Abstract
In the Soconusco region of Mexico, the abundance of larger-bodied, carnivorous fish decreased relative to smaller-bodied, omnivorous/herbivorous fish between the terminal Late Archaic (4700–4000 BP [2700–2000 BCE]) and late Early Formative (3300–3200 BP [1300–1200 BCE]). The exact reason is unknown, occurring during a time of change when plant-based food production became a larger proportion of the diet and settlement patterns were shifting. Here, we address whether the same diversity of fish harvested during the terminal Late Archaic persisted into the Formative Period and if it reflects a change in where fish were being harvested. Using seven previously identified and published zooarchaeological assemblages dating to between the Middle Archaic and Middle Formative periods, we analyzed changes in diversity (NISP versus NTAXA, evenness, and richness) and habitat exploitation (nestedness and proportion of aquatic environment). We find differences in taxonomic diversity, changes in where fish were harvested along the coast, and the degree of exploitation intensity. Finally, we conclude the Chantuto society may have shifted their focus from marine to more freshwater species in conjunction with early low-level food production and agricultural production. We suggest the shift in focus during the late Early Formative and Middle Formative to estuarine–riverine environments may have occurred in conjunction with agricultural intensification.
Introduction
As early as the Middle Archaic (7500–5500 BP [5500–3500 BCE]), coastal resources such as shellfish and fish were established in the diet of coastal inhabitants along the Pacific coast of the Soconusco region of Mexico such as the Chantuto society (Voorhies Citation2004). Coastal resources persisted in the diet even as plant-based food production became a larger proportion of the diet between the Late Archaic (5500–3500 BP [3500–1500 BCE]) and Middle Formative periods (2900–2600 BP [900–600 BCE]). Between the terminal Late Archaic (4500–3500 BP [2500–1500 BCE]) and late Early Formative (3200–3000 BP [1300–1000 BCE]), the abundance of larger-bodied, carnivorous fish decreased relative to smaller-bodied, omnivorous/herbivorous fish (Voorhies and Kennett Citation2011). Shifts from larger, carnivorous to smaller, omnivorous/herbivorous fish within a zooarchaeological assemblage may have multiple causes. One cause is a shift in the local habitat such as due to changing climate, sea surface temperature (SST), or change in sea level. Overfishing or high human harvesting pressures (Pauly et al. Citation1998), often attributed to changes in human population size and density in the archaeological record (Reitz, Quitmyer, and Marrinan Citation2009), may result in this shift. Similarly, switching fishing technologies from individual-capture (i.e., hook) to mass-capture (i.e., nets) technologies can result in a taxonomic shift (Giovas et al. Citation2016).
Neither sea level nor climatic change appear to explain this shift in relative abundance during the terminal Late Archaic. While a decrease in the relative abundance of freshwater- to saltwater-associated fish may indicate a rise in sea level toward the late Middle Archaic (Voorhies Citation2004), a similar change did not occur during the Late Archaic. Furthermore, there are no extant data supporting a change in climate or SST during either the Middle or Late Archaic periods.
Similarly intriguing is the variable evidence for overfishing. In conjunction with other lines of evidence, decreases in the size of shellfish or fish may indicate high human harvesting pressures; however, the only potential evidence for this interpretation is the decline in size over time of Cynoscion albus (Wake and Voorhies Citation2015, 159, Figure 9.4) based on otolith measurements. Shellfish size stayed the same and, in fact, may have increased over the course of the Late Archaic (Voorhies and Kennett Citation2021); however, it is unknown if this trend is due to a change in harvesting practices, changes in cold water upwelling or other nutrient-influencing factors. Similarly, vertebrae measurements from multiple species of both large, carnivorous and smaller, omnivorous/herbivorous fish found no significant change in fish vertebrae size (Wake and Voorhies Citation2015). Changes in the trophic level of a fish species may also indicate higher human harvesting pressures (Boethius and Ahlström Citation2018). At Tlacuachero, there is a change moving from below to above the floors from higher trophic level resources to lower trophic level resources (Wake and Voorhies Citation2015, 161) which could be interpreted as an, at least localized, example of higher human harvesting pressures.
Lacking immediate evidence for a change in sea level or climate and with variable evidence for overharvesting, the possibility remains that the change in distribution of high to low trophic fishes reflects a change in harvesting practices, such as a switch in fishing technologies, the season of harvest, or a change in where fish are being harvested from.
Here, we first characterize the diversity of fish harvested in the Middle to Late Archaic and address if the same degree of diversity persisted into the Formative Period. We then identify where fish are being harvested from and consider the results within the context of changing fishing technologies and season of harvest. We used previously published zooarchaeological assemblages from the sites of Cerro de las Conchas, Campón, Tlacuachero, and Zapotillo (Voorhies Citation2004, Citation2015); Paso de la Amada (Wake et al. Citation2021); La Blanca (Wake and Harrington Citation2002); and Salinas La Blanca (Coe and Flannery Citation1967, Table 7) to measure diversity as changes in sample size (measured as NISP, number of identified specimens), richness (or NTAXA, number of identified taxa), and evenness (or the degree to which the assemblage is dominated by one taxon). We measured the nestedness of fish from each site during the Archaic Period as well as the Formative Period to measure if the fish at any one site are a subset of taxon represented at all other sites included in the analysis (Alsgaard Citation2020; Lyman Citation2008). Non-nested results can indicate differences in diet breadth (Jones Citation2004), foraging efficiency (Wolverton et al. Citation2015), or habitats (Jones Citation2016) when used in conjunction with measures of taxonomic diversity. Finally, we assess the relative proportion of fish caught in marine and/or brackish environments closer to the coast from estuarine or lagoonal systems, compared to freshwater environments, such as rivers or lakes. As behaviors and settlement structures were shifting to focus more on agricultural resources, fishing strategies may have included more riverine, lacustrine, or other freshwater resources which would have been spatially closer to hypothesized agricultural production areas further inland. Not only would these resources have been located closer to areas of agricultural production, but they would have easily facilitated the use of mass-capture techniques such as nets, allowing for a high capture rate and reducing the risk of falling below the necessary harvest rate across all dietary resources (Winterhalder and Goland Citation1997).
Background
Barbara Voorhies and Douglas Kennett recovered fish and shellfish remains from five shellmounds within the Chantuto-Panzacola lagoon system in the Acapetahua zone of the Soconusco. The shellmounds, Cerro de las Conchas, Campón, Tlacuachero, and Zapotillo, span the Middle to terminal Late Archaic periods (7500–3800 BP). They contain evidence of early coastal exploitation along the Pacific Coast of Mexico (Voorhies Citation2004, Citation2015) by the Chantuto society which also incorporated low-scale production of wild and domestic food plants farther inland by 6500 BP (Kennett et al. Citation2010). Significantly, at Tlacuachero, there is evidence for a change from a focus on larger carnivorous fish (fish with a high nutritional value) to smaller omnivorous/herbivorous fish (fish with a lower nutritional value) between the Late Archaic and terminal Late Archaic periods (5500–3500 BP [3500–1500 BCE]; Voorhies Citation2015; Voorhies and Kennett Citation2011).
By the terminal Late Archaic (3,500 BP) shellmound construction ceased and coastal populations along the Soconusco began to build earthen mounds (Voorhies, Gasco, and Cackler Citation2011) on the inland side of today’s estuary. Unlike the majority of the shellmounds, the earthen mounds have evidence of residential occupation and paleobotanical indicators of plant cultivation (Voorhies, Gasco, and Cackler Citation2011). There is evidence for corresponding changes in the seasons when shellfish were harvested (Kennett & Voorhies Citation1995; Kennett & Voorhies Citation1996) and for an increased investment in small-scale food production (Voorhies and Kennett Citation2021). However, the subsistence base remained focused on a combination of coastal and terrestrial resources, although the diversity of wild resources may have increased from the Late Archaic (5500–3500 BP [3500–1500 BCE]) to the Early Formative periods (3400–3000 BP [1400–1000 BCE]; Lesure, Sinensky, and Wake Citation2021).
Solid evidence for sedentary ranked villages appeared by the late Early Formative with sites such as Paso de la Amada, El Varal, Cantón Corralito, and El Silencio (Rosenswig Citation2006). Sophisticated ceramics are present—the earliest currently recorded in Mesoamerica (Clark and Gosser Citation1995)—as is evidence of contact with the Olmec on the Gulf Coast of Mexico (Rosenswig Citation2012). Although investment increased in small-scale food production, the subsistence base remained focused on both coastal and terrestrial resources, with the addition of domestic dog (Wake Citation2004; Wake and Harrington Citation2002). Sites in the Soconusco adopted a maize-dependent agriculture by the end of the Early Formative Period and moving into the Middle Formative, when the sites of La Blanca (Love Citation2002) and Izapa arose (Rosenswig et al. Citation2015). Based on carbon and nitrogen stable isotope data from human remains, the role of coastal resources in the diet may have decreased during this period as the proportion of domesticated resources in the diet increased (Blake et al. Citation1992; Reynaga Citation2010).
To evaluate whether the focus on small fish (e.g., Pacific fat sleeper [Dormitator latifrons]) during the terminal Late Archaic persisted into the Formative periods was due to a change in fishing technologies, we statistically analyzed the previously published zooarchaeological data from the Middle Archaic Cerro de las Conchas shellmound; the Late Archaic shellmounds of Campón, Tlacuachero, and Zapotillo; the late Early Formative site Paso de la Amada; and the Middle Formative sites Salinas La Blanca and La Blanca using the number of identified taxon, assemblage richness, assemblage evenness, site nestedness, and the proportion of fish caught using mass-capture technologies.
Methods
We used sample size, assemblage richness, assemblage evenness, and nestedness to characterize the diversity of diets and the taxonomic structure of the fish assemblages from sites aged between the Middle Archaic and the Middle Formative in the Soconusco region of Mexico. We used these analytical tools to assess changes in taxonomic composition using previously published assemblages from Cerro de las Conchas (Voorhies et al. Citation2002; Wake, Anikouchine, and Voorhies Citation2004), Tlacuachero (Wake and Voorhies Citation2015), Campón (Wake, Anikouchine, and Voorhies Citation2004), Zapotillo (Cooke et al. Citation2004), Paso de la Amada (Wake 2021), La Blanca (Wake and Harrington Citation2002), and Salinas La Blanca (Coe and Flannery Citation1967) (see ). We excluded assemblages from the Soconusco region without fish (e.g., La Victoria), those reported using only MNI (minimum number of individuals) (i.e., Campón, Aquiles Serdán, and a portion of the Tlacuachero assemblage), or that are pending publication (i.e., El Chorro and El Grillo). The assemblages we included are used in all our analyses with the exception of Campón. Because Campón is reported using only MNI, we were able to include its assemblage only in our analyses of nestedness and the proportion of mass-captured fish. We also analyzed the Tlacuachero fauna from different contexts separately. We analyzed the Tlacuachero faunal data in Cooke et al. (Citation2004) separately from the Tlacuachero faunal data in Wake and Voorhies (Citation2015) to account for differences in screen size. We further split the Tlacuachero (2015) assemblage by its three contexts (see Voorhies Citation2015, ): the below floor context (4.6–6.8 m below datum, 5050–4870 cal BP) referring to unconsolidated bedded marsh clam shell, the floors context (4.2–4.6 m below datum; 4855–4405 cal BP) which refers to three floors, each separated by unconsolidated shell, and the above floor context (1.6–4.2 m below datum; 4380–4235 cal BP) of unconsolidated bedded marsh clam shells. The floors coincide with several subsistence changes including: a change in the season of shellfish collection, the presence of maize phytoliths and increase in disturbance plant taxa, the presence of expedient manos and metates, and a shift in focus from large carnivorous to small omnivorous/herbivorous fishes (Voorhies Citation2015, 187–8). Finally, the collection method used at Salinas La Blanca is unclear. As different collection methods, especially from older excavations such as Salinas La Blanca which was excavated in 1962 (Coe and Flannery Citation1967), can produce different taxonomic distributions, especially with regard to the recovery of small fish remains which can often become overlooked (e.g., Nagaoka Citation2005; Partlow Citation2006), the NISP from this site may not represent the true taxonomic distribution from the site. We emphasize that insight into past subsistence practices at Salinas La Blanca may be impacted by older excavation methodology.
Table 1. Data overview of each zooarchaeological assemblage, the associated statistical analyses, the original faunal analysts, screen size, and citation. Richard Cooke analyzed the Tlacuachero assemblage screened through graded sieves ≥ 2mm. Thomas Wake analyzed the assemblage Tlacuachero assemblage screened through 5mm mesh.
Comparison of NISP and NTAXA
We first analyzed the relationship between sample size (NISP) and richness, or the number of total taxa (NTAXA), to assess the differences in taxonomic diversity among the sites (; ). We expected the number of individual specimens and the number of taxa to increase until the inflection point where the number of taxa level off despite an increasing number of specimens (Lyman Citation2008). This relationship is linear when logged. Deviations from this relationship can indicate different populations within an assemblage due to different local environments (Alsgaard Citation2020), changes in diet breadth (Grayson and Delpech Citation1998), differences in climate (Grayson Citation1998), or differences in taphonomy (Lyman Citation2015). This analysis can identify whether the taxa at any one site are a subset of the taxa at all other sites included in the analysis. Another reason to perform this analysis is because richness (NTAXA) is affected by sample size (Grayson Citation1984; Jones Citation2016). Plotting NISP and NTAXA on a biplot enabled us to evaluate deviations from the expected outcome.
Figure 1. Relationship between log(NISP) and log(NTAXA) with 95% confidence intervals in light grey for Middle Archaic (Cerro de las Conchas), Late Archaic (Tlacuachero 2004; Tlacuachero 2015 below floor, floor, and above floor; and Zapotillo), late Early Formative (Paso de la Amada), and Middle Formative (La Blanca and Salinas La Blanca) sites.
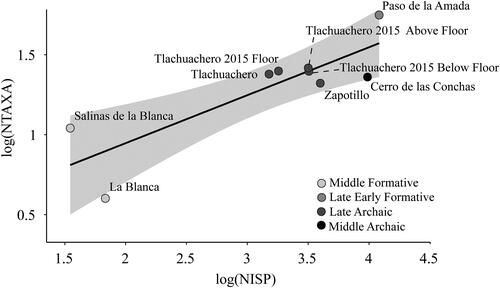
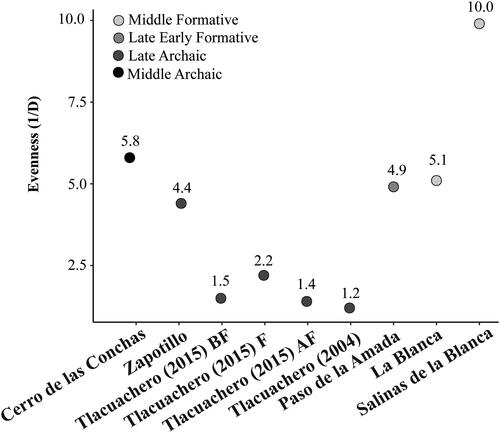
Table 2. NISP/MNI, NTAXA, and evenness values for each site.
Richness
We measured richness using the NTAXA (Lyman Citation2008) identified for a specific level within an assemblage (; ). Increases in richness, or number of identified taxa, can indicate a higher taxonomic diversity when evenness is accounted for (Jones Citation2004). We included specimens identified to the genus level or lower when we calculated NTAXA. We used this cutoff because the life history characteristics of fish (i.e., such as how or where a fish forages) can vary between genera and species. By limiting the calculation of richness to genera-level identifications or lower, we reduced the number of grouped life history characteristics.
Evenness
To calculate evenness or the degree to which the assemblage is dominated by one taxon (), we used the reciprocal of Simpson’s dominance index (1/D; Lyman Citation2008; Magurran Citation1988). Unlike other indices, Simpson’s dominance index does not assume all taxa are equally represented in an assemblage—thus accurately reflecting archaeological contexts—and instead measures the degree to which an assemblage is dominated by one taxon (Jones Citation2004). The higher the value, the greater the evenness.
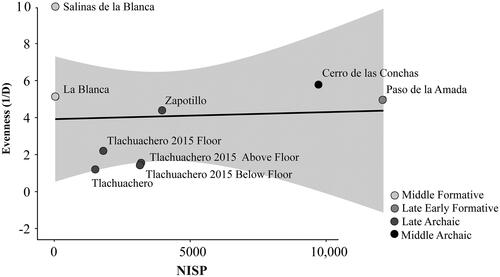
To account for the fact evenness can be driven by sample size (Jones Citation2004), we plotted sample size on a biplot with evenness to evaluate for a linear relationship (). We used the strength of the linear relationship to assess if sample size was driving the evenness values. We used the coefficient of determination to assess the strength of the relationship.
Figure 2. Relationship between sample size and evenness for Middle Archaic Cerro de las Conchas (Middle Archaic), Late Archaic (Tlacuachero 2004; Tlacuachero 2015 below floor, floor, and above floor; and Zapotillo), late Early Formative (Paso de la Amada), and Middle Formative (La Blanca and Salinas La Blanca) sites.
Nestedness
We used nestedness to measure whether the taxa at any one site are a subset of taxon represented at all other sites we analyzed. As the archaeological sites we included are all in the same or similar contexts (i.e., estuarine environments), we expected all the sites were nested. A nested result suggests taxa were harvested from similar habitats during the time periods covered by these assemblages. If the results are not nested, different taxonomic populations may have been harvested from different habitats.
We calculated nestedness by creating a presence–absence matrix, calculating the nestedness metric, and testing the metric’s statistical significance. Nestedness is calculated using a presence–absence matrix allowing us to include datasets that do not report NISP. In this analysis, we included the Campón site, which is reported only in MNI. We also calculated nestedness between the levels of the shellmound sites of Cerro de las Conchas, Tlacuachero (2004, 2015), Campón, and Zapotillo.
In our presence–absence matrix, each site is represented as a column and each species present at a site is a row (Supplementary Table S1). In the case of the analyses of site levels, each level is represented as a column and each species within the level is a row. We used the NeD nestedness program (Strona et al. Citation2014) to calculate NODF, a nestedness metric based on overlap and decreasing fill (Almeida-Neto et al. Citation2008) which calculates nestedness independently for columns (i.e., sites) and rows (i.e., species), rather than the presence or absence of a species alone (Alsgaard Citation2020). While the NODF metric can be calculated for individual rows and columns separately, we calculated only the NODF metric for the whole matrix. We used the NeD nestedness program to generate a series of null model matrices to determine whether the calculated NODF value is more likely to occur than would be expected by random chance. We used the EE null model to generate null matrices (Ulrich, Almeida-Neto, and Gotelli Citation2009). This null model generates null matrices the same size as the inputted matrix. It randomly fills the null matrix until the number of inputted data points is equivalent to the inputted matrix. It assumes all sites and species occur with equal probability, regardless of the actual matrix values. We generated 500 null matrices for each test.
We analyzed the nestedness of the Archaic Period assemblages from Cerro de las Conchas, Campón, Tlacuachero, and Zapotillo (). Non-nested results may be due to taxonomic changes related to changes in the climate or differences in harvesting practices. We also analyzed the nestedness of the Archaic and the Formative assemblages (Paso de la Amada, La Blanca, and Salinas La Blanca). The objective was to measure if there was a change in the taxonomic community moving from the Archaic Period to the Formative Period, when maize was becoming a larger proportion of the diet. Finally, we analyzed the nestedness of the Archaic and the Formative assemblages excluding Salinas La Blanca (Paso de la Amada and La Blanca) to account for any potential impact of older excavation methods at Salinas La Blanca.
Table 3. Results of the nestedness analysis for the Archaic Period layers of shellmound sites and sites spanning the Archaic and Formative Periods.
Fish habitat
Finally, to address if fish were being procured more from marine or freshwater environments, we quantified habitat use for the top five most abundant species, genera or family from each site following the simplified categorization of aquatic habitats described by Wake, Bishop, and Lesure (Citation2021, Table 14.8) including: marine–lower estuary, estuary–river, lagoon–swamp, and swamp. This categorization ranges from most saline (marine–lower estuary) to most freshwater (swamp). We calculated the relative abundance of the entire assemblage, but only considered the top five most abundant resources in this analysis.
Results
In the following section, we present the results of the statistical analyses.
Comparison of NISP and NTAXA
depicts a moderately strong relationship between log(NISP) and log(NTAXA) when all sites are included ( and ; R2 = 0.57). It is possible there are two distinct NISP and NTAXA relationships (e.g., Grayson and Delpech Citation1998): a low-slope relationship including the Archaic Period sites and Salinas La Blanca, and a high-slope relationship including the Formative Period sites. The low-slope relationship includes the shellmound sites (Tlacuachero, Zapotillo, Cerro de las Conchas) and sites where shells were very abundant (Salinas La Blanca); they have a higher richness compared to the number of identified taxa (NISP) identified at these sites. However, it is important to recognize that the relatively high richness at Salinas La Blanca may be a result of older excavation methods and/or hand collection.
Richness
The comparison of NISP and NTAXA indicates the degree of richness is proportional to the number of identified specimens for each assemblage. In other words, richness increased with sample size, as expected (see ). However, Salinas La Blanca and Paso de la Amada departed from this pattern. Salinas La Blanca has a higher-than-expected richness for the number of identified specimens. While is it possible the relatively higher richness of Salinas La Blanca is due to the unknown collection strategy, we would expect a lower richness value if the assemblage was hand collected (Trusler Citation2013). Paso de la Amada has a higher-than-expected richness for the number of identified specimens.
Evenness
The coefficient of determination () is extremely weak, indicating no relationship between sample size and evenness. The least even assemblages are the contexts from Tlacuachero (see ) followed by Paso de la Amada. Given the relative abundance of the identified specimens, it is possible these low evenness values were driven by the high proportion of catfish (Ariopsis sp.) and sleepers (Eleotridae) in the assemblages. Salinas La Blanca is the most even assemblage; however, we cannot rule out that the relatively high evenness value of Salinas La Blanca is due to the older excavation methodology.
Nestedness
The nestedness analysis indicates all the Archaic Period assemblages are subsets of one another. Similarly, all the Archaic and Formative Period assemblages are subsets of one another. All the results are nested (). The Archaic Period sites are relatively more nested with one another (NODF = 56.70) then when Formative Period sites are also included (NODF = 46.66). We exclude Salinas La Blanca in the final nestedness analysis (NODF = 38.716) to account for any affect older excavation methods may have on the results.
Fish habitat
When considered by aquatic environment, there are different foci overtime and between sites (, ). Most striking is the high proportion of fish caught from the marine–lower estuary from Cerro de las Conchas. It is the only site where the fish are dominated by species likely caught from the marine–lower estuary. At both Tlacuachero and Zapotillo, the fish are overwhelmingly dominated by fish caught from swamp and/or lagoon–swamp environments. Moving to Paso de la Amada, but also at La Blanca and Salinas La Blanca, the highest proportion of fish come from estuarine–riverine environments. Notably, this assessment does not take into account within-site variation (such as described at Paso de la Amada by Wake et al. Citation2021), but it meant to capture any broad temporal patterns.
Figure 4. Proportion of the five most abundant species, genera or family per aquatic environment at each site. Values used in calculation are reported in .
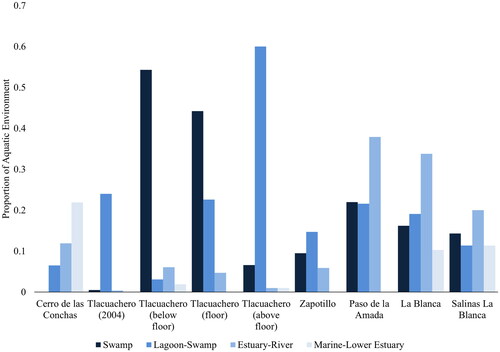
Table 4. The top five most abundant fish species, genera or family from each site. Rank refers to the relative abundance at the site. Taxon refers to the taxonomic category. Aquatic environment refers to the category assigned to the fish species based on Wake et al. Citation2021. For those taxaonomic categories not previously described, we consulted Fishbase (accessed 2023). NISP/MNI refers to the number of identified specimens or minimum number of individuals, respectively. Proportion of diet is the relative abundance compared to the whole diet for those site recorded in NISP. Proportion of diet was used to calculate .
Discussion
The relationship between logged NISP and NTAXA between the Archaic and Formative periods suggests a single sloped relationship. While Salinas La Blanca has a higher richness value than expected for its sample size, we cannot rule this out as an artifact of excavation methodology rather than a true reflection of past subsistence practices.
When Salinas La Blanca is excluded, differences in richness do not seem to follow any specific temporal trends with La Blanca, a Middle Formative site, having the lowest richness and Paso de la Amada, the highest. Interestingly, despite variations in sample size, the Middle and Late Archaic sites have similar richness values suggesting the breadth of subsistence strategies remains consistent during this period. However, there are distinct differences in evenness.
Excluding Salinas La Blanca, the highest evenness values come from the Middle Archaic site of Cerro de las Conchas, followed by the Early Formative sites of La Blanca and Paso de la Amada, and the Late Archaic site of Zapotillo. All contexts from Tlacuachero have similar low evenness values. When compared to data on the aquatic environments of the top five most harvested fish species, it may be that the relatively higher evenness values are being driven by more equal exploitation from multiple environments. This is compared to Tlacuachero where most of the harvested fish are coming primarily from the same one or two aquatic environments.
The relative exploitation from these different environments may also be underlying the nestedness values. While all sites are nested, the relative degree of nestedness is different when comparing the Archaic, Archaic and Formative, and Archaic and Formative (excluding Salinas La Blanca) groups. The Archaic Period sites are relatively more nested with one another (NODF = 56.7) then when Formative Period sites are also included (NODF = 46.7). When Salinas La Blanca is removed, the nestedness value drops to NODF = 38.716 in the final nestedness analysis. When compared with , the proportion of estuarine–riverine fish in the Formative Period sites is higher than in the Archaic Period, which focused primarily on swamp/lagoon–swamp species. This environmental variation may be underlying the difference in nestedness values.
Figure 5. Evenness values calculated as the inverse of Simpson’s D (1/D), NTAXA values, and proportion of aquatic environment for Cerro de las Conchas (Middle Archaic), Tlacuachero and Zapotillo (Late Archaic), Paso de la Amada (late Early Formative), La Blanca (Middle Formative), and Salinas La Blanca (Middle Formative).
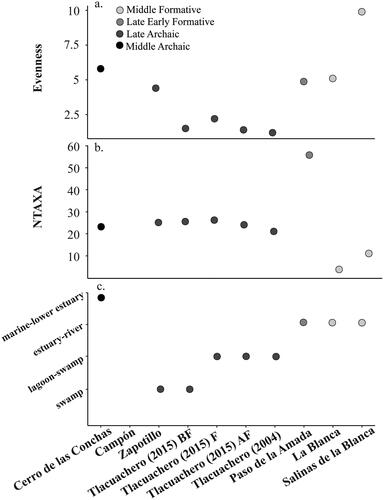
The first striking observation from the analysis of aquatic habitats is the dominance of marine–lower estuarine fish in the assemblage of Cerro de las Conchas. It is the only site where the highest proportion of fish come from this environment, driven by Lutjanus sp. It is likely at least some species were harvested by hook as Cerro de las Conchas is one of two shell midden sites in the area with turtle shell fishhooks (Voorhies Citation2004) and today Lutjanus sp. is captured by hook rather than net. Notably, the assemblage is driven by larger carnivorous fish that would have been harvested closer to the marine–lower estuarine environment. While fish were also being harvested from the lagoon–swamp and estuary–river environments, the lower marine environment was also being highly exploited.
At Tlacuachero, we see a strong focus on swamp and/or lagoon–swamp environments both below, during, and following floor construction. The aquatic habitat difference is primarily driven by the exploitation of tropical gar below the floor construction and pacific fat sleeper above the floor construction (Voorhies and Kennett Citation2011, Citation2021; Wake and Voorhies Citation2015). This taxonomic difference may also indicate a technological and seasonal shift in fish exploitation, with pacific fat sleeper primarily caught by net and highly seasonal in rivers and estuaries, where they move toward the end of the rainy season (Vega-Villasante et al. Citation2021). The dominance of the pacific fat sleeper continues with Zapotillo; however, relatively more evenly when compared to fish harvested from swamp and estuarine–riverine environments.
At Paso de la Amada, and potentially the other formative sites of La Blanca and Salinas La Blanca, the highest proportion of fish are harvested from estuarine–riverine environments. At Paso de la Amada, this is primarily driven by catfish exploitation (Ariidae and Ariopsis sp.), while at La Blanca and Salinas La Blanca, it is driven by catfish and snook (Ariidae and Centropomus sp.). However, these sites also have relatively high evenness values. These high evenness values may be explained by more equal exploitation from a number of different environments (i.e., swamp, lagoon–swamp, estuary–river, and marine–lower estuary).
Conclusions
Why then do we see a higher proportion of fish from relatively freshwater environments, specifically swamp and lagoon–swamp environments, from Campón, Tlacuachero, and Zapotillo and later, a high proportion of fish from estuarine–riverine environments at Paso de la Amada and potentially La Blanca and Salinas La Blanca? One option could be a change in fishing technology. Fish from freshwater environments, including tropical gar, cichlids, and pacific fat sleeper, were likely caught primarily using nets. An experimental study along the Santa María River in Panama using mass-capture (i.e., commercial and experimental gill nets) and individual-capture techniques (hooks) found the majority of fish species were caught using nets in both an estuarine and riverine environment (Cooke and Rodríguez Citation1994). While catfish were also occasionally caught by hook, the highest proportion were still taken by net. Similar results were found collecting fish in the Heyuate Swamp (Lesure and Wake Citation2011, 81, ). However, it should be noted that two turtle shell fishhook fragments were also recovered from under the floors at Tlacuachero (Voorhies Citation2015). This being said, there are not enough data to be able to assign specific changes in technology to a change from the marine–lower estuary to more freshwater environments. Many of the species at all sites are easily caught by nets, and even those, such as catfish, which can be caught by hook and line, could be harvested using either technology.
Lacking sufficient data on technological changes, we hypothesize the change from marine lower to swamp and lagoon–swamp environments represents a change from coastal resource harvesting focused primarily on marine coastal resources to harvesting that included fish exploitation as part of an overall dietary strategy that incorporated food production further inland at sites such as Vuelta Limon (Voorhies Citation2015).
Previous analyses of the Cerro de las Conchas dataset have noted the presence of large, marine fish (compared to Late Archaic contexts) with evidence for year-round site use (compared to the primarily wet season during the terminal Late Archaic). We support previous observations regarding these data that they both represent a change from fishing in the marine–lower estuary area to further inland and that they may represent a change in gender roles (Voorhies and Kennett Citation2011). We contribute another complementary interpretation that the Chantuto people shifted their focus from marine to more freshwater species in conjunction with agricultural production.
Recently summarized by Palka (Citation2024), there is evidence for fishing alongside agricultural activities and potential evidence for aquaculture during periods of intensive agricultural activity, such as among the Classic Period Maya, including evidence for flood-plain agriculture along riverbanks and raised field agriculture in swamps. Canals, from either raised fields or specifically for these purposes, attract gar, catfish, cichlids, and mojarra that are easily captured (Hickling Citation1961, 231). These same species are easily harvested from lake or swamp environments, which would have required less initial investment (Rivas and Odum Citation2019). The beginnings of these intensified practices may have developed alongside low-level food production or early agriculture itself, indicated by a shift from marine–lower estuarine resources to less saline or freshwater resources in the late to terminal Late Archaic. Rather than traveling to the coast itself, it may have been easier and, if using nets, more efficient to exploit these freshwater environments alongside agricultural production. Furthermore, mass-capture techniques may have been used as a way to increase fish surpluses to reduce the risk of falling below the minimum necessary harvest in the future (Winterhalder and Goland Citation1997), which could have been useful for mitigating food short falls during the period of early maize domestication. During the Early Formative, when the proportion of fish from estuarine–riverine environments, specifically catfish, but also cichlids, increases, it is possible this could be an indication of fishing from agricultural field canals or canals built for this purpose. The increase in both the proportion of catfish and cichlids, which were exploited at lower levels in the Archaic Period assemblages, may be higher during the Early Formative due to fishing from anthropogenically modified environments. Catfish and cichlids both thrive in shallow water environments including anthropogenically modified canals. Their increased proportion in Early Formative and Middle Formative contexts may indicate their increase in abundance due to modified environments driven by agricultural intensification and/or population increases.
We propose that these changes may be associated with agricultural intensification (sensu Boserup Citation1965) and spatial relation to where agricultural is practiced on the landscape, but additional data are required to fully evaluate this novel hypothesis. Understanding where agriculture occurred and the relative distance between agricultural production areas and fish harvesting locations at the site level could provide insight into this potential link between agricultural production and freshwater fishing activities.
Supplemental Material
Download PDF (93.9 KB)Acknowledgements
We would like to thank the dissertation committee (Dr Emily Lena Jones, Dr James Boone, Dr Bruce Huckell, Dr Ronda Brulotte, and Dr Viorel Atudorei) of Dr Asia Alsgaard who provided extremely helpful comments and insight into earlier forms of this work. We would like to extend additional thanks to Dr Barbara Voorhies who reviewed and gave valuable feedback on an early draft. Furthermore, we recognize and appreciate the zooarchaeologists who identified the faunal remains discussed here, including Dr Thomas Wake, Dr Richard Cooke, and Dr Elizabeth Reitz.
Supplemental Material
Supplemental S1. 1 Nestedness matrix used to calculate the NeD nestedness metric.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Almeida-Neto, M., P. Guimarães, P. R. Guimarães, Jr, R. D. Loyola, and W. Ulrich. 2008. A consistent metric for nestedness analysis in ecological systems: Reconciling concept and measurement. Oikos 117 (8):1227–39. doi:10.1111/j.0030-1299.2008.16644.x.
- Alsgaard, A. 2020. A meta-analysis approach to understanding Maya fish use on the Yucatán peninsula. Journal of Ethnobiology 40 (4):499–518. doi:10.2993/0278-0771-40.4.499.
- Blake, M., B. S. Chisholm, J. E. Clark, B. Voorhies, and M. W. Love. 1992. Prehistoric subsistence in the Soconusco region. Current Anthropology 33 (1):83–94. doi:10.1086/204038.
- Boethius, A., and T. Ahlström. 2018. Fish and resilience among Early Holocene foragers of southern Scandinavia: A fusion of stable isotopes and zooarchaeology through Bayesian mixing modelling. Journal of Archaeological Science 93:196–210. doi:10.1016/j.jas.2018.02.018.
- Boserup, E. 1965. The conditions of agricultural growth. New York: Routledge.
- Clark, J. E., and D. Gosser. 1995. Reinventing Mesoamerica’s first pottery. In The emergence of pottery: Technology and innovation in ancient societies, ed W. K. Barnett and J. W. Hoopes, 209–22. Washington, DC: Smithsonian Institution Press.
- Coe, M. D., and K. V. Flannery. 1967. Early cultures and human ecology in south coastal Guatemala. Washington, DC: Smithsonian Press. doi:10.5479/si.00810223.3.1
- Cooke, R. G., M. Jiménez, C. Tapia, and B. Voorhies. 2004. A closer look at the Late Archaic fish fauna. In Coastal collectors in the Holocene: The Chantuto people of southwest Mexico, ed. B. Voorhies, 207–99. Gainesville: University Press of Florida.
- Cooke, R., and G. T. Rodríquez. 1994. Marine and freshwater fish amphidromy in a small tropical river on the Pacific coast of Panama: A preliminary evaluation based on gill-net and hook-and-line captures. In Fish exploitation in the past. Proceedings of the 7th meeting of the ICAZ fish remains working group. ed. W. Van Neer, 99–106. Sciences Zoologiques no274. Tervuren, Belgium: Annales du Museé Royal de l‘Afrique Centrale.
- Giovas, C. M., S. M. Fitzpatrick, O. Kataoka, and M. Clark. 2016. Prey body size and anthropogenic resource depression: The decline of prehistoric fishing at Chelechol ra Orrak, Palau. Journal of Anthropological Archaeology 41:132–46. doi:10.1016/j.jaa.2015.12.001.
- Grayson, D. K. 1984. Quantitative zooarchaeology: Topics in the analysis of archaeological faunas. Orlando, FL: Academic Press.
- Grayson, D. K. 1998. Moisture history and small mammal community richness during the latest Pleistocene and Holocene, northern Bonneville Basin, Utah. Quaternary Research 49 (3):330–4. doi:10.1006/qres.1998.1970.
- Grayson, D. K., and F. Delpech. 1998. Changing diet breadth in the early Upper Palaeolithic of southwestern France. Journal of Archaeological Science 25 (11):1119–29. doi:10.1006/jasc.1998.0339
- Jones, E. L. 2004. Dietary evenness, prey choice, and human-environment interactions. Journal of Archaeological Science 31 (3):307–17. doi:10.1016/j.jas.2003.08.011.
- Jones, E. L. 2016. In search of the broad spectrum revolution in Paleolithic southwest Europe. New York: Springer.
- Hickling, C. F. 1961. Tropical inland fisheries. New York, NY: John Wiley and Sons.
- Kennett, D., and B. Voorhies. 1995. Middle Holocene periodicities in rainfall inferred from oxygen and carbon isotopic fluctuations in prehistoric tropical estuarine mollusk shells. Archaeometry 37 (1):157–70. doi:10.1111/j.1475-4754.1995.tb00734.x
- Kennett, D., and B. Voorhies. 1996. Oxygen isotopic analysis of archaeological shells to detect seasonal use of wetlands on the southern Pacific Coast of Mexico. Journal of Archaeological Science 23 (5):689–704. doi:10.1006/jasc.1996.0065.
- Kennett, D. J., D. R. Piperno, J. G. Jones, H. Neff, B. Voorhies, M. K. Walsh, and B. J. Culleton. 2010. Pre-pottery farmers on the Pacific coast of southern Mexico. Journal of Archaeological Science 37 (12):3401–11. doi:10.1016/j.jas.2010.07.035.
- Lesure, R. G., and T. A. Wake. 2011. Archaic to formative in Soconusco. In Early Mesoamerican social transformations: Archaic and formative lifeways in the Soconusco Region. ed. R. G. Lesure, 67–93. Oakland, CA: University of California Press.
- Lesure, R. G., R. J. Sinensky, and T. A. Wake. 2021. The end of the Archaic in the Soconusco region of Mesoamerica: A tipping point in the local trajectory toward agricultural village life. In Preceramic Mesoamerica, ed. J. C. Lohse, A. Borejska, and A. A. Joyce, 481–05. New York: Routledge.
- Love, M. W. 2002. Early complex society in Pacific Guatemala: Settlements and chronology of the Rio Naranjo, Guatemala. Papers of the New World Archaeological Foundation 66. Provo: Brigham Young University.
- Lyman, L. R. 2008. Quantitative Paleozoology. Cambridge: Cambridge University Press.
- Lyman, L. R. 2015. On the variable relationship between NISP and NTAXA in bird remains and in mammal remains. Journal of Archaeological Science 53:291–6. doi:10.1016/j.jas.2014.10.027
- Magurran, A. E. 1988. Ecological diversity and its measurement. Princeton, NJ: Princeton University Press.
- Nagaoka, L. 2005. Differential recovery of Pacific Island fish remains. Journal of Archaeological Science 32 (6):941–55. doi:10.1016/j.jas.2004.12.011
- Palka, J. W. 2024. Ancestral Maya domesticated waterscapes, ecological aquaculture, and integrated subsistence. Ancient Mesoamerica 35 (1):208–36. doi: 10.1017/S0956536122000402.
- Partlow, M. A. 2006. Sampling fish bones: A consideration of the importance of screen size and disposal context in the north Pacific. Arctic Anthropology 43 (1):67–79. doi:10.1353/arc.2011.0064
- Pauly, D., V. Christensen, J. Dalsgaard, R. Froese, and F. Torres. Jr. 1998. Fishing down marine food webs. Science (New York, N.Y.)279 (5352):860–3. doi: 10.1126/science.279.5352.860.
- Reitz, E. J., I. R. Quitmyer, and R. A. Marrinan. 2009. What are we measuring in the zooarchaeological record of Prehispanic fishing strategies in the Georgia Bight, USA? The Journal of Island and Coastal Archaeology 4 (1):2–36. doi:10.1080/15564890802349894
- Reynaga, D. K. M. 2010. Pre-Columbian diets in the Soconusco revisited: A dietary study through stable isotope analysis. MA thesis, University of British Columbia.
- Rivas, A. E., and W. G. B. Odum. 2019. Ethnography and archaeology of water in the Maya lowlands. Open Rivers 14:93–110.
- Rosenswig, R. M. 2006. Sedentism and food production in early complex societies of the Soconusco, Mexico. World Archaeology 38 (2):330–55. doi:10.1080/00438240600694115
- Rosenswig, R. M. 2012. Materialism, mode of production, and a millennium of change in southern Mexico. Journal of Archaeological Method and Theory 19 (1):1–48. doi:10.1007/s10816-010-9101-0.
- Rosenswig, R. M., A. M. VanDerwarker, B. J. Culleton, and D. J. Kennett. 2015. Is it agriculture yet? Intensified maize-use at 1000cal BC in the Soconusco and Mesoamerica. Journal of Anthropological Archaeology 40:89–108. doi: 10.1016/j.jaa.2015.06.002.
- Strona, G., D. Nappo, F. Boccacci, S. Fattorini, and J. San-Miguel-Ayanz. 2014. A fast and unbiased procedure to randomize ecological binary matrices with fixed row and column totals. Nature Communications 5 (1):4114. doi:10.1038/ncomms5114.
- Trusler, A. K. 2013. The impact of recovery methods on taxonomic richness in roman faunal assemblages. Archaeometry 56 (6):1075–84. doi:10.1111/arcm.12066
- Ulrich, W., M. Almeida-Neto, and N. J. Gotelli. 2009. A consumer’s guide to nestedness analysis. Oikos 118 (1):3–17. doi:10.1111/j.1600-0706.2008.17053.x
- Vega-Villasante, F., L. E. Ruiz-González, O. Chong-Carrillo, M. E. R. Basto-Rosales, D. J. Palma-Cancino, A. Tintos-Gómez, C. E. Montoya-Martínez, L. D. Kelly-Gutiérrez, S. R. Guerrero-Galván, J. T. Ponce-Palafox, et al. 2021. Biology and use of the Pacific fat sleeper Dormitator latifrons (Richardson, 1844): state of the art review. Latin American Journal of Aquatic Research 49 (3):391–403. doi:10.3856/vol49-issue3-fulltext-2637
- Voorhies, B. 1976. The Chantuto people: An Archaic Period society of the Chiapas littoral, México. Utah: New World Archaeological Foundation.
- Voorhies, B., ed. 2004. Coastal collectors in the Holocene: The Chantuto people of southwest Mexico. Florida: University Press of Florida.
- Voorhies, B., ed. 2015. An Archaic Mexican shellmound and its entombed floors. California: UCLA Cotsen Institute of Archaeology Press.
- Voorhies, B., J. Gasco, and P. Cackler. 2011. Prehistoric settlement in the south Pacific coast of Chiapas, Mexico. Papers of the New World Archaeological Foundation. Provo, UT: New World Archaeological Foundation.
- Voorhies, B., and D. Kennett. 2011. A gender-based model for changes in subsistence and mobility during the terminal Late Archaic Period on the coast of Chiapas, Mexico. In Early Mesoamerican social transformations: Archaic and Formative lifeways in the Soconusco region, ed. R. Lesure, 27–46. Berkeley: University of California Press.
- Voorhies, B., and D. J. Kennett. 2021. Preceramic lifeways on the Mesoamerican south Pacific coast. In Preceramic Mesoamerica, ed. J. C. Lohse, A. Borejska, and A. A. Joyce. New York: Routledge.
- Voorhies, B., D. J. Kennett, J. G. Jones, and T. A. Wake. 2002. A Middle Archaic archaeological site on the west coast of Mexico. Latin American Antiquity 13 (2):179–200. doi:10.2307/971913
- Wake, T. A. 2004. A vertebrate archaeofauna from the Early Formative Period site of Paso de la Amada, Chiapas, Mexico: Preliminary results. In Maya zooarchaeology: New directions in method and theory, ed. K. F. Emery, 209–22. Los Angeles, CA: Cotsen Institute of Archaeology.
- Wake, T. A., N. Anikouchine, and B. Voorhies. 2004. Food procurement and processing: Fish and game remains at the shellmound sites. In Coastal collectors in the Holocene: The Chantuto people of southwest Mexico, ed. B. Voorhies, 154–206. Gainesville: University Press of Florida.
- Wake, T. A., K. J. Bishop, and R. G. Lesure. 2021. The Faunal Remains of Paso de la Amada. In Paso de la Amada: An early Mesoamerican ceremonial center, ed. R. G. Lesure, 309–46. Los Angeles, CA: Cotsen Institute of Archaeology Press.
- Wake, T. A., and L. R. Harrington. 2002. Appendix II: Vertebrate faunal remains from La Blanca, Guatemala. In Early complex society in Pacific Guatemala: Settlements and chronology of the Rio Naranjo, Guatemala, ed. M. Love, 237–52. Provo, UT: Brigham Young University.
- Wake, T. A., and B. Voorhies. 2015. The Tlacuachero vertebrate fauna. In An Archaic Mexican shellmound and its entombed floors, ed. B. Voorhies, 145–70. Los Angeles, CA: Cotsen Institute of Archaeology Press.
- Winterhalder, B., and C. Goland. 1997. An evolutionary ecology perspective on diet choice, risk, and plant domestication. In People, plants, and landscapes: Studies in paleoethnobotany, ed. K. J. Gremillion, 123–60. Tuscaloosa: University of Alabama Press.
- Wolverton, S., C. Otaola, G. Neme, M. Giardina, and A. Gil. 2015. Patch choice, landscape ecology, and foraging efficiency: The zooarchaeology of Late Holocene foragers in Western Argentina. Journal of Ethnobiology 35 (3):499–518. doi:10.2993/etbi-35-03-499-518.1.