Abstract
Introduction. Ingestion of a small amount of concentrated hydrogen peroxide can cause cerebral air gas embolism (CAGE). Hyperbaric oxygen therapy (HBOT) is the standard of care in the treatment of CAGE. We report a case of CAGE after accidental ingestion of 33% hydrogen peroxide treated with HBOT resulting in reversal of both the clinical and radiologic abnormalities. Case report. A 48 year-old male took two sips of 33% hydrogen peroxide. A short time later, he developed hematemesis, left sided hemiplegia, confusion, and left homonymous hemianopsia. Initial laboratory studies, chest x-ray, and brain CT were normal. MRI demonstrated areas of restricted diffusion and T2 hyperintensities in multiple vascular territories consistent with ischemia due to CAGE. Eighteen hours after arrival, the patient underwent HBOT at 3 atmospheres absolute (ATA) for 30 minutes and 2.5 ATA for 60 minutes with clinical improvement. Follow-up MRI at six months demonstrated resolution of the hyperintensities. Discussion. A search of MEDLINE from 1950 to present revealed only two cases of CAGE from ingestion of concentrated hydrogen peroxide treated with HBOT. Both cases, similar to ours, had complete resolution of symptoms. Of the seven reported cases of CAGE from hydrogen peroxide that did not undergo HBOT, only in one patient was there a report of symptom resolution. Conclusion. Ingestion of even a small amount of concentrated hydrogen peroxide can result in cerebral air gas embolism. Hyperbaric oxygen therapy may be of benefit in reversing the symptoms and preventing permanent neurological impairment.
Introduction
Hydrogen peroxide is an oxidizing agent available in most households and found in many industrial settings. Used as topical disinfectants, solutions of hydrogen peroxide typically contain low concentrations, either 3% or 6%. Higher concentrations, such as 33% or 35%, are used as bleaching agents and extremely high concentrations, 80 to 90%, are used with catalysts or other solutions to fuel rocket engines (Citation1). Some practitioners of “alternative medicine” suggest that hydrogen peroxide be used in the treatment of patients with HIV infection, cancer, and other disease processes; although these claims are unfounded (Citation2). With its wide range of uses, hydrogen peroxide results in numerous exposures reported to poison centers every year. Over a 36 month period beginning in January 1989, the Utah Poison Control Center reported 325 hydrogen peroxide exposure cases, or 0.34% of all calls (Citation3). Most exposures to peroxide are benign but toxic exposures occurring as a result of ingestion have been reported (Citation3). A rare, but known, complication of concentrated hydrogen peroxide ingestion is cerebral artery gas embolism (CAGE) (Citation4–9). We report a patient who unintentionally ingested 33% hydrogen peroxide and presented with symptoms and magnetic resonance imaging (MRI) findings suggestive of multiple cerebral arterial gas emboli; and his subsequent response to treatment with hyperbaric oxygen therapy.
Case report
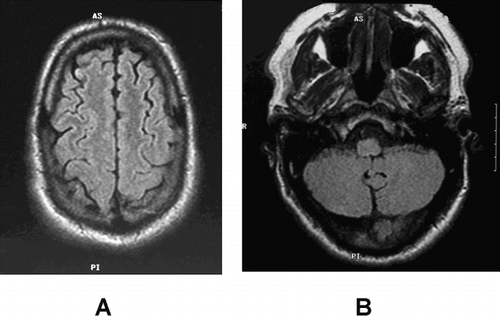
A 48-year-old right-handed man unintentionally ingested two “sips” an unlabeled container of 33% hydrogen peroxide with two acetaminophen tablets. He had been previously healthy and had a past medical history positive for hypertension but no history of stroke or other neurological abnormality. Soon after ingestion, the patient vomited and went to the local emergency department. Several minutes after arriving in the emergency department, the patient had hematemesis consisting of 50 to 75 milliliters of foamy red fluid. After vomiting, the patient complained of a headache, problems with his vision and had difficulties following commands. Two hours later, he developed left sided weakness and confusion. His initial vital were reported to be normal except a systolic blood pressure of 186/90 mmHg. A neurological examination demonstrated a left homonymous hemianopsia, a left sided facial droop, and motor weakness. Strength examination showed a left sided hemiparesis with approximately 1/5 strength in all muscles affecting arms and legs equally. Initial laboratory studies including a complete blood count, a basic metabolic profile, prothrombin time and activated partial thromboplastin time were within normal laboratory values. Chest radiograph, electrocardiogram and non-contrast head CT were likewise interpreted as normal. MRI of the brain, performed twelve hours after ingestion of hydrogen peroxide, demonstrated multiple areas of hyperintensity on fluid attenuated inversion recovery images (FLAIR) ( and ) and multiple matching areas of restricted diffusion on diffusion weighted images ( and ). More specifically the abnormalities were seen in the following areas: the parietal lobes affecting the right greater than the left; right occipital lobe; bilateral frontal lobes; left posterior temporal lobe; along the left central sulcus; and bilateral cerebellar hemispheres right greater than left. These findings were consistent with multiple areas of cerebral ischemia.
Fig. 1. MRI of the brain 12 hours after concentrated hydrogen peroxide ingestion demonstrating hyperintensities in (A) the right occipital cortex, left central sulcus and bilateral frontal lobes and in bilateral cerebellar hemispheres (B) on FLAIR imaging, areas of restricted diffusion in the bilateral cerebellar hemispheres (C) and in the right occipital cortex (D).
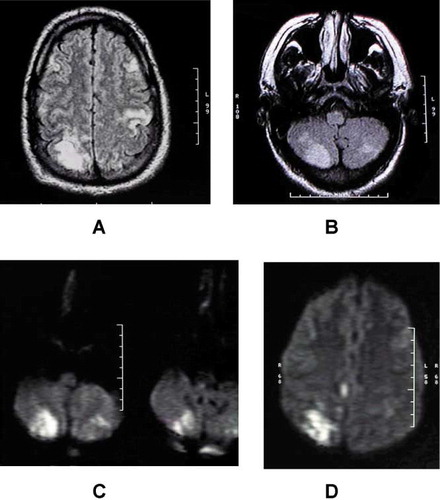
Given the patient's history of hydrogen peroxide ingestion, the local physician contacted the regional poison control center who recommended emergent hyperbaric oxygen therapy (HBOT). Eighteen hours after arrival, the patient underwent HBOT in a monoplace chamber pressured with 100% oxygen at a depth of 3 atmospheres absolute (ATA) for 30 minutes and 2.5 ATA for 60 minutes. Improved vision and strength were noted in the patient immediately following HBOT. The patient remained hypertensive throughout his hospitalization, and was treated with a daily nitro patch. By hospital day 4, the patient had complete resolution of the previous physical exam findings and was discharged home. Follow-up MRI of the brain 6 months later showed only a small area of T2 hyperintensity in the right occipital lobe ( and ). Fourteen days after discharge the patient resumed his jobs as a truck driver and farmer. Since discharge, the patient noted his only residual deficit was occasional difficulties with long-term memory, although formal testing was not obtained.
Discussion
Ingestions of 3% hydrogen peroxide are typically benign with mucosal toxicity being the most commonly reported sign (Citation10). At concentrations of 30% or higher, ingestion of even small amounts of peroxide can be potentially fatal. In previous reports ingestion of concentrated hydrogen peroxide has resulted in the following complications: hemorrhagic gastritis (Citation11,Citation12), respiratory collapse (Citation11,Citation13); pneumomediastinum (Citation14); seizures (Citation9, Citation11); portal venous gas (Citation12,Citation15); air in the right ventricle of the heart (Citation15,Citation16); and death (Citation15–17). Cerebral air gas embolism (CAGE), alone or in combination with other complications, may also develop after ingestion of a small amount of concentrated hydrogen peroxide (Citation4–9,Citation11). Similar to other reported cases, our case involved the ingestion of a relatively small amount (two “sips” per history) of concentrated (33%) hydrogen peroxide. Our patient also showed signs of gastritis, with hematemesis, similar to other cases of CAGE (Citation6,Citation11).
There have been three proposed mechanisms by which orally ingested hydrogen peroxide could produce an arterial gas embolism. First, oxygen bubbles formed, or forced, in the venous circulation could pass directly to the arterial circulation through a patent foramen ovale or intracardiac defect (Citation8). Secondly, oxygen bubbles or undissociated hydrogen peroxide could pass through a pulmonary arterio-venous fistula or undergo transpulmonary transportation over the pulmonary capillary bed (Citation7,Citation18). And lastly, aspiration could result in hydrogen peroxide itself, or oxygen gas bubbles, being absorbed into the pulmonary veins (Citation8). In our patient, echocardiography was not performed so the mechanism for the appearance of the cerebral arterial gas emboli is unclear.
Diagnosis of CAGE is typically based on a temporal relationship to a known cause, such as open heart surgery, barotrauma related to scuba diving, suicidal injection of air (Citation19), or as in this case ingestion of hydrogen peroxide (Citation20). Further support for the diagnosis can come from radiological imaging with non-contrast head CT if gaseous bubbles can be directly visualized in the intracranial circulation (Citation7), or with MRI if diffusion weighted and FLAIR images show multiple areas of cerebral ischemia (Citation5,Citation8). In our case, the patient had a reportedly normal head CT, while the MRI showed multiple areas of restricted diffusion on diffusion weighted images.
Successful treatment of CAGE from concentrated hydrogen peroxide has been has been demonstrated after hyperbaric oxygen therapy (HBOT) in two previous articles (Citation7, Citation9). Benefit from HBOT is presumed to occur by decreasing the volume of the gas in the intra-cranial vasculature allowing its redistribution, absorption, and subsequent vascular reperfusion (Citation7,Citation21). While early treatment with HBOT would seem ideal, treatment in previous reported cases occurred at 11 and 20 hours after ingestion and still resulted in reported benefits (Citation7,Citation9). This is similar to cases of CAGE from other causes where excellent clinical outcomes have been reported with delays in hyperbaric treatment ranging from 11 up to 60 hours (Citation22,Citation23). In our case, the patient underwent HBOT at 18 hours with some improvement noted immediately afterward and complete resolution by 4 days after treatment. Despite the reports of benefit, the role of HBOT in hydrogen peroxide ingestions is based solely on case reports. Of the eight previous reports involving 10 patients, including ours, of CAGE from concentrated hydrogen peroxide ingestion, three patients' received HBOT and seven did not. In all the patients receiving HBOT there was complete resolution of symptoms (Citation7,Citation9). In the 7 patients not undergoing HBOT: one died (Citation4), four had persistent hemiparesis (Citation4,Citation6,Citation8,Citation11), one had spastic quadriplegia and seizures (Citation4) and only in one case was there reported to be complete resolution of previous deficits: hemiparesis, and a six nerve palsy (Citation5). Based on these reports, the relatively low risk involved in HBOT, and its established role as treated for CAGE from other causes (Citation20): Physicians should consider HBOT as first line therapy in all cases of CAGE from concentrated hydrogen peroxide ingestion.
Current recommendations for HBOT in cerebral air gas embolisms involve use of Navy Dive Table 6, which takes patients to a depth of 3 ATA for 60 minutes followed by 120 minutes at 2 ATA with several air breaks interspersed throughout taking a total of 285 minutes to complete. In the two previous cases where HBOT was used for hydrogen peroxide induced CAGE, treatments consisted of 49 minutes at 3 ATA (dive stopped secondary to claustrophobia) and 3 ATA for 30 minutes followed by 2 ATA for 60 minutes (Citation7,Citation9). Our protocol was similar to the latter with the patient taken to a depth of 3ATA for 30 minutes and 2.5 ATA for 60 minutes in a 100% oxygen environment.
In conclusion, ingestion of concentrated hydrogen peroxide can result in clinical and radiological evidence of cerebral air gas embolism. Treatment with hyperbaric oxygen therapy, even if delayed, may be of benefit and should be recommended in patients with CAGE.
References
- http://www.peroxidepropulsion.com/article/2 Accessed 8/14/2007.
- S Green. Oxygenation Therapy: Unproven Treatments for Cancer and Aids. Scientific Review of Alternative Medicine 1998; 2:6–13.
- KF Dickson, and EM Caravati. Hydrogen peroxide exposure—325 exposures reported to a regional poison control center. Clin Toxicol 1994; 32:705–714.
- BC Ashdown, DD Stricof, ML May, SJ Sherman, and RF Carmody. Hydrogen peroxide poisoning causing brain infarction: neuroimaging findings. Am J Roentgenol 1998; 170:1653–1655.
- S Furtado, S Jodoin, W Hu, and R Sevick. Accidental hydrogen peroxide ingestion. Can J Neurol Sci 2002; 29:276–277.
- T Ijichi, T Itoh, R Sakai, K Nakaji, T Miyauchi, R Takahashi, S Kadosaka, M Hirata, S Yoneda, Y Kajita, and Y Fujita. Multiple brain gas embolism after ingestion of concentrated hydrogen peroxide. Neurology 1997; 48:277–279.
- ME Mullins, and JT Beltran. Acute cerebral gas embolism from hydrogen peroxide ingestion successfully treated with hyperbaric oxygen. Clin Toxicol 1998; 36:253–256.
- SJ Sherman, LV Boyer, and WA Sibley. Cerebral infarction immediately after ingestion of hydrogen peroxide solution. Stroke 1994; 25:1065–1067.
- SJ Vander Heide, and JP Seamon. Resolution of delayed altered mental status associated with hydrogen peroxide ingestion following hyperbaric oxygen therapy. Acad Emerg Med 2003; 10:998–1000.
- MC Henry, J Wheeler, HC Mofenson, TR Caraccio, M Marsh, GM Comer, and AJ Singer. Hydrogen peroxide 3% exposures. Clin Toxicol 1996; 34:323–327.
- TP Giberson, JD Kern, DW Pettigrew3rd, CC EavesJr., and JF HaynesJr.. Near-fatal hydrogen peroxide ingestion. Ann Emerg Med 1989; 18:778–779.
- TA Luu, MT Kelley, JA Strauch, and K Avradopoulos. Portal vein gas embolism from hydrogen peroxide ingestion. Ann Emerg Med 1992; 21:1391–1393.
- J Sansone, N Vidal, R Bigliardi, A Voitzuk, V Greco, and K Costa. Unintentional ingestion of 60% hydrogen peroxide by a six-year-old child. J Toxicol Clin Toxicol 2004; 42:197–199.
- LR Dye, and DW Dobler. Accidental ingestion of 35% hydrogen peroxide resulting in air in the soft tissues of the neck and significant esophageal injury. Int J Med Toxicol 2002; 5:7.
- DW Christensen, WE Faught, RE Black, GA Woodward, and OD Timmons. Fatal oxygen embolization after hydrogen peroxide ingestion. Crit Care Med 1992; 20:543–544.
- SJ Cina, JC Downs, and SE Conradi. Hydrogen peroxide: a source of lethal oxygen embolism. Case report and review of the literature. Am J Forensic Med Pathol 1994; 15:44–50.
- GV Giusti. Fatal poisoning with hydrogen peroxide. Forensic Sci 1973; 2:99–100.
- M Black, J Calvin, KL Chan, and VM Walley. Paradoxic air embolism in the absence of an intracardiac defect. Chest 1991; 99:754–755.
- DK Doostan, SL Steffenson, and ER Snoey. Cerebral and coronary air embolism: an intradepartmental suicide attempt. J Emerg Med 2003; 25:29–34.
- E Fukaya, and HW Hopf. HBO and gas embolism. Neurol Res 2007; 29:142–145.
- SJ Mitchell, M Benson, L Vadlamudi, and P Miller. Cerebral arterial gas embolism by helium: an unusual case successfully treated with hyperbaric oxygen and lidocaine. Ann Emerg Med 2000; 35:300–303.
- PM Winter, HJ Alvis, and AA Gage. Hyperbaric treatment of cerebral air embolism during cardiopulmonary bypass. JAMA 1971; 215:1786–1788.
- H Bitterman, and Y Melamed. Delayed hyperbaric treatment of cerebral air embolism. Isr J Med Sci 1993; 29:22–26.