?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
A thermodynamic analysis is performed on a solar-pond assisted reverse osmosis-electrodialysis system to produce freshwater and a hydroponic fertilizer solution for sustainable desalination and waste recovery. The reverse osmosis unit desalinates seawater to produce freshwater and a waste concentrated salt solution rich in many useful ions and minerals. The reverse osmosis unit’s waste stream is sent to an electrodialysis unit that utilizes monovalent membranes to reject magnesium, calcium and sulfate ions. These ions are beneficial for plant growth and the product can be used as a hydroponic fertilizer solution for agricultural purposes. A solar pond is integrated into the system to reduce the power requirements. The solar pond utilizes energy from the sun to store thermal energy in a large body of concentrated salt water with temperatures up to 90°C in the lower convecting zone. This thermal energy is utilized in an organic Rankine cycle with the working fluid R113 to create a self-sustaining desalination and waste treatment system running on solar energy. The system produces about 4.52 kg/s of freshwater and 4.75 kg/s of hydroponic solution. The overall energy and exergy efficiencies of the integrated system are found to be 20.36% and 2.55%, respectively.
Introduction
Climate change, population growth, and human activity are all leading to freshwater shortages around the world. By 2050, it is predicted that one in four people will originate from a country affected by freshwater shortages (Global water crisis: Facts, FAQs, and how to help | World Vision Citationn.d.). This not only affects human health, but also the agriculture sector, as it accounts for 70% of the world’s water consumption. It is essential to make freshwater production processes more sustainable and minimize waste to guarantee resources for future generations. Electrodialysis (ED) uses selective ion exchange membranes to separate ions from water using an applied electrical current. It can be used for treating seawater (35,000 ppm), groundwater (1000–10,000 ppm) and wastewater streams. ED is better suited for treating brackish water below 3,000 ppm whereas Reverse Osmosis (RO) is better suited for salinities above 3,000 ppm (Moran Citation2018). The specific power requirement for RO desalination is 2–6 kW/m3 (Zhao et al. Citation2019). For ED, it is between 10–25 kW/m3 and 0.4–4 kW/m3 (Electrodialysis – an overview | ScienceDirect Topics Citationn.d.) for seawater and brackish water, respectively. The benefit of ED compared to RO is that it has a much higher recovery rate of around 85–90% (Electrodialysis – an overview | ScienceDirect Topics Citationn.d.). Additionally, ED is more suitable for agriculture and farming use in remote areas as local plants can be set up in areas off the grid.
Solar-based power generation systems are some of the most promising methods to meet the worlds energy demands in a sustainable way (Hosseini Citation2019). Since ED and RO require electrical energy, solar ponds can be implemented in combination with heat engines. Solar ponds are large bodies of saline water that can be used to store thermal energy from the sun. There are two types of solar ponds: Convecting shallow ponds and non-convecting deep ponds (El-Sebaii et al. Citation2011). Salinity gradient ponds are a type of non-convecting solar pond, and they absorb and store solar energy in a saltwater reservoir. It contains three layers known as the upper convective zone (UCZ), non-convective zone (NCZ), and lower convective zone (LCZ) (Khalilian, Pourmokhtar, and Roshan Citation2018). The UCZ layer at the top has a low salt concentration, and the LCZ layer has a very high salt concentration close to saturation (Njoku, Agashi, and Onyegegbu Citation2017). The NCZ is the middle layer in which the salt concentration increases with depth and prevents water movement into the upper and lower layers (Njoku, Agashi, and Onyegegbu Citation2017). Therefore, there is no convection, and the only heat transfer takes place through conduction (Njoku, Agashi, and Onyegegbu Citation2017). The NCZ acts as an insulator because water has a low thermal conductivity, allowing the LCZ to absorb and store solar radiation (Njoku, Agashi, and Onyegegbu Citation2017). The density and temperature of the NCZ also increases from top to bottom with an increasing concentration of salt (Bezir et al. Citation2009). This results in the LCZ reaching temperatures above 90°C while the temperature in the UCZ remains at around 30°C (Wagner Citation2013). This temperature difference can be utilized in an organic Rankine cycle-based heat engine to generate electricity.
For a large-scale electrical power production of 5 MW, areas up to 1 km2 are required for solar ponds; sites such as the Dead Sea, Great Salt Lake, and the Salton Sea exist and can be utilized for such projects (Wittenberg Citation1981). There are several studies coupling solar ponds with thermal and membrane desalination. Ghaebi and Rostamzadeh (Ghaebi and Rostamzadeh Citation2020) performed a theoretical study on a salinity-gradient solar pond integrated with organic Rankine cycle (ORC) for RO desalination and found that the system provided 4 m3/h of freshwater and 29.6 kW of net electricity. El Mansouri et al. (El Mansouri et al. Citation2020) performed a feasibility analysis of RO desalination run by solar ponds in the Mediterranean and semi-arid conditions; the optimization results found that freshwater with a salinity of 376.6 mg/L can be produced with 2.1 kWh/m3 specific energy consumption. Additionally, it was found that a solar pond constructed in a semi-arid land would be 33.6% smaller than one constructed in the Mediterranean (El Mansouri et al. Citation2020). Salata and Coppi (Salata and Coppi Citation2014) found that 1000–4000 m2 of solar pond area is required per m3 of desalinated water produced. Other papers studied the hybridization of solar ponds with forward osmosis (FO) (Monjezi, Mahood, and Campbell Citation2017), lithium extraction (Yu et al. Citation2015) and multi-stage flash (MSF) distillation (Agha, Rice, and Wheldon Citation2000).
Since the agriculture sector is essential for human life, fertilizer production can be made more sustainable. Hydroponic agriculture is beneficial as less water is used overall and it would be particularly useful for hot regions where water is scarce. According to Loganathan Naidu, and Vigneswaran (Loganathan, Naidu, and Vigneswaran Citation2017), Na, Ca, Mg, K, Li, Sr, Br, B, and U can potentially be extracted from seawater if suitable methods are found. Out of these, Mg, Ca, K, and Br can be used for fertilizer production. Currently, methods for mining minerals from seawater include vacuum or solar evaporation, ED, membrane distillation crystallization (MDC), and adsorption/desorption/crystallization (Loganathan, Naidu, and Vigneswaran Citation2017). Conventional cation and anion-selective ED membranes cannot be used to separate ions of the same charge. Selective monovalent ion membranes need to be utilized to separate specific ions from divalent ions (Loganathan, Naidu, and Vigneswaran Citation2017). There is also focus on the production of novel monovalent selective membranes for ions such as Mg2+ and struvite using alkyl spacers and zwitterions (Irfan, Wang, and Xu Citation2019). A lab study in the University of South Carolina Research Foundation produced NaCl, Mg(OH)2, and Br2 from RO brine waste using ED (Desalination and Water Purification Research and Development Program Citation2006). Monovalent selective membranes were used in the study that allowed Na+, Cl−, and Br− to pass through and reject divalent Ca2+, Mg2+ and SO42- ions (Desalination and Water Purification Research and Development Program Citation2006). The freshwater stream produced from the ED unit had an Mg concentration much greater than seawater, which was precipitated as Mg(OH)2 with the addition of NaOH. Crystallization was used for the concentrated stream from the ED unit to produce pure NaCl and the solution was further treated with Cl2 to produce Br2 (Desalination and Water Purification Research and Development Program Citation2006). To optimize the Mg extraction, the Ca ions need to be removed from the seawater to prevent membrane scaling. A study by Natasha, Firdiyono, and Sulistiyono et al. (Natasha, Firdiyono, and Sulistiyono Citation2017) suggested that ammonium bicarbonate can be used to remove Ca ions without precipitating the Mg.
Moreover, another study used sodium carbonate and sodium hydroxide sequentially for the removal of Ca and Mg ions, respectively (Sorour, Hani, and Shaalan Citation2016). There are several studies focusing on the recovery of K+ ions from seawater. Guo et al. (Gonzalez, Grágeda, and Ushak Citation2017) used modified clinoptilolite in column sorption-elution to recover K+ ions with a concentration of 7.5720 g/L from desalination by-products. An additional study uses precipitation to recover Mg2+, K+, and SO42- simultaneously from seawater by adding disodium phosphate (1.2% volume), calcium chloride (2% volume), and sodium hydroxide (2% volume) (Pujiastuti et al. Citationn.d.). Other studies also investigated using RO brine waste as a precipitant to recovery phosphorus, which can also be used in the fertilizer industry (Tian et al. Citation2016).
In this paper, a thermodynamic analysis is carried out on a novel system, in which the waste from RO seawater desalination is used to produce a hydroponic fertilizer solution with the assistance of a solar pond. The concentrated waste stream from a RO unit enters an ED unit to produce a hydroponic fertilizer solution rich in minerals by the use of monovalent membranes. The electricity requirement for the ED and RO units is fulfilled by utilizing solar energy through a solar pond-based organic Rankine cycle. There are previous studies combining solar ponds with membrane technologies such as RO and FO (El Mansouri et al. Citation2020); however, there is a lack of studies with ED. Moreover, this study incorporates renewable energy from a solar pond in an integrated system, which has not been previously studied. It incorporates waste reduction by producing a hydroponic solution required for hydroponic greenhouses for sustainable agriculture. The novelty is based on developing a unique integrated system in a minimal waste manner to produce freshwater, hydroponic fertilizer solution, and electricity using solely solar energy. The objectives accomplished in this study are listed as follows:
A theoretical system is to be designed to recover valuable minerals from RO desalination waste brine in the form of a hydroponic fertilizer solution.
A solar pond is to be integrated into the system to generate electricity from an organic Rankine cycle (ORC) to satisfy the power requirements of the ED and RO units.
Mathematical and thermodynamic calculations are to be carried out on the system.
Energy balances, entropy balances, and exergy balances on the system components are to be performed and the efficiencies of the individual units as well as the overall system are to be calculated.
A sensitivity analysis is to be conducted to identify how changing input parameters such as temperature, pressure, solar irradiance and salinity affect the system performance.
System description
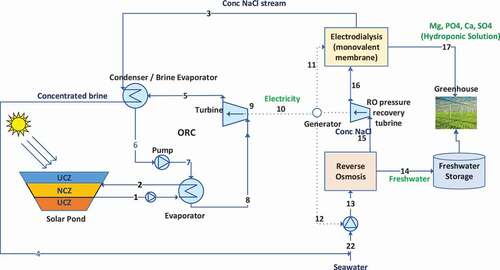
In the theoretical system diagram shown in , a RO unit is used to desalinate a seawater stream to produce fresh water and a brine waste stream. RO is a membrane desalination technology that uses pressure to separate the ions from saltwater to produce fresh water. It is an energy-intensive process as high pressure is required. A pressure recovery turbine is also included in the system to lower the pressure of the stream exiting the RO unit and reutilize the recovered energy. The brine waste stream from the RO unit is sent to an ED unit with specialized monovalent membranes (Neosepta CMS and ACS univalent-selective ion-exchange membranes (Tokuyama Corp.) (Desalination and Water Purification Research and Development Program Citation2006)). ED units use ion-exchange membrane technology and an anode and cathode to separate ions from a solution into different streams: Concentrated and dilute. In this case, the membranes allow bivalent ions like Mg2+, Ca2+, and SO42- to pass and reject the Na+ and Cl− ions (Desalination and Water Purification Research and Development Program Citation2006). These ions enter the dilute stream, which can be used as a hydroponic fertilizer solution for agricultural purposes. The ions are beneficial for healthy plant growth, and the hydroponic solution ensures that less water is used overall in the agricultural process. Since both the ED and RO units require energy input, a solar pond is utilized with an ORC to produce electrical energy. The solar pond is a large body of saline water that absorbs the energy from the sun and heats up the water in levels. The sun hits the UCZ which has the lowest temperature of 25°C and the lowest concentration of salt. The water increases in salinity and temperature as it moves toward the bottom of the solar pond up to 90°C. The difference between the heat in the UCZ and LCZ is to be utilized in an ORC. The ORC includes a pump, turbine, condenser, evaporator, and generator to produce electricity by utilizing the different temperature water in the solar pond. The hot stream from the bottom layer of the solar pond is circulated through the evaporator, providing energy to the working fluid (R113) in the ORC.
Analysis
The system shown in is mathematically modeled on Engineering Equation Solver (EES) (F-Chart-Software, EES: Engineering Equation Citation2020) software by analyzing each unit. The thermodynamic balance equations used in the model are presented in . The ED and RO systems are modeled by utilizing simple equations without detailed membrane calculations to carry out the thermodynamic analysis. The solar pond is simulated based on a model provided by Khalilian et al. (Khalilian, Pourmokhtar, and Roshan Citation2018). The ORC is also modeled on EES (F-Chart-Software, EES: Engineering Equation Citation2020). The working fluid used in the ORC is R113. Finally, after the thermodynamic analysis, a sensitivity analysis is also performed on individual units and as well the entire system to understand how modifying the temperature, pressure, salinities, and solar irradiance affect the energy and exergy efficiencies of the system.
Table 1. Balance and efficiency equations for the evaporator, pump, condenser, and turbine
Table 2. Balance and efficiency equations for ED, RO and solar pond
Reverse osmosis/electrodialysis system
The RO and ED units are modeled on EES (F-Chart-Software, EES: Engineering Equation Citation2020) software with the built-in seawater fluid package. The thermodynamic balances are applied, and the efficiencies of the overall system and individual units are calculated.
The main assumptions considered during the analysis are written below:
The surrounding air temperature is T0 = 25°C and the surrounding pressure is P0 = 101 kPa.
The pumps and turbines are isentropic.
Seawater salinity is taken as 35 g/kg.
Simplified balances are performed on the ED and RO units.
The dilute product stream from ED has a salinity of 35 g/kg.
The electrodialysis cell uses Neosepta CMS and ACS univalent-selective ion-exchange membranes (Tokuyama Corp.) (Desalination and Water Purification Research and Development Program Citation2006)
The ionic composition in the ED dilute is: 64, 2.53, 164, 24.5, 241, 0, 100, 2.75 mM for Na+, K+, Mg2+, Ca2+, Cl−, Br−, SO42- and HCO3- (Desalination and Water Purification Research and Development Program Citation2006).
The recovery rate (R) for both the electrodialysis unit and reverse osmosis unit is found by the equation where Qp is the product flow rate (m3/s) and Qf is the feed flow rate (m3/s) (Lee et al. Citation2002):
The solute rejection (SR) for both units can be calculated by an equation where Cf is the feed inlet concentration, and Cp is the concentration of the product freshwater outlet (Lee et al. Citation2002).
The pumping pressure (P) for the RO unit can be found by the following equation, where Posmotic is the osmotic pressure of seawater.
Both units are modeled on EES (F-Chart-Software, EES: Engineering Equation Citation2020) according to the assumptions and input variables mentioned in .
Table 3. Input values for RO and ED units
Solar pond
The one-dimensional solar pond is modeled on EES (F-Chart-Software, EES: Engineering Equation Citation2020) software with the built-in seawater library containing its thermophysical properties. The mathematical model used in this system is provided by Khalilian et al. (Khalilian, Pourmokhtar, and Roshan Citation2018). The model was then modified to include an ORC to utilize the heated saline water and the concentrated waste output from the desalination unit. The stream used in the organic Rankine cycle is taken from the NCZ and pumped into the evaporator after which renters the solar pond. summarizes the input values that were used to model the solar pond.
Table 4. Input values for the solar pond analysis
For the solar pond, the following assumptions are made:
The average solar irradiation is I = 400 W/m2.
The temperature of the sun is Tsolar = 6000 K.
The factor 0.85 represents the reduction in radiation absorbed by the solar pond.
The UCZ temperature is equal to the surrounding temperature.
The side walls do not allow the heat to pass.
The temperature of the ground is equivalent to the surrounding temperature.
The physical properties where T is in K and C is in kg/m3 are calculated by:
The salinity in the NCZ layers increases with depth and it is calculated as follows:
The remainder solar radiation is calculated as follows:
The coefficient of reflection is calculated as follows:
The following equation represents the relationship between incidence and refraction angles:
The solar irradiation underwater at different levels in the solar pond is represented by the following equation:
The transmission function h(z) is represented by the following equation:
Organic Rankine cycle
An ORC is used to generate electricity by using working fluids with low boiling temperatures instead of steam. The working fluid is first pumped from a saturated liquid state in the condenser to the evaporator. It is then evaporated in the evaporator by using the heated stream from the solar pond. The working fluid is then expanded in the turbine, where it rotates the shaft and generates electricity. Finally, the working fluid is condensed back into the saturated liquid state (Zeynali, Akbari, and Khalilian Citation2019). The heated stream from the NCZ in the solar pond (stream 1) is sent to the evaporator, where it evaporates the working fluid. The chosen working fluid is R113, as it was found to be most efficient based on previous literature (Safarian and Aramoun Citation2015). However, the adverse effects of this refrigerant on human health and the environment must be acknowledged. It contains Carbon tetrachloride, Chloroform and Trichloroethylene, which are chemicals that can lead to cancer and congenital disabilities. It also causes ozone depletion, which can lead to increased rates of skin cancer (R-113 | A-Gas in the Americas Citationn.d.).
→The EES(F-Chart-Software, EES: Engineering Equation Citation2020) model developed for the ORC cycle is based on the assumptions made by Safarian and Aramoun (Safarian and Aramoun Citation2015). summarizes the main input values used in the ORC. shows the thermophysical properties of different working fluids, which are used in the sensitivity analysis.
Table 5. ORC input parameters
Table 6. ORC working fluid thermophysical properties
The net power input into the system is calculated by the following:
Results and discussion
This section presents the results of the entire system as well as the validation of the ORC cycle and summarizes the main findings and interpretations. The energy and exergy efficiencies of individual systems, as well as the overall system, are found. Moreover, several input parameters are modified in a sensitivity analysis to find their effects on the efficiencies and output parameters.
Integrated system results
The system was set up to have a net power of zero where the ED, RO, and pumps ran on the energy utilized from the solar pond alone. shows a summary of system state points and the individual enthalpy, entropy, and exergy values of the streams that were calculated.
Table 7.. Summary of stream enthalpy, entropy, exergy values
shows the work inputs and outputs for individual components to produce 4.213 kg/s of freshwater and 4.424 kg/s of hydroponic solution with the ionic composition of 64, 2.53, 164, 24.5, 241, 0, 100, 2.75 mM for Na+, K+, Mg2+, Ca2+, Cl−, Br−, SO42- and HCO3- respectively (Tian et al. Citation2016). The power produced by the solar pond via the ORC is 49.77 kW. The ED unit requires 23.3 kW of power to produce the hydroponic solution as a product and a concentrated stream that is recycled. The RO unit requires 44.71 kW of power which is much higher than the ED unit. However, the RO recovery turbine generates 18.86 kW of power, resulting in the RO unit having a decreased overall power input of 25.9 kW to desalinate seawater with a mass flow rate of 10.53 kg/s.
Table 8. Work inputs/outputs for the ORC system
shows the energy and exergy efficiencies of the different units in the system. The ED unit has the highest energy efficiency of 68.97%, and the solar pond has the lowest energy efficiency of 22.97%. This is because a lot of the available solar energy is wasted and not absorbed by the water. Some of it is lost to the ground, which is assumed to be at ambient temperature. The energy efficiency of the organic Rankine cycle is also found to be 19.75%, which is comparable to other works in the literature (Safarian and Aramoun Citation2015).
Table 9. The energy and exergy efficiencies of the ED, RO, and solar pond
shows the efficiencies and the specific energy requirements of the system and main subunits. The overall energy efficiency of the system is around 19.29% which is significantly higher than the exergy efficiency of 2.38%. The specific energy of the ED unit was set to be 1.5 kWh/m3 to model the system and the RO specific energy is calculated to be 2.94 kWh/m3 which is comparable to other works in the literature (Karabelas et al. Citation2018).
Table 10. Overall system efficiencies and specific energy requirements
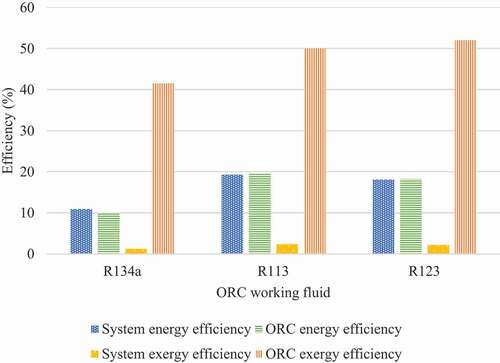
The working fluid can also influence the efficiency of the system, so a comparative analysis is performed. shows a comparison between different refrigerants (R134a, R113 and R123) which can be used in the ORC cycle. The mathematical calculations were performed for all three and the results showed that R113 had the highest overall energy and exergy system efficiencies; therefore, this refrigerant will be kept as the base case for this system analysis.
Sensitivity analysis
The following section consists of a sensitivity analysis performed on the system by using the developed mathematical code on EES software (F-Chart-Software, EES: Engineering Equation Citation2020). Several parameters such as salinity, temperature, solar irradiance, and heat input were modified to find their effects on the system.
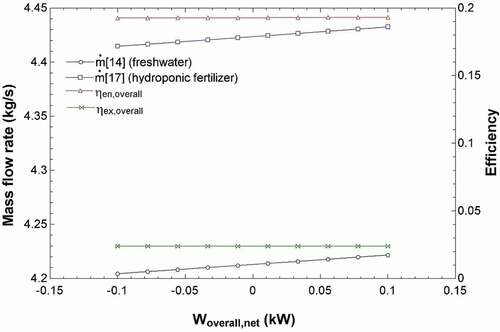
shows the effect of the seawater salinity into the system (stream 22) on the efficiencies and work requirements of the overall system in addition to the ED and RO units. The seawater salinity does not have much of an effect on the system’s efficiencies; however, when analyzing the work inputs, the salinity of the seawater input increases the power requirement of the RO pump slightly. The RO recovery turbine also produces more power, according to . shows the effect of the net work of the system on the mass flow rates of the hydroponic solution (Stream 17) and freshwater solution (Stream 14) as well as the overall energy and exergy efficiencies. The base case was carried out at a net work of 0 kW where all the power produced from the solar pond was utilized by the RO and ED units. The graph shows the efficiency to be increasing where the net work in is positive. In this case, the net calculation was based on the EquationEquation 12(12)
(12) to find the net power input. The graph shows that the efficiencies and the mass flow rates of the products to increase with a more positive net work; this means that in this case, external power would be required from another source.
Figure 3. Effects of the salinity of seawater inlet (stream 22) on the work requirements of the ED and RO units and the work produced by the RO turbine.
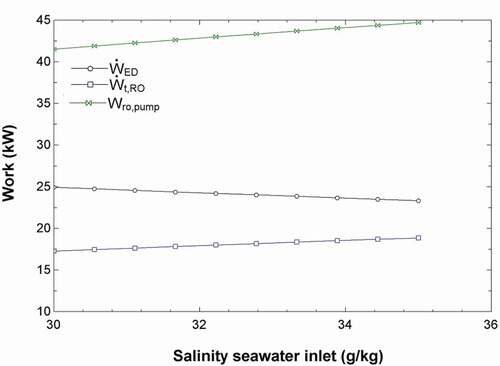
Figure 4. Effects of the net work of the system on the mass flow of the freshwater and hydroponic fertilizer solutions (y1 axis) and on the efficiencies of the overall system (y2 axis).
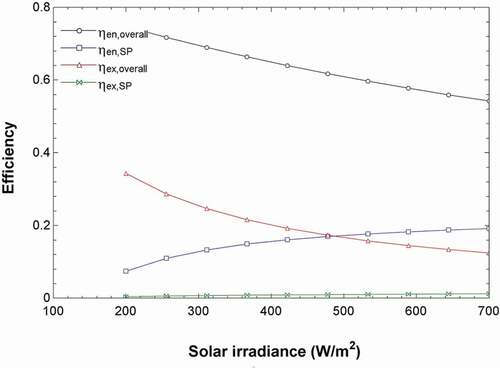
shows that as the solar irradiance is increased, the temperature of the NCZ layers in the solar pond is increased. This is because more energy is absorbed per area by the water, allowing it to increase in temperature. The temperatures of the UCZ and LCZ zone were fixed as an assumption, so they do not change. Additionally, shows that the energy and exergy efficiency of the solar pond slightly increases with increasing irradiance. This is because more energy is absorbed by the solar pond per area allowing it to reach higher temperatures.
Figure 5. Effects of the solar irradiance on the temperature of the UCZ and the NCZ’s of the solar pond.
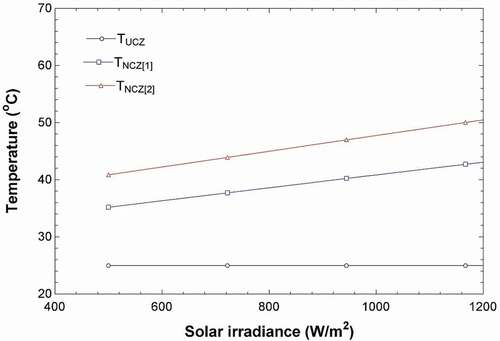
Figure 6. Effects of the solar irradiance on the solar pond and overall system energy and exergy efficiencies.
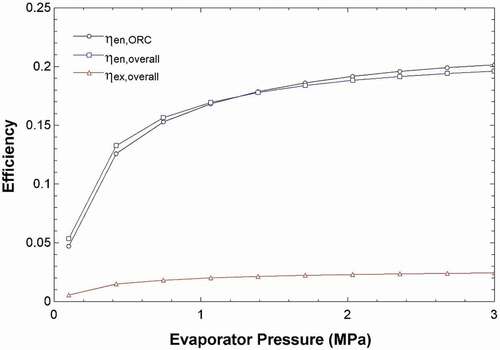
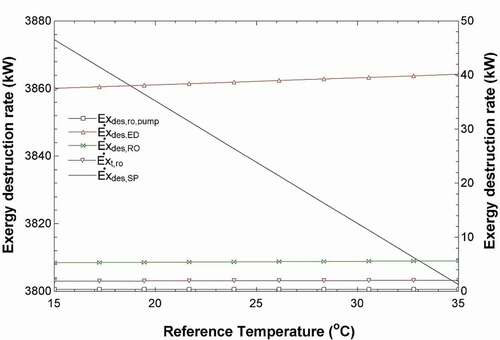
The exergy destruction shows the resource degradation, and it can be caused by the mixing of streams with different temperatures, pressures and chemical compositions. When energy or material steams are released into the surroundings, the exergy associated is the exergy loss for the entire system (Kotas Citation1985). According to , changing the ambient temperature does not affect the exergy destructions of most of the units in the system. It only slightly increases them, as shown by the axis on the right. However, the ambient temperature does decrease the exergy destruction of the solar pond, as shown on the left y-axis of the graph. The solar pond has interacting streams with a wide range of temperatures so the change in ambient temperature makes more of a difference. Additionally, shows that increasing the pressure of the evaporator outlet increases the energy efficiency of the ORC and the entire system where it levels out to around 20%. The exergy efficiency also increases, however, at a much lower rate.
Figure 7. Effects of changing the system ambient temperature on the exergy destruction rates of the RO pump, ED unit, RO unit, RO turbine (y2 axis) and the solar pond (y1 axis).
Since the system is set up to be self-sustaining, the net power requirement is 0 kW; this results in the mass flow rates of the products being calculated based on the energy from the solar pond. shows that as more energy enters the evaporator via stream 1 from the solar pond, the mass flow rates of the hydroponic solution (stream 17) and the freshwater (stream 14) increase. This is because as more energy is fed into the evaporator of the ORC, the power output is higher, allowing the ED and RO units to run at a higher capacity. Moreover, shows that as the energy into the evaporator of the ORC increases, the energy efficiency of the solar pond and the overall efficiency slightly increases. The energy efficiency of the ORC does not change, and the overall efficiency is affected by the solar pond. This is because the temperature of stream 2 is fixed at 40°C, which is the stream returning to the solar pond after providing heat to the evaporator. Therefore, changes made to the heat extracted from the solar pond will allow the temperature of the stream entering the evaporator from the solar pond to be increased (stream 1). A larger difference in temperatures will allow the efficiency of the solar pond to increase according to the energy efficiency equation of the solar pond presented in .
Figure 9. Effects of the heat into the ORC evaporator on the mass flow rates of the product freshwater (stream 14) and hydroponic solution (stream 17).
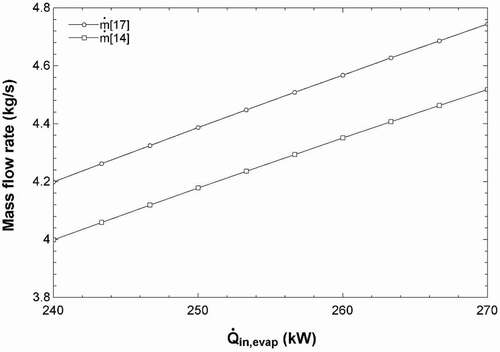
Figure 10. Effects of the heat into ORC evaporator on the efficiencies of ORC, solar pond and complete system.
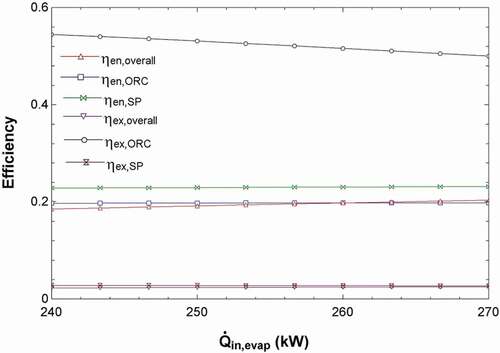
Therefore, as a result of , the energy extracted from the solar pond will be set to 270 kW, which results in the temperature of stream entering the ORC evaporator to be 86.4°C. The resulting solar pond efficiencies are found to be 23.15% for energy and 2.7% for exergy. Moreover, the ORC energy efficiency is found to be 19.77% and the exergy efficiency is found to be 50.03. This increases the overall efficiencies of the system to be 20.36% and 2.54% for energy and exergy respectively. The exergy efficiency is low for the overall system due to the solar pond having a low exergy efficiency as shown in . A higher exergy efficiency would improve the energy quality of the system making it more sustainable. Therefore, it is necessary to carry out further optimization studies to increase the exergy efficiency. The energy efficiencies of the solar pond and ORC are comparable to those in literature (Mosaffa and Farshi Citation2021).
The final system run by the solar pond produces 4.52 kg/s and 4.75 kg/s of freshwater and hydroponic fertilizer solution, respectively. This system produces 4.52 kg/s of hydroponic solution which is able to produce around 203,632 tomatoes/year since the hydroponic requirement for tomatoes is about 35 L/kg on average (Massa et al. Citation2010).
Conclusion
In this study, a novel integrated system is designed and modeled to produce freshwater and a hydroponic fertilizer solution for agriculture whilst utilizing solely solar energy. The integrated system consists of a RO unit, ED unit, a solar pond and an ORC. The seawater is desalinated using an RO unit, which produces a concentrated waste brine stream in addition to a freshwater stream. This waste stream contains many useful minerals, which can be extracted for fertilizer production. Therefore, the concentrated waste stream is sent to an ED unit, which uses specialized monovalent membranes to separate the useful ions and produce a hydroponic fertilizer solution containing ions like Mg2+, Ca2+ and SO42-. A hydroponic solution fertilizer is particularly useful for water conservation and space management in agriculture. The system is made more sustainable by utilizing renewable solar energy via a solar pond to run the ED and RO units. A thermodynamic analysis is carried out on the system, including energy, entropy and exergy balances. Moreover, a sensitivity analysis is conducted where various parameters such as salinity, temperature, solar irradiance, and heat input are studied.
The main findings of this work are listed as follows:
The specific energy requirement of the RO desalination unit is 2.94 kWh/m3 and 1.5 kWh/m3 for the ED unit.
The system requires 75.45 kW to run the system for hydroponic solution and freshwater production.
The system is designed to be self-sustaining; therefore, the solar pond generates the same amount of power to produce 4.52 kg/s and 4.75 kg/s of freshwater and hydroponic fertilizer solution, respectively.
The RO, ED, solar pond, and ORC energy efficiencies are 42.06%, 69.97%, 23.15%, and 19.77%, respectively.
Similarly, the exergy efficiencies of the RO, ED, solar pond, and ORC are 27.05%, 7.35%, 2.7%, and 50.02%, respectively.
The overall energy efficiency of the system is 20.36%, and the overall exergy efficiency is 2.55%.
In future work, an experimental investigation can be done to validate the theoretical results. Additionally, a life cycle assessment can be carried out to find the overall sustainability of the system in the long term in comparison to conventional desalination and fertilizer production routes. Moreover, a cost analysis can be performed to understand the overall cost of the system and its economic feasibility.
Nomenclature
Table
Acknowledgments
The authors acknowledge the support provided by the Hamad Bin Khalifa University, Qatar Foundation. This publication was made possible by a graduate sponsorship research award (GSRA) from Qatar National Research Fund with number GSRA6-2-0617-19102. Open Access funding provided by the Qatar National Library.
Disclosure statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Additional information
Funding
Notes on contributors
Namra Mir
Namra Mir did her BSc in Chemical Engineering from Qatar University and MSc in Sustainable Energy from Hamad Bin Khalifa University, Qatar. Currently, she is working as a research assistant at Hamad Bin Khalifa University, Qatar.
Yusuf Bicer
Dr. Yusuf Bicer received his PhD in the area of mechanical engineering from the University of Ontario Institute of Technology in Oshawa, Canada. He completed his BS in Control Engineering (2012) and master’s degree in Energy Science and Technology (2014) at Istanbul Technical University, Turkey. Currently, Dr. Bicer is an assistant professor of sustainable development in the College of Science and Engineering at Hamad Bin Khalifa University in Doha, Qatar.
References
- Agha, K. R., G. Rice, and A. E. Wheldon. 2000. The thermal characteristics and economic analysis of a solar pond coupled low temperature multi stage desalination plant part I: Thermal characteristics. International Journal of Solar Energy 20 (4):255–74. doi:https://doi.org/10.1080/01425910008914359.
- Bezir, N. Ç., N. Özek, R. Kayal, A. K. Yakut, A. Şencan, and S. Kalogirou. 2009. Theoretical and experimental analysis of a salt gradient solar pond with insulated and reflective covers, energy sources, part A recover. Util. Environ. Eff 31:985–1003. doi:https://doi.org/10.1080/15567030802089011.
- Desalination and Water Purification Research and Development Program, 2006.
- El Mansouri, A., M. Hasnaoui, A. Amahmid, and S. Hasnaoui. 2020. Feasibility analysis of reverse osmosis desalination driven by a solar pond in Mediterranean and semi-arid climates, energy convers. Manag 221:113190. doi:https://doi.org/10.1016/j.enconman.2020.113190.
- El-Sebaii, A. A., M. R. I. Ramadan, S. Aboul-Enein, and A. M. Khallaf. 2011. History of the solar ponds: A review study. Renewable and Sustainable Energy Reviews 15 (6):3319–25. doi:https://doi.org/10.1016/j.rser.2011.04.008.
- Electrodialysis - an overview | ScienceDirect Topics, (n.d.). https://www.sciencedirect.com/topics/earth-and-planetary-sciences/electrodialysis (accessed November 17, 2019).
- Engineering Equation Solver F-chart software: Engineering software, (2020). 6–8. http://www.fchartsoftware.com/ees/ (accessed May 11, 2020).
- Ghaebi, H., and H. Rostamzadeh. 2020. Performance comparison of two new cogeneration systems for freshwater and power production based on organic Rankine and Kalina cycles driven by salinity-gradient solar pond, renew. Energy 156:748–67. doi:https://doi.org/10.1016/j.renene.2020.04.043.
- Global water crisis: Facts, FAQs, and how to help | World Vision, (n.d.). https://www.worldvision.org/clean-water-news-stories/global-water-crisis-facts ( accessed September 27, 2020).
- Gonzalez, A., M. Grágeda, and S. Ushak. 2017. Assessment of pilot-scale water purification module with electrodialysis technology and solar energy, appl. Energy 206:1643–52. doi:https://doi.org/10.1016/j.apenergy.2017.09.101.
- Hosseini, S. E. 2019. Development of solar energy towards solar city utopia, energy sources, part a recover. util. environ. Eff 41:2868–81. doi:https://doi.org/10.1080/15567036.2019.1576803.
- Irfan, M., Y. Wang, and T. Xu. 2019. Novel electrodialysis membranes with hydrophobic alkyl spacers and zwitterion structure enable high monovalent/divalent cation selectivity. Chemical Engineering Journal 383:123171. doi:https://doi.org/10.1016/j.cej.2019.123171.
- Karabelas, A. J., C. P. Koutsou, M. Kostoglou, and D. C. Sioutopoulos. 2018. Analysis of specific energy consumption in reverse osmosis desalination processes. Desalination 431:15–21. doi:https://doi.org/10.1016/j.desal.2017.04.006.
- Khalilian, M., H. Pourmokhtar, and A. Roshan. 2018. Effect of heat extraction mode on the overall energy and exergy efficiencies of the solar ponds: A transient study. Energy 154:27–37. doi:https://doi.org/10.1016/j.energy.2018.04.120.
- Kotas, T. J. 1985. Exergy analysis of simple processes, in: Exergy method therm. Plant Anal 99–161. doi:https://doi.org/10.1016/b978-0-408-01350-5.50011-8.
- Lee, H. J., F. Sarfert, H. Strathmann, and S. H. Moon. 2002. Designing of an electrodialysis desalination plant. Desalination 142 (3):267–86. doi:https://doi.org/10.1016/S0011-9164(02)00208-4.
- Loganathan, P., G. Naidu, and S. Vigneswaran. 2017. Mining valuable minerals from seawater: A critical review, Environ. Environmental Science: Water Research & Technology 3 (1):37–53. doi:https://doi.org/10.1039/c6ew00268d.
- Massa, D., L. Incrocci, R. Maggini, G. Carmassi, C. A. Campiotti, and A. Pardossi. 2010. Strategies to decrease water drainage and nitrate emission from soilless cultures of greenhouse tomato, Agric. Agricultural Water Management 97 (7):971–80. doi:https://doi.org/10.1016/J.AGWAT.2010.01.029.
- Monjezi, A. A., H. B. Mahood, and A. N. Campbell. 2017. Regeneration of dimethyl ether as a draw solute in forward osmosis by utilising thermal energy from a solar pond. Desalination 415:104–14. doi:https://doi.org/10.1016/j.desal.2017.03.034.
- Moran, S. 2018. Clean water unit operation design, in: An appl. guid. to water effl. In Treat. Plant Des, Marinakis, Kostas, 69–100. Elsevier. doi:https://doi.org/10.1016/b978-0-12-811309-7.00007-2.
- Mosaffa, A. H., and L. G. Farshi. 2021. Thermodynamic feasibility evaluation of an innovative salinity gradient solar ponds-based ORC using a zeotropic mixture as working fluid and LNG cold energy. Applied Thermal Engineering 186:116488. doi:https://doi.org/10.1016/j.applthermaleng.2020.116488.
- Natasha, N. C., F. Firdiyono, and E. Sulistiyono. 2017. Impurities Removal in Seawater to Optimize the Magnesium Extraction. IOP Conference Series: Materials Science and Engineering,176; 012039. Surakarta, Indonesia:Eng., Institute of Physics Publishing. doi:https://doi.org/10.1088/1757-899X/176/1/012039.
- Njoku, H. O., B. E. Agashi, and S. O. Onyegegbu. 2017. A numerical study to predict the energy and exergy performances of a salinity gradient solar pond with thermal extraction, Sol. Energy 157:744–61. doi:https://doi.org/10.1016/j.solener.2017.08.079.
- Pujiastuti, C., K. Sumada, Y. Ngatilah, and P. Hadi, REMOVAL OF Mg 2+, K +, SO4-2 IONS FROM SEAWATER BY PRECIPITATION METHOD, (n.d.). doi:https://doi.org/10.1051/conf/2016.
- R-113 | A-Gas in the Americas, (n.d.). https://www.agas.com/us/products-services/refrigerants/products/cfcs-1/r-113/ (accessed October 20, 2021).
- Safarian, S., and F. Aramoun. 2015. Energy and exergy assessments of modified organic rankine cycles (ORCs). Energy Reports 1:1–7. doi:https://doi.org/10.1016/j.egyr.2014.10.003.
- Salata, F., and M. Coppi. 2014. A first approach study on the desalination of sea water using heat transformers powered by solar ponds, Appl. Energy 136:611–18. doi:https://doi.org/10.1016/j.apenergy.2014.09.079.
- Sorour, M. H., H. A. Hani, and H. F. Shaalan. 2016. Separation of calcium and magnesium using dual precipitation/chelation scheme from saline solutions, Desalin. Desalination and Water Treatment 57 (48–49):22818–23. doi:https://doi.org/10.1080/19443994.2015.1114170.
- Tian, X., G. Wang, D. Guan, J. Li, A. Wang, J. Li, Z. Yu, Y. Chen, and Z. Zhang. 2016. Reverse osmosis brine for phosphorus recovery from source separated urine, Chemosphere. https://doi.org/10.1016/j.chemosphere.2016.09.037.
- Wagner, L. 2013. Overview of energy storage technologies, in: Futur. energy improv. In Letcher, Trevor M., Sustain. Clean Options Our Planet 613–31. Elsevier Inc. doi:https://doi.org/10.1016/B978-0-08-099424-6.00027-2.
- Wittenberg, L. J. 1981. SALT-GRADIENT SOLAR PONDS: DESIGN, CONSTRUCTION AND POWER PRODUCTION Janzen, A. F, and R. K, Swartman. https://doi.org/10.1016/b978-0-08-025388-6.50057-x. London, Ontario, Canada: Pergamon Press Canada Ltd. 411–42.
- Yu, J., M. Zheng, Q. Wu, Z. Nie, and L. Bu. 2015. Extracting lithium from Tibetan Dangxiong Tso Salt Lake of carbonate type by using geothermal salinity-gradient solar pond, Sol. Energy 115:133–44. doi:https://doi.org/10.1016/j.solener.2015.02.021.
- Zeynali, A., A. Akbari, and M. Khalilian. 2019. Investigation of the performance of modified organic Rankine cycles (ORCs) and modified trilateral flash cycles (TFCs) assisted by a solar pond, Sol. Energy 182:361–81. doi:https://doi.org/10.1016/j.solener.2019.03.001.
- Zhao, D., L. Y. Lee, S. L. Ong, P. Chowdhury, K. B. Siah, and H. Y. Ng. 2019. Electrodialysis reversal for industrial reverse osmosis brine treatment, sep. purif. Technol 339–47. doi:https://doi.org/10.1016/j.seppur.2018.12.056.