Abstract
Introduction. Mortality from ingestion of the mushroom Amanita phalloides still remains as high as 8–10%. In critical patients, liver dialysis can bridge the patient to liver transplantation, which may be a lifesaving procedure. We report the use of 13C-methacetin breath test (13C-MBT) in monitoring hepatic function in a case of A. phalloides poisoning. Case report. A 33-year-old woman ate mushrooms that she had picked. After 8 h, she developed nausea and vomiting, abdominal cramps, and diarrhea, which lasted for another 24 h. On the third day, features of liver injury were seen. Pharmacologic therapy failed and she underwent liver dialysis on days 4 and 5. A 13C-MBT was used to evaluate hepatic functional reserve before the first and after the second dialysis. A liver transplantation on day 6 was successful. Discussion. The breath test results showed that at 40 min after substrate ingestion the mean 13C-MBT cumulative oxidation percentage was 10.5 ± 3.8% in healthy controls, whereas in our patient this parameter decreased from 0.09% on the fourth day to 0.02% on the fifth day. Conclusions. 13C-MBT is a simple, non-invasive diagnostic tool which may be useful as a predictor of outcome and as a marker of the severity of liver damage.
Introduction
Amanita phalloides poisoning may cause acute liver failure. This diagnosis should be suspected in patients with a history of severe gastrointestinal symptoms (nausea, vomiting, diarrhea, abdominal cramping), which occurs within hours to a day of ingestion (Citation1). Amanitin can be detected in the serum up to 36 h and in the urine up to 72 h following mushroom ingestion (Citation2,3). Amatoxins are excreted in breast milk (Citation4).
The critical problem of individual patients with acute liver failure is to determine the prognosis. Daily monitoring of serum aspartate aminotransferase, alanine aminotransferase activity, and bilirubin levels are the basic indicators of hepatocyte necrosis. Given the variable course of liver failure, these routine liver tests do not predict clinical outcome in individual patients. In acute liver failure, many prognostic criteria have been proposed and are widely used. King's College Hospital, London, England and Clichy criteria are the most commonly used to define critical liver injury and are useful in clinical classification for a liver transplant (Citation5–7).
Liver dialysis with “Prometheus” system (FPSA, fractioned plasma separation and adsorption treatment) is an extracorporeal technique that eliminates albumin-bound and water-soluble toxins from the blood. This treatment offers time for liver regeneration or acts as a bridging therapy to liver transplantation. There are no commonly used criteria when to begin FPSA treatment. Some reports (Citation5–8) show that the presence of hepatic encephalopathy grade ≥II, hepatorenal syndrome, or serum bilirubin > 10 mg/dL may be an indication for death from liver failure, and therefore, a bridging technique such as dialysis treatment may be an option. Another problem is how long and how frequently liver dialysis should be applied to achieve long-lasting health improvement or to delay multiple organ failure.
The use of additional, non-invasive liver testing methods such as 13C-breath tests have been proposed and have been shown to be practical and non-invasive in evaluating hepatic functional capacity (Citation9–15). Orally administered labeled 13C-substrate is selectively metabolized within the liver. Among different substances used in liver breath testing, methacetin was found as the substrate which undergoes extensive first-pass clearance and is rapidly metabolized by cytochrome P450 (isoform CYP1A2) into CO2 by single dealkylation (Citation9,16,17). The 13C-methacetin breath test (13C-MBT) has been shown to be practical, non-toxic, and non-invasive in evaluating hepatic functional reserve before and after liver transplant (Citation18). In view of this, we investigated the usefulness of 13C-MBT in monitoring hepatic function in a case of A. phalloides poisoning.
Case report
A 33-year-old woman ate mushrooms that she had picked. After 8 h, she developed nausea and vomiting, abdominal cramps, and diarrhea, which lasted for another 24 h. She was admitted to the Emergency Department of the Regional Hospital on the second day after eating the mushrooms with suspected A. phalloides poisoning. Amanita phalloides spores were not found in the gastric fluid, but Russula and Tricholoma spores were found in diarrhea fluids. No electrolyte or metabolic disturbances or any other features of organ injury were present. The patient was admitted to the Internal Medicine Department for observation. On the beginning of the third day following ingestion, gastrointestinal symptoms had resolved, but the features of liver injury were seen (). The clinical course was consistent with amanitin-induced liver failure.
Table 1. Laboratory data: days II–V after Amanita phalloides ingestion
During first 3 days of the poisoning, the patient was treated with intravenous hydration. On the beginning of the third day, when biochemical features of the liver injury were noticed, crystalline penicillin (1 million units/kg/day), hydrocortisone (200 mg/day), N-acetylcysteine (150 mg/kg/day), vitamin K (10 mg/day), and l-ornithine (5 g/day) were administered intravenously. Due to the signs of ongoing liver damage, the patient was transferred to the Toxicology Department in Krakow. On admission, the patient complained of severe weakness, drowsiness, and abdominal diffuse pain. Her vital signs were as follows: blood pressure 120/70 mmHg, pulse 90 beats/min, and respiratory rate 16 breaths/min. Body temperature was normal. She was slightly jaundiced and had grade I hepatic encephalopathy (according to the West Haven criteria). Slight tenderness was present above the right epigastrum. The liver was not enlarged. Laboratory studies and encephalopathy degree during hospital stay and Prometheus-FPSA treatment are summarized in .
Because of rapid worsening in her coagulation profile (INR > 6.5) and the development of hepatic encephalopathy (grade II/III), the patient was placed on the high urgency waiting list for liver transplant.
The patient was treated with hepatoprotectives (silibinin 20 mg/kg/day IV and N-acetylcysteine 70 mg/kg/day IV), preparations decreasing ammonia production (l-ornithine 25 g/day IV, lactulose 3 × 15 mL orally, and neomycin 750 mg four times daily orally), and a gastroprotective (ranitidine 150 mg/day IV), and metabolic disturbances (hypokalemia and hypoglycemia) were corrected. Fresh frozen plasma (six units) and vitamin K 10 mg/day IV were added to increase the prothrombin level to allow for safe central vein catheterization and insertion of a hemodialysis catheter. On both the fourth and fifth day following ingestion, liver dialysis was performed using Prometheus system (i.e., FPSA treatment). We used standard treatment kit: albumin filter (AlbuFlow AF 01), capillary dialyzer (FX 50), and adsorbers (Prometh 01 and 02). Mean blood flow during dialysis was 200 mL/min, plasma flow 300 mL/min, and dialysate flow 300 mL/min. Heparin (500–750 IU/h) was added to the blood circuit.
FPSA treatment was monitored using RR, ECG, blood saturation, glucose and potassium levels, and activated partial thromboplastin time (APTT). At the beginning and the end of the treatment, blood samples were taken to control biochemical values (). On the fifth day following ingestion, slight enlargement of the liver with increased echogenicity of the parenchyma and ascites was found on the ultrasound examination. Treatment was considered ineffective because of irreversible liver damage manifested as rapid progression of the encephalopathy and worsening of the patient's coagulation profile. King's College criteria for urgent liver transplantation were fulfilled.
A cadaveric donor liver graft was found on the sixth day following ingestion and a successful liver transplantation was performed. The original liver was removed and histopathological examination showed massive central lobular cell necrosis.
Methods
13C-methacetin breath test
A 13C-MBT was used to assess cytochrome P450-dependent liver function. The test was performed before the first and after the second Prometheus treatment (interval 48 h). The results of MBT were compared with data obtained in a control group consisting of healthy persons with no clinical and biochemical evidence of hepatic, gastrointestinal, endocrine, or respiratory abnormalities and no history of chronic alcohol or drug consumption (modulating capacity on cytochrome P450 activity).
The 13C-MBT was performed after an overnight fast. All tested healthy controls (10 men and 11 women) were asked to be seated to avoid any physical activity during the testing period. Before intake of the substrate, two breath samples were collected into a test tube (Exetainer vials, Labco Ltd., High Wycombe, Buckinghamshire, England) to estimate the baseline amount of 13C (i.e., the pre-dose ratio of 13CO2/12CO2). One hundred milligrams of 13C-methacetin (99 atom% 13C, Cambridge Isotope Laboratories, Inc., Andover, MA, USA) dissolved in 50 mL of water was administrated orally and exhaled air samples were collected at 10, 20, 30, 40, 50, and 60 min, thereafter. To check the clearance of 13C-methacetin, 1 day after the 13C-MBT had been performed we collected the baseline breath samples in the control group once again. In each patient, the ratios of 13CO2/12CO2 found in the baselines 24 h after 13C-methacetin administration were not statistically different (− 26.1 ± 0.7).
In our case, 13C-methacetin was administered through a nasogastric tube directly into the stomach. Under these circumstances, exhaled breath samples were collected into plastic bags with the use of modified Ambu-like face mask equipped with suitable valves. All collected breath samples were sent for analysis to Department of Physiology, Jagiellonian University Medical College, Krakow.
Measurements of 13C content (13CO2/12CO2) in collected breath samples were done using an isotope ratio mass spectrometer (Heliview, Seoul, South Korea) and the increase in exhaled 13CO2 was expressed as delta over baseline value (DOB; delta/1000). Final results were re-calculated and expressed in percentage of administered dose of 13C recovered per hour (% 13C dose/h) and finally in cumulative percentage of administered dose of 13C recovered over time (% 13C cumulative dose) which reflects methacetin cumulative oxidation percentage over time. The results of the 13C-MBT in control group were not statistically different in both genders. We assumed that small variations in the activity of cytochrome P-450 between genders were not of importance in the presented case.
The use of 13C-MBT was approved by the local ethics committees and all individuals provided written informed consent prior to the enrollment in the study.
Calculations and statistical analysis
Body surface area was calculated for each subject following the formula of Haycock (Citation19). Statistical significance and analysis were determined with paired Student t-test. The changes in the cumulative recovery dose (13C-methacetin oxidation percentage) were measured over six intervals of 10 min each both in controls and in the patient, in whom 13C-MBT was repeated in 2 successive days (days 4 and 5). Comparison between results obtained on the fourth and fifth day (rate of changes in % 13C cumulative dose) was performed using the analysis of covariance (ANCOVA) (Citation20).
Results
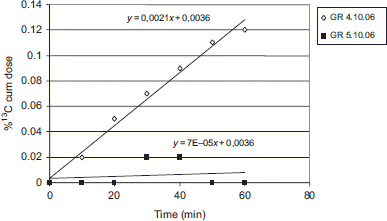
DOB values of the Amanita-poisoned patient significantly differed from healthy controls (p < 0.001) on both testing days (on the fourth and fifth day after mushroom ingestion) and at all time points of breath sampling (10–60 min). Highest DOB value in the patient's breath sample was found on the fourth day at 30 min time point and reached only 0.16 parts per thousand versus 20.70 ± 5.9 parts per thousand in controls. The mean cumulative percent 13C-dose recovery (CPDR) in controls was significantly different from MBT results in the patient tested on the fourth and fifth day. The cumulative recovery after 60 min in the patient tested on the fourth day was 0.12% compared with 16.9 ± 4.7% (p < 0.001) in healthy controls. The results of the 13C-MBT found in the reported case and in the control group are summarized in .
Table 2. Cumulative percent 13C-dose recovery in control group and in the reported case (GR)
The results showed that 40 min after substrate ingestion the mean 13C-MBT cumulative oxidation percentage was 10.5 ± 3.8% in the healthy controls, whereas in our patient this parameter decreased from 0.09% on the fourth day to 0.02% on the fifth day and came close to the baseline value just before liver transplantation.
shows the subtle differences between CPDR patient's results obtained in two consecutive days (the fourth and the fifth day). The data indicate that the methacetin cumulative oxidation on the fifth day increased minimally in time, while the day before (the fourth) it had been clearly rising. The ANCOVA showed that the discrepancy in these two trends was significant (p < 0.0001) and that the difference in the cumulative doses was increasing by 0.02% on the fourth day, in 10 min intervals.
Discussion
The main toxin from the species A. phalloides is α-amanitin, a cyclic non-ribosomal peptide of eight amino acids, which is in the A. phalloides, Amanita virosa, and several other Amanita species of mushrooms (Citation21). α-Amanitin is a potent inhibitor of RNA polymerases blocking the production of mRNA and protein synthesis.
The most common criteria of severe liver failure that indicate poor prognosis are hepatic encephalopathy (III/IV), serum total bilirubin level > 25 mg/dL, oliguria, severe coagulopathy (prothrombin time ≥100 s) with bleeding, and refractory hypoglycemia (Citation2,3,5,7). Other factors of poor prognosis are factor V <10%, lactic acidosis, gastrointestinal bleeding, and age <10 years. In a retrospective study (Citation5), survival following amatoxin poisoning was evaluated. In this study two markers, prothrombin index < 25% and serum creatinine > 106 μmol/L (> 1.2 mg/dL), between days 3 and 10 following mushroom ingestion, were the best predictors of fatal outcome. In our case, the prothrombin index was 11.6%, despite the administration of fresh frozen plasma. We did not observe any increase in serum creatinine concentration because liver dialysis clears dialyzable molecules from the blood. We noted oliguria, indicating the development of renal failure. Liver transplantation restored the normal renal function.
In our study we used the 13C-MBT, which quantitatively evaluates the cytochrome P450-dependent liver function (Citation14). The breath tests are based on the oral administration of the exogenous substrate. The substrate (labeled with stable isotope, e.g., 13C) is mainly or almost exclusively metabolized by the liver. The appearance of 13CO2 in exhaled breath indicates that substrate has undergone oxidation. Clinical studies have investigated the breath test with different substrates as a diagnostic tool for the evaluation of hepatic functional mass (Citation9,12,15,22). Most of the studies concern the patient with advanced liver cirrhosis on a list for liver transplant or after the postoperative phase (Citation5,18). The use of 13C-methacetin as a breath test substrate has numerous advantages: low cost, safety, high rate of blood extraction, and rapid clearance. The results of 13C-MBT are usually expressed as a cumulative percentage of the administered dose of 13C recovered over time, which corresponds to 13C-methacetin cumulative oxidation percentage and reflects metabolic liver capacity. 13C-MBT discriminates the hepatic functional mass not only between the control group and patients with chronic liver disease but also between patients with different degrees of chronic liver disease (Citation15,22).
In our case of advanced fulminant hepatic failure, 13C-MBT revealed showed that in A. phalloides poisoning the liver functional mass was damaged. Results of MBT performed on both the fourth and fifth day and expressed as 13C-methacetin cumulative oxidation percentage were of extreme low value, close to the baseline (pre-dose) values. Nevertheless, covariance analysis demonstrated a small amount of hepatic oxidative activity on the fourth day (). The cumulative recovery percentage was statistically significant compared with 13C-MBT results on the fifth day when the absence of any liver activity was evident. In view of this, the methacetin test may be useful in monitoring hepatic damage from Amanita poisoning.
Conclusions
13C-MBT appears to be a potentially useful tool for assessing the degree of liver injury after Amanita poisoning. It is difficult to say from only one case if low exhaled 13CO2 levels correlate with the degree of liver histopathology. 13C-MBT may be an auxiliary indicator for optimal timing of initiation and intensity of liver dialysis therapy as bridging therapy to liver transplant. Future studies may clarify the role of 13C-MBT in monitoring the efficacy of albumin dialysis, as liver functional recovery may be facilitated by the extracorporeal elimination of endotoxins.
References
- Thomson Micromedex. Micromedex Healthcare Series. USA: Thomson Micromedex, (1974–2006).
- A Jaeger, F Jehl, and F Flesch. Toxicokinetics of alpha and beta amanitins in Amanita phalloides poisoning. Communication: XII Congress of EAPCC, Bruxelles, Belgium, (1988); 27–30.
- A Jaeger, F Jehl, and F Flesch. (1993). Kinetics of amatoxins in human poisoning: therapeutic implications. Clin Toxicol 31 (1):63–80.
- HG Bivins, R Bivins, R Lammers, DB McMicken, and O Wolowodiuk. (1985). Mushroom ingestion. Ann Emerg Med 14:101–106.
- M Genzert, N Felenhauer, and T Zilker. (2005). Indication of liver transplantation following amatoxin intoxication. J Hepatol 42:202–209.
- TI Huo, JC Wu, WY Sheng, CY Chan, SJ Hwang, TZ Chen, and SD Lee. (1996). Prognostic factor analysis of fulminant hepatic failure in an area endemic for hepatitis. J Gastroenterol Hepatol 11 (6):560–565.
- JG O'Grady, GJ Alexander, KM Hayllar, and R Williams. (1989). Early indicators of prognosis in fulminant hepatic failure. J Gastroenterol Hepatol 97 (2):439.
- J Polson, and WM Lee. (2005). AASLD position paper: the management of acute liver failure. Hepatology 41 (5):1179–1197.
- A Armuzzi, M Candelli, MA Zocco, A Andreoli, A De Lorenzo, EC Nista, L Fini, L Miele, F Cremonini, IA Cazzato, A Grieco, G Gasbarrini, and A Gasbarrini. (2002). Breath testing for human liver function assessment. Aliment Pharmacol Ther 16:1977–1996.
- M Becker. (1998). 13C breath tests for measurement of liver function. Gut 43 (3):25–27.
- B Braden, D Faust, U Sarrazin, S Zeuzem, CF Dietrich, WF Caspary, and C Sarrazin. (2005). 13C-methacetin breath test as liver function test in patients with chronic hepatitis C virus infection. Aliment Pharmacol Ther 21 (2):179–185.
- D Festi, S Capodicasa, L Sandri, L Colaiocco-Ferrante, E Staniscia, A Vestito, P Simoni, G Mazzella, P Portincasa, E Roda, and A Colecchia. (2005). Measurement of hepatic functional mass by means of 13C-methacetin and 13C-phenylalanine breath tests in chronic liver disease: comparison with Child–Pugh score and serum bile acid levels. World J Gastroenterol 11 (1):142–148.
- H Fischer, and K Wetzel. (2002). The future of 13C-breath tests. Food Nutr Bull 23 (3):53–56.
- S Klatt, C Taut, D Mayer, G Adler, and K Beckh. (1997). Evaluation of the 13C-methacetin breath test for quantitative liver function testing. Z Gastroenterol 35 (8):609–614.
- S Lara-Baruque, M Razquin, I Jimenez, A Vazquez, JP Gisbert, and JM Pajares. (2000). 13C-phenylalanine and 13C-methacetin breath test to evaluate functional capacity of hepatocyte in chronic liver disease. Dig Liver Dis 32 (3):226–232.
- EC Nista, L Fini, A Armuzzi, M Candelli, MA Zocco, IA Cazzato, G Merra, R Finizio, L Miele, A Grieco, G Gasbarrini, and A Gasbarrini. (2004). 13C-breath tests in the study of microssomal function. Eur Rev Med Pharmacol Sci 8:33–46.
- H Shrin, V Aeed, and M Sori. (2006). Utility of methacetin breath test in the evaluation of the extent of hepatic injury in an animal model. Gastroenterology 130:A828.
- A Petrolati, D Festi, G De Berardinis, L Colaiocco-Ferrante, D Di Paolo, G Tisone, and M Angelico. (2003). 13C-methacetin breath test for monitoring hepatic function in cirrhotic patients before and after liver transplantation. Aliment Pharmacol Ther 18 (8):785–790.
- G Haycock, G Schwartz, and D Wisotsky. (1978). Geom for measuring body surface area. J Pediatr 93:62–66.
- RR Sokal, and FJ Rhalf. Biometry, the Principles and Practice of Statistics in Biological Research. NY, USA: W.H. Freeman and Company, (1998).
- JG Lim, JH Kim, and CY Lee. (2000). Amanita virosa induced toxic hepatitis: report of three cases. Yonsei Med J 41:416–421.
- B Pfaffenbach, O Gotze, C Szymanski, D Hagemann, and RJ Adamek. (1998). The 13C-methacetin breath test for quantitative noninvasive liver function analysis with an isotope-specific nondispersive infrared spectrometer in liver cirrhosis. Dtsch Med Wochenschr 123 (49):1467–1471.