 270 μg/kg from 400 Austrian students. At the time of admission to hospital, a notable steady increase in the mercury concentration is observable, which is not only depending on the date of IV administration of metallic mercury, but also on the lag period between injections of mercury and its inclusion in hair. Our patient did not appear for follow-up examinations. Therefore, his condition and the course of mercury have not documented beyond the data presented here.
Conclusion
This case report demonstrates mild acute toxicity following intravenous administration of unknown amounts of elemental mercury. Clinical manifestations of mercury intoxication were supported by monitoring the mercury concentration in whole blood and urine. Chelation produced a demonstrable increase in the urinary excretion of mercury. With respect to the amounts of mercury evidently injected, the renal elimination of approximately 1 mg mercury per day is extremely small.
Notes
3. PubMed, National Library of Medicine and the National Institutes of Health, 18 January 2007.
5. Bertram HP. Spurenelemente: Analytik, ökologische und medizinisch-klinische Bedeutung, Urban und Schwarzberg, München, Wien, Baltimore, 1992:140–141.
9. Hruby K, Schiel H. Antidotarium 2005/2006: Therapie akuter Vergiftungen, 9. Ausgabe
References
- McFee RB, Caraccio TR. Intravenous mercury injection and ingestion: clinical manifestations and management. Clin Toxicol 2001; 7: 733–738
- Risher JF, Amler SN. Mercury exposure: Evaluation and intervention the inappropriate use of chelating agents in the diagnosis and treatment of putative mercury poisoning. Neuro Toxicol 2005; 26: 691–699
- Givica-Perez A, Santana-Montesdeoca JM, Diaz-Sanchez M, Martinez-Lagares FJ, Castaneda WR. Deliberate, repeated self-administration of metallic mercury injection: case report and review of the literature. Clin Toxicol 2001; 11: 1351–1354
- Vallant B, Kadnar R, Goessler W. Development of a new HPLC method for the determination of inorganic and methylmercury in biological samples with ICP-MS detection. J Anal At Spectrom 2007; 22: 322–325
- Hohage H, Otte B, Westermann G, Witta J, Welling U, Zidek W, Heidenreich S. Elemental mercurial poisoning. South Med J 1997; 10: 1033–1036
- Eyer F, Felgenhauer N, Pfad R, Drasch G, Zilker T. Neither DMPS nor DMSA is effective in quantitative elimination of elemental mercury after intentional IV injection. Clin Toxicol 2006; 44: 395–397
- Winker R, Schaffer AW, Konnaris C, Barth A, Giovanoli P, Osterode W, Rüdiger HW, Wolf C. Health consequences of an intravenous injection of metallic mercury. Int Arch Occup Environ Health 2002; 75: 581–586
- Baxter DC, Rodushkin I, Engström E, Klockare D, Waara H. Methylmercury measurement in whole blood by isotope-dilution GC-ICPMS with 2 sample preparation methods. Clin Chem 2007; 53: 111–116
- Tobin DJ. Hair in Toxicology: An important Bio-Monitor, The Royal Society of Chemistry. Cambridge 2005; 21