Abstract
Context: The prevalence of sensitive skin among the general population in industrialized countries is reported to be over 50%. Sensitive skin subjects often report significant reactions to contact with cosmetics, soaps and other consumer products.
Objective: This paper describes the overall skin compatibility and mildness program for a newly developed, lightly fragranced, colorant free laundry product (i.e. Arm & Hammer™ Sensitive Skin plus Skin-Friendly Fresh Scent), specially formulated for individuals with sensitive skin. The skin mildness of the product was compared to Arm & Hammer™ Free & Clear liquid laundry detergent with no fragrance or colorant, and an established history of safe use by sensitive skin consumers.
Materials and methods: The test material was a liquid laundry product with a light scent formulated for sensitive skin consumers (Arm & Hammer™ Sensitive Skin plus Skin-Friendly Fresh Scent). The product was compared to commercially marketed products for sensitive skin with a history of skin safety in the marketplace, including: a very similar product formulation (Arm & Hammer™ Free & Clear with no fragrance), and several selected competitors’ products. Studies were conducted among individuals with self-assessed sensitive skin (based on a questionnaire) using standard protocols for the Human Repeat Insult Patch Test (HRIPT), 10-Day Cumulative Irritation, the Wrist Band Wear test, and the Safety In-Use testing. Responses in all protocols were evaluated by visual scoring of potential dermatologic reactions, and recording any sensory effects at the time of the examination. In addition, sensory effects collected from panelists’ daily diaries were also evaluated.
Results: The HRIPT confirmed that neither the fragrance alone, nor the product formulation with fragrance, induced contact sensitization in sensitive skin subjects. The 10-Day cumulative irritation study conducted using sensitive skin subjects showed highly favorable skin compatibility, and the test product was comparable to the control product (Arm & Hammer Free & Clear) and other nonirritant controls. In the Wrist Band Wear test, exposure to laundered fabrics under exaggerated conditions gave similar results for the test and control products, with no objective signs of skin irritation, and no self-reported persistent adverse sensory effects. Very mild, transient and isolated sensory effects were noted in daily diaries by a small proportion of subjects, and were similar for the test and control products. The Safety In-Use tests evaluated 4-week exposure to product and laundered fabrics under realistic use conditions. There were no clinically objective signs of skin irritation, and reports of transitory, mild sensory effects were minimal and similar for the test and controls.
Discussion and conclusion: A comprehensive skin safety program on a lightly scented sensitive skin laundry formulation (i.e. Arm & Hammer™ Sensitive Skin plus Skin-Friendly Fresh Scent) conducted among panels of self-assessed sensitive skin subjects demonstrated that the presence of a light fragrance did not adversely impact skin compatibility in any of the testing protocols when the product was compared to a similar product with no fragrance. The lightly fragranced product demonstrated overall skin compatibility and mildness when tested in a self-assessed sensitive skin population, and compared favorably to currently marketed sensitive skin products.
Introduction
Sensitive skin is identified as a hypersensitivity to stimuliCitation1,Citation2, and is defined clinically by a set of unpleasant and subjective sensory perceptions including tightness, stinging, burning, tingling, pain and itchingCitation3–5. Often, objective signs of any skin irritation are absentCitation6. Sensitive skin was initially referred to in 1947Citation7,Citation8, and for many years it was thought to be an unusual condition confined to a few individuals. More recently it has become evident that a large portion of the population experiences this phenomenonCitation9. Sensitive skin subjects experiencing this condition report exaggerated reactions when their skin is in contact with cosmetics, soaps and other consumer products, and often report worsening condition after exposure to dry and cold climateCitation2.
In a recent review Richters, et al., summarized physiologic factors that may lead to sensitive skin as:Citation1 sensory hyper-reactivity,Citation2 impaired barrier function,Citation3 inflammatory or vascular responsiveness, andCitation4 atopic predispositionCitation10. However, host factors (e.g. life style choices, gender, age and anatomic site), and environmental factors (e.g. climate) are also known contributorsCitation3,Citation4,Citation11,Citation12. Several investigators are evaluating the role of a specific sensory receptor; the transient receptor potential vanilloid-1 (TRPV1). TRPV1 is a nonselective, thermo-sensitive cation channel that responds to heat and low pH, and is related to nociception, neurogenic inflammation, and pruritusCitation13,Citation14. The expression of this channel has been found to be upregulated in subjects with sensitive skin, and correlates with the intensity of the symptomsCitation15,Citation16. Topical application of the TRPV1 antagonist 4-t-butylcyclohexanol has been demonstrated to have an anti-stinging/anti-burning effect in a capsaicin-induced sting testCitation14.
The unpleasant sensory reactions that are associated with sensitive skin, and the absence of objective signs and symptoms in most individuals, mean that the condition is largely self-reported. As a result, surveys have been a popular approach to evaluating the prevalence of this condition among the general population. A review of the literature published in 2012 identified over 15 surveys on the prevalence of self-assessed sensitive skin conducted in over 20 geographic regions in Europe, North American and Latin AmericaCitation17. Since that time, the scientific community has continued to evaluate this condition in other countries, including ChinaCitation18, MexicoCitation19, JapanCitation20, Brazil and RussiaCitation21. Estimates of the overall prevalence of this condition vary as a result of differences in the approaches to conducting the surveys and in the wording of specific questions. However, the general consensus is the prevalence of sensitive skin among the general population is over 50%Citation2,Citation7. There is some variation depending on the specific geographic regionCitation5, which could be related to differing weather patterns. Higher rates may be reported in some geographies due to a higher percentage of the population having a fair skin phenotype that is more closely associated with sensitive skinCitation22. Cultural factors may also play a roleCitation5. Aggressive advertising of products targeted for sensitive skin may lead to a higher level of understanding and acceptance of this conditionCitation17. In addition, cultures with a greater emphasis on appearance and fashion may have a higher level of exposure to potential irritants that trigger adverse sensory responsesCitation23.
Manufacturers of consumer products have long conducted programs on products and ingredients to confirm skin compatibility and mildnessCitation24,Citation25. However, with the recent realization of the high prevalence of sensitive skin in industrialized societies, manufacturers are developing products especially formulated for this large subgroup of consumers. The overall approach is similar to that previously described for other consumer productsCitation24–26 and involves a stepwise evaluation of skin mildness and compatibility, with a comparative approach to confirmatory testing in which newly developed products are compared to reference, commercially marketed products with an established history of safe use. However, when products are specifically formulated for the sensitive skin consumer, additional testing is warranted to ensure mildness for this population.
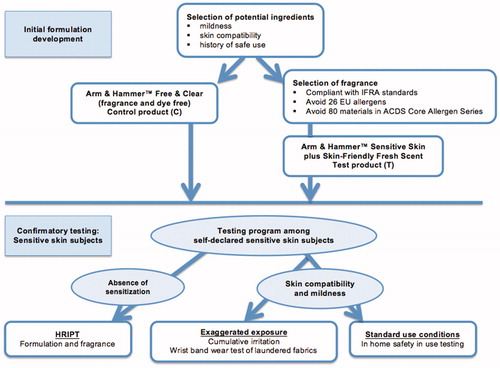
The objective of this program was to evaluate the skin compatibility of a laundry product developed for the sensitive skin consumer. Further, we wanted to determine if the addition of a light scent to a sensitive skin laundry product would adversely affect the skin compatibility and mildness of the product. A testing program was developed to compare the scented, dye-free formula (Arm & Hammer™ Sensitive Skin plus Skin-Friendly Fresh Scent, or A&H Sensitive Skin + Scent), to the safely marketed, fragrance- and dye-free formula (Arm & Hammer™ 2X Laundry Detergent, Sensitive Skin). The overall approach to the program is outlined in . Initial development of the laundry detergent test formulations used carefully selected ingredients with excellent overall skin compatibility, a demonstrated mildness to skin, and a long history of safe use in consumer products developed for similar uses. Product versions containing a light scent were developed to appeal to those sensitive skin consumers who prefer a dermatologically safe, scented product. The fragrance was specially formulated to: (1) contain a minimal number of ingredients, (2) ensure that all ingredients comply with the International Fragrance Association (IFRA) usage standards and guidelines (accessible at: http://www.ifraorg.org), (3) contain none of the 26 fragrance allergens recognized by the European Union (EU) as having the potential to cause allergyCitation27, and (4) contain no fragrance ingredients included on the American Contact Dermatitis Society (ACDS) Core Allergen Series GroupCitation28. We conducted a confirmatory testing program on the Arm & Hammer™ Sensitive Skin plus Skin-Friendly Fresh Scent (A&H Sensitive Skin + Scent) formulation to evaluate skin compatibility and mildness among the target consumer population, i.e. individuals with sensitive skin. Standard test protocols were used to assess gentleness and mildness among sensitive skin consumers under expected use conditions and exaggerated exposure conditions. Endpoints measured in these studies included objective signs of irritation, and a recording of sensory effects.
Figure 1. Approach to developing products for sensitive skin individuals. Formulations are developed by selecting ingredients and fragrances with extensive safety data and a long history of safe use and mildness in similar consumer products. Resulting formulations are subjected to confirmatory testing using volunteer panels of self-assessed sensitive skin individuals. Tests should include evaluations for contact sensitization (HRIPT), and test protocols designed to evaluate skin compatibility and mildness of the product and laundered fabrics under exaggerated exposure conditions (Cumulative Irritation test for the product and Wrist-Band Wear test for laundered fabric) and expected use conditions (In Home Safety In-Use test).
Methods
Materials tested
Information on the formulations and other materials used in the skin mildness program are shown in . The composition of the formulations is proprietary, but a concentration range of key components is provided in . The test product (T: Arm & Hammer™ Sensitive Skin plus Skin-Friendly Fresh Scent) contained a proprietary, mild fragrance blend (W). Other materials in the test product were the same as the control product (C: Arm & Hammer™ Free & Clear (fragrance-free, dye-free).
Table 1. Liquid laundry detergent formulations used in safety program.
The skin compatibility and mildness studies conducted with the test product are shown in . The dilutions used in the HRIPT and cumulative irritation studies are provided. For the wrist band and safety in use tests, exposure was to laundered fabric, as detailed in the protocol descriptions below. Other controls in the HRIPT and cumulative irritation studies included selected competitors’ products targeted for sensitive skin consumers (E: Tide™ Free And Gentle Liquid Laundry Detergent, Procter & Gamble Co., Cincinnati, OH; F: All™ Free Clear Liquid Detergent, Henkel, Wilton, CT; H: Purex™ Free and Clear Laundry Detergent, The Dial Corporation, Scottsdale, AZ). Low irritancy (negative) controls were included in the cumulative irritation test: 0.9% sodium chloride, and undiluted baby oil (Johnson & Johnson™, New Brunswick, NJ).
Subjects
All the studies were conducted among healthy adult volunteers with self-declared sensitive skin. Subjects were classified as sensitive skin based on a self-assessed history of short-term skin intolerance or skin reactivity following contact with cleaning products (e.g. laundry detergents, fabric softeners, or dishwashing and household cleaning products), or personal care products (e.g. cosmetics, lotions, shaving products and hair products). The participants were specifically asked to identify specific laundry and fabric softener products that they perceived caused adverse reactions in the past. Volunteers were excluded from participation for reasons including: any systemic or dermatologic disease or disorder which could interfere with the conduct of the study or increase the subject’s risk of adverse reactions, pregnancy or nursing, taking anti-inflammatory, corticosteroids or other medications that may interfere with test results, or participation in a patch test study within the previous 28 days. Selected participants signed an informed consent document, and could withdraw from the studies at any time. All study protocols were reviewed and pre-approved by board-certified dermatologists and the test facilities’ Institutional Review Board (IRB), and were conducted in accordance with Good Clinical Practice (GCP) Regulations (21 CFR 50: Protection of Human Subjects-Informed Consent and ICH-GCP Consolidated Guidelines, May 9, 1997 Federal Register). A summary of the number and mean age of participants for each study is provided in .
Table 2. Summary of number and mean age of sensitive skin participants completing each study.
Human repeat insult patch test (HRIPT)
The test was conducted at a contract facility (Reliance Clinical Testing Services, Inc., Irving TX 75062) using a standard HRIPT protocol (similar toCitation29,Citation30). Study participants were 95 self-declared sensitive subjects age 20–60. Test products were diluted in deionized distilled water to result in solutions equivalent to the recommended use concentrations on the package labels. This concentration has been demonstrated in previous tests conducted by the Company on liquid detergent formulations to be minimally irritating under occlusive patch conditions. In addition to test and control products, the fragrance itself was diluted in mineral oil to the concentration intended for the final formulation (0.8%). Test solutions were prepared fresh each day. For application, 0.2 ml of each test article solution was applied to the back between the left scapula and the spinal mid-line using occlusive patches of nonwoven cotton (2 cm × 2 cm Parke-Davis Readi Bandages). Test and control skin test sites were randomized. The induction phase consisted of nine 24-h patches at a single site on the back with a 24-h rest between (48 h on weekends). The patch sites were graded for skin responses prior to each application and at the removal of the test patches. Approximately, 14 days after the last induction application, 24-h challenge patches were applied to previously unpatched sites on the back. Reactions were scored daily for 4 days (i.e. at 24, 48, and 72 and 96 h) after removal of the challenge patch. Visual assessment was conducted by an expert grader (at 24, 48 and 96 h) and a board certified dermatologist (at 72 h) under a 100 watt incandescent bulb using a scale where “0” indicated no visible reaction, “0.5” was a barely perceptible reaction, “1” was mild, “2” was moderate, “3” was marked, and “4” was severe. The presence of edema and/or papules, spreading or vesicles was also noted.
Cumulative irritation study
Study participants were 26 self-declared sensitive subjects age 18–65. The study was conducted at a contract testing facility (Hill Top Research, St. Petersburg, FL) using the facility’s standard Cumulative Irritation protocol. Test articles were diluted in deionized distilled water to the recommended use concentrations. Patches were applied on the back, and sites were randomized for the test and control articles. For application, 0.2 ml of each test article solution was applied using occlusive patches of nonwoven cotton (Webril®) covered by and secured on all sides by hypoallergenic tape (Blenderm™). Approximately, 23 h after application test materials were removed, test sites were evaluated, and fresh test patches were re-applied to the same test site. Panelists were exposed to test substances for 10 consecutive days. Skin reactions were evaluated by visual assessment prior to any treatment (Day 1), and after removal of each of the test material application (Day 2–10).
Visual assessment was conducted using a standardized grading scale for erythema of “0–7” where “0” is no apparent cutaneous involvement and “7” is a strong reaction spreading beyond test siteCitation31. Glazing, fissuring and erosions were scored on a separate scale of “0–3”, and the numerical equivalent added to the erythema score to produce a transformed daily numerical score. This resulted in a maximum transformed numerical score of “10” for a test site (erythema of “7” and erosions of “3”). The same grader was used throughout an experiment, and the grader was not aware of treatment assignments. If a test site exhibited a strong reaction at any site (i.e. a daily numerical score of “3” or greater), the test material was not reapplied at that site. It is noteworthy that none of the test or control articles produced individual scores approaching “3” throughout the course of the study.
The study was conducted under the supervision of a board certified dermatologist. Scoring was conducted daily. Cumulative transformed irritation scores were determined for the panel for each test material. Using this evaluation method and panel size, a cumulative irritation score of 0–24 indicates a mild article with no irritation; 25–95 indicates probably mild in normal use; 96–213 indicates possibly mild in normal use; 214–276 indicates a cumulative irritant; and 277–300 indicates a primary irritant.
Statistical analyses were conducted by the test facility to compare the cumulative irritation scores for days 1, 7, and 10. Initially, a Friedman rank sum test was performed. If significant differences were found using this approach, a Fishers Least Significant Difference test was performed.
Wrist band wear
Study participants were 33 self-declared sensitive subjects aged 18–65. The parallel-group, double-blind, randomized study was conducted at a contract facility (Harrison Research Labs, Union, NJ 07083). The protocol was similar to one published for evaluating the skin compatibility to laundry detergentsCitation32. Four different sets of swatches were prepared by pre-laundering; two sets were prepared by laundering fabric three times in the recommended use concentration of each of the products (referred to as 1X samples), and two sets were prepared by laundering three times in solutions that were triple the recommended use concentration (referred to as 3X samples).
Laundered fabric swatches were applied to each wrist (randomly determined), and held in place using tape and an elastic terry-cloth wristband. Subgroup 1 consisted of 17 subjects who received 1X samples of the test product T on one wrist and 1X samples of the control product C on the other. Subgroup 2 consisted of 16 subjects and received the 3X test and control samples. Subjects were instructed to wear the test samples for 8 h before removing them. Samples were applied daily for 4 total days. A trained grader conducted dermatologic evaluations of the test sites prior to each sample application (including a baseline evaluation prior to the first application), and on the morning of day 5 after 4 full days of exposure.
Safety in-use
Two independent parallel-group, double-blind, randomized studies were conducted at contract facilities (Harrison Research Labs, Union, NJ 07083 and Clinical Research Laboratories, Piscataway, NJ, 08854). The two studies used the identical protocol, and test and control products. The participants were healthy volunteers with self-declared sensitive skin aged 18–63. Sixty-four subjects completed the first study (32 for test product T, and 32 for control product C). The second was completed by 105 subjects (53 for T, and 52 for C).
In each of the two studies, participating subjects received an examination by a board certified dermatologist prior to any exposure to the test or control products to establish baseline scores for erythema, edema and dryness. The examination included an evaluation of the hands, legs, arms, chest and back. A total of 23 sites per subject were rated for these three parameters. After the dermatological examination, subjects were provided test or control product and instructed to use it as the only laundry detergent for a 4-week period, including a minimum of 2 laundry loads per week, and a minimum of 2–3 min twice per week of hand laundry and/or stain pretreatment using the assigned detergent. The subjects kept a daily diary to record the date and time of the laundry, including hand laundry and pretreatment, the amount of detergent used, and any adverse sensory reactions related to product use. After 2 and 4 weeks of test product use, dermatologic evaluations were conducted, and subjects were asked to rate the intensity of any sensory reactions, such as, itching, dryness and stinging/burning.
All the dermatologic evaluations (erythema, edema and dryness) and the sensory reactions (itching, dryness, stinging/burning) were rated on a scale of “0–3”, where “0” indicated none, “0.5” was barely perceptible, “1” was mild, “2” was moderate, “3” was severe. Statistical analyses were conducted by the test facility using the Chi-Square test to compare responses of the test groups for baseline, week 2 and week 4 evaluations.
Results
HRIPT for contact sensitization
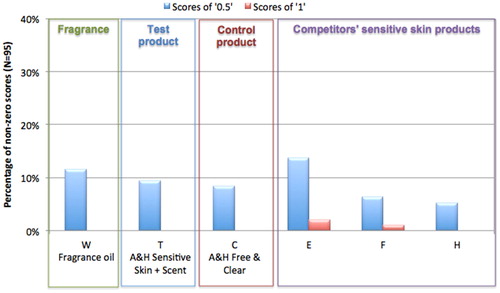
The HRIPT was conducted to confirm the fragranced formulation did not cause contact sensitization in self-declared sensitive skin subjects. Test and control products were evaluated at the recommended use concentrations under occlusive patch. None of the test or control samples produced responses indicating sensitization. illustrates the low level of responses at challenge (48-h after removal of the challenge patch). There were a small percentage of scores of “0.5”, indicative of barely perceptible reactions, and no scores of “1” after challenge with the fragrance oil alone (W), the test product (T), or the key control product (C). All of the competitors’ sensitive skin products were also negative for sensitization (test samples E, F and H).
Figure 2. HRIPT on self-assessed sensitive skin subjects: 48-h challenge results. The HRIPT was conducted using a standard test protocol as described in the methods section among 95 self-declared sensitive subjects. Test samples included the fragrance oil alone (W), A&H Sensitive Skin + Scent (T), A&H Free and Clear (C), and three competitors’ sensitive skin formulations (E, F and H). Additional information on the test samples is provided in . The graph shows the percentage of non-zero scores (“0.5” and “1”) at challenge (48-h after challenge patch removal).
Cumulative irritation
Results of the 10-day cumulative irritation study are shown in . The test product (T) produced no evidence of irritation, even with the exaggerated exposure conditions of occlusive patches repeated daily. Results were not significantly different from the key control product (C: A&H Free & Clear), or from the nonirritant controls (I: 0.9% saline, and J: baby oil). The cumulative irritation score of ≤5 for the test formulation resulted in a classification of “mild, with no irritation” (classification is detailed in the methods section).
Figure 3. Cumulative irritation study on self-assessed sensitive skin subjects: dermatologic evaluation of reactions. Patches containing 0.2 ml of laundry product solutions were applied daily for 10 consecutive days using a standard protocol, as described in the methods section. Test samples included the test product (T: A&H Sensitive Skin + Scent), the key control product (C: A&H Free & Clear), nonirritant controls (saline and baby oil), and competitors’ sensitive skin formulations (E, F and H). Additional information on the test samples is provided in . Reactions were scored on separate scales for erythema and glazing/fissuring. The sum of these scores represented the cumulative total irritation score (a). Dividing this total by the number of subjects produced the mean transformed irritation score (b).
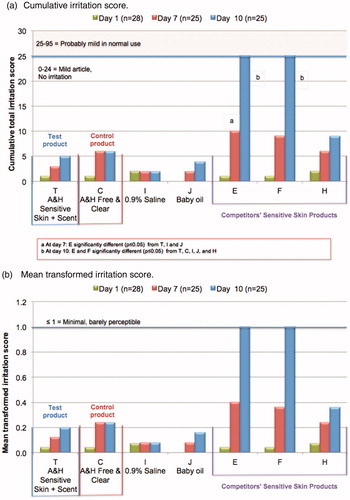
Each of the competitors’ products formulated for sensitive skin produced scores that indicated they were mild to the skin in this sensitive skin population. However, control products E, and F produced cumulative irritation scores that were significantly different (p ≤ 0.05) from the A&H Sensitive Skin + Scent product (T). Two of the control products (E and F) produced cumulative irritation scores of 25, placing them just barely in the category of “probably mild in normal use” (i.e. cumulative score of 25–95).
The mean cumulative irritation score is plotted in . On the scoring scale that was used in this study, a “1” indicates minimal, barely perceptible erythemaCitation31. All the test and control materials produced a mean score that was at or below “1”.
Wrist band wear
In the wrist band wear test sensitive skin subjects were exposed to fabrics laundered in either the test (T: A&H Sensitive Skin + Scent) or control (C: A&H Free & Clear) product using 1X or 3X the recommended use concentrations. Fabrics were worn on the wrist in close proximity to the skin for prolonged periods (8 h per day for 4 days), thus providing an exaggerated exposure compared to the exposures expected under normal consumer use conditions.
Results of the wrist band wear test are summarized in . The dermatologic evaluations showed that none of the subjects exhibited any erythema, dryness, or edema during the dermatologic examinations as a result of exaggerated exposure to the fabric swatches laundered with either the test product (T) or the control product (C) at 1X or 3X recommended use concentration, i.e. all scores were “0”. Further, none of the study subjects reported any sensory responses (burning, stinging, itching, dryness, other) at the time of the evaluation visits.
Table 3. Wrist band wear testing on self-assessed sensitive skin subjects: summary of results.
Daily diaries were reviewed for evidence of transient adverse sensory reactions that may not have been present at the evaluation visits. Reported effects appeared to be isolated incidents that were very mild in nature and short in duration. Even among this sensitive skin population, the incidence of reported sensory effects was very low. Two subjects out of the 17 who participated (i.e. 12%) reported slight to moderate itching to the 1X samples of the test product (T) compared to three subjects reporting slight itching to 1X samples of the control product (C) (17%). As a result of wearing the 3X samples, three subjects reported mild itching to test product T (19%) and four subjects to control C (25%).
Safety in-use
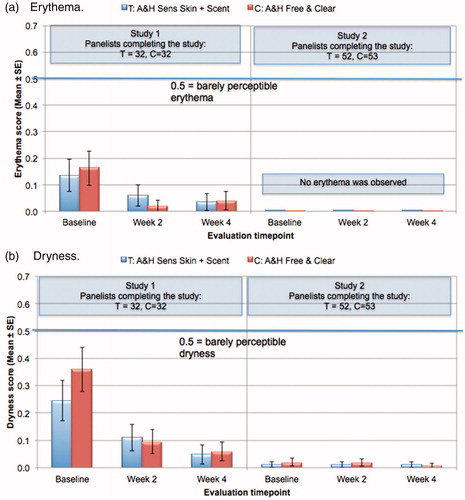
Results of the Safety In-Use testing are summarized in . After 2- and 4-weeks of home product usage, dermatologic evaluations were conducted on 23 sites per subject, including sites on the hands, legs, arms, chest and back. Evidence of dermatologic reactions was very low for both the products (test and control) in both the studies. At the 2- and 4-week evaluation, mean ratings for erythema () were consistently below 0.2, and mean ratings for dryness () were consistently below 0.4. There was no evidence of edema at any time in either study (data not shown). There were no significant differences between the test and control products in either of the two studies for any of the dermatologic parameters.
Figure 4. Safety in-use studies on self-assessed sensitive skin subjects: dermatologic evaluation. Two studies were conducted using panels of self-assessed sensitive skin subjects, with identical test protocols and products. Products for the home use study included the test (T: A&H Sensitive Skin + Scent) and control (C: A&H Free & Clear) products. Additional information on the test samples is provided in . In each study, one groups of subjects received the test product (T: A&H Sensitive Skin + Scent), and one group received the control product (C). Subjects were instructed to use the provided product as the only laundry detergent for a 4-week period. Prior to the start of the study, and after 2 and 4 weeks of test product use, each panelist received dermatologic evaluations on a total of 23 sites per subject. Skin sites were rated for erythema and dryness. Means are shown in plots (a) and (b), respectively. Dermatologic scores were very low throughout the study, with no significant differences between the test and control groups.
Reports of sensory effects at the skin evaluation time points were low, with a small number of subjects in both groups (test and control) reporting mild dryness (). There were no reports of itching or burning. When daily diary reports were reviewed for sensory comments, a small number of subjects reported transient, mild itching, redness or dryness. Statistical analysis indicated no differences between test groups.
Table 4. Safety in-use studies on self-assessed sensitive skin subjects: reports of subjective effects.
Discussion
Consumers with sensitive skin can have intolerance to products that other consumers can use with no adverse consequences. As early as 1977, Frosch and Kligman described unexpected, adverse sensory reactions among consumers to products that had been thoroughly evaluated for irritation and sensitizationCitation33. In an unpublished research study conducted by the Church & Dwight Co., Inc., we found that 17% of the laundry detergent users self-reported that they had previously had reactions to laundry detergents. Among individuals with sensitive skin the percentage is higher. Farage reported that over 50% of the individuals with self-declared sensitive skin have experienced adverse skin reactions to laundry products at some time compared to approximately 20% of individuals not claiming sensitive skinCitation34. This program (summarized in ) was designed to evaluate a lightly scented laundry product for mildness and skin compatibility among sensitive skin consumers.
Table 5. Summary of skin compatibility program performed among sensitive skin subjects.
Results in the HRIPT demonstrated an absence of contact sensitization to the product formulation and the fragrance (). This result was expected based on the selection of only those ingredients with extensive safety data, including evaluations of contact sensitization potential, and a long history of safe use and mildness in similar consumer products.
The exaggerated exposure to product solution in the 10-day cumulative irritation study among sensitive skin subjects resulted in classification of the test products as ‘mild, causing no irritation’ (). Importantly, the lightly fragranced test product (T, with 0.8% fragrance) was very similar to the fragrance-free control (C), demonstrating that the inclusion of fragrance in the formulation did not alter the skin mildness or compatibility of the product. Although all of the tested materials were mild in this study, the test product produced significantly lower cumulative irritation scores than two of the competitors’ commercially marketed sensitive skin products.
During the laundering process, materials from laundry product have the potential to deposit on fabricCitation24,Citation35. With modern fabrics and laundering methods, residues on laundered fabric represent a major source exposure to detergent components. Therefore, evaluating laundered fabric is an important part of any overall skin compatibility program. However, the objective signs of irritation, such as, erythema, dryness and edema, that are the typical endpoints for these types of studies are likely not sufficient for sensitive skin consumers. In fact, subjective sensory perceptions are often the only symptom experienced by these individualsCitation3–5. A careful review of sensory responses is essential in evaluating products for sensitive skin consumers.
In the Wrist Band Wear test fabric is laundered under extreme conditions to maximize deposition of formulation components, i.e. use of a three-fold concentrated wash solution. Exposure conditions are also extreme, with exposure via elastic wrist bands worn continuously 8 h per day for 4 days. In the wrist band test conducted among sensitive skin subjects there was no evidence of either skin irritation or persistent adverse sensory effects as a result of exposure to fabrics laundered in the test product (T, with 0.8% fragrance). The small number of mild, transient sensory effects recorded in the participants’ daily diaries was similar to those recorded for the control product (C with no fragrance) (). The in-home Safety In-Use test among sensitive skin subjects provided a 4-week duration of exposure under actual expected product use conditions. Subjects were exposed to the product (during hand laundry and pre-treating), and to clothing and bedding fabrics laundered in the product. There was no evidence of erythema or dryness among sensitive skin panelists using either the test (T) or control (C) product (). During the safety in-use studies, very few adverse sensory reactions were reported at either the skin evaluation intervals or in the daily diary entries ().
In the Safety In-Use Study 1 (), the mean scores for erythema and dryness appear to decrease after 2 and 4 weeks of product use. This apparent decrease is very marginal and, likely insignificant. Scores throughout both in-use tests were very low. At the baseline dermatological evaluations (prior to product exposure), mean scores for erythema and dryness were less than 0.5, which is defined as “barely perceptible”. We do not believe the minor decrease in mean scores represents a meaningful improvement in skin condition. However, both the test and control products were specifically formulated for the sensitive skin consumer. It is possible that use of a sensitive skin product in place of a product that was not specifically formulated for sensitive skin may result in an improvement in skin condition for some individuals.
The important observation from both the wrist band and safety in-use testing confirm that the addition of the light fragrance to the sensitive skin product did not alter the skin mildness of the product for individuals with sensitive skin ().
Individuals with sensitive skin are known to react to cosmetic, personal care and other household products. With increasing evidence of the high prevalence of sensitive skin among the general population, manufacturers of consumer products are focusing more and more on developing products to minimize adverse reactions among the sensitive skin consumer. Such efforts will allow the sensitive skin consumer to comfortably enjoy the performance benefits of modern formulation technology.
Conclusions
We conducted a comprehensive skin compatibility and mildness program using volunteer test panels consisting of individuals with self-declared sensitive skin in order to determine if the addition of a light scent to a sensitive skin laundry product would adversely affect the skin compatibility and mildness of the product. The program included confirmatory testing for the absence of contact sensitization (HRIPT), and evaluations of skin effects from exaggerated exposures to product solutions (Cumulative Irritation test) and laundered fabrics (Wrist Band Wear test). Skin compatibility under expected use and exposure conditions was also evaluated in 4-week Safety In-Use testing. In all test protocols, the lightly scented sensitive skin laundry product was mild to skin and comparable to marketed sensitive skin products with a history of safety and skin compatibility in the marketplace. The addition of the light scent chosen for the product (i.e. Arm & Hammer™ Sensitive Skin plus Skin-Friendly Fresh Scent) had no adverse effects on skin compatibility or mildness.
Declaration of interest
The program was sponsored by the Church & Dwight Co., Inc., and evaluated products developed or commercialized by the company.
Acknowledgements
The authors would like to thank Terresa L. Nusair, PhD, of the Health and Environmental Safety Alliance (Cincinnati, Ohio) for the preparation of this manuscript. The authors would also thank Howard Maibach, MD, for his review of and valuable feedback on this manuscript.
References
- Primavera G, Berardesca E. Sensitive skin: mechanisms and diagnosis. Int J Cosmet Sci 2005;27:1–10
- Berardesca E, Farage M, Maibach H. Sensitive skin: an overview. Int J Cosmet Sci 2013;35:2–8
- Misery L, Loser K, Ständer S. Sensitive skin. J Eur Acad Dermatol Venereol 2016;30(Suppl 1):2–8
- Stander S, Schneider SW, Weishaupt C, et al. Putative neuronal mechanisms of sensitive skin. Exp Dermatol 2009;18:417–423
- Farage MA, Maibach HI. Sensitive skin: closing in on a physiological cause. Contact Derm 2010;62:137–149
- Saint-Martory C, Roguedas-Contios AM, Sibaud V, et al. Sensitive skin is not limited to the face. Br J Dermatol 2008;158:130–133
- Buhé V, Vié K, Guéré C, et al. Pathophysiological study of sensitive skin. Acta Derm Venereol 2016;96:314–318
- Bernstein ET. Cleansing of sensitive skin; with determination of the pH of the skin following use of soap and a soap substitute. J Invest Dermatol 1947;9:5–9
- Farage MA, Katsarou A, Maibach HI. Sensitive skin: sensory, clinical, and physiological factors. In: Barel AO, Paye M, Maibach HI, eds. Handbook of cosmetic science and technology. 4th ed. Boca Raton, FL: Taylor and Francis; 2014:59–70
- Richters R, Falcone D, Uzunbajakava N, et al. What is sensitive skin? A systematic literature review of objective measurements. Skin Pharmacol Physiol 2014;28:75–83
- Farage MA, Maibach HI. Sensitive skin: new findings yield new insights. In: Baran R, Maibach HI, eds. Textbook of cosmetic dermatology. New York, NY: Taylor & Francis; 2010:73–83
- Berardesca E, Fluhr JW, Maibach HI. What is sensitive skin? In: Berardesca E, Fluhr JW, Maibach HI, eds. Sensitive skin syndrome. New York, NY: Taylor & Francis; 2006:1–6
- Kueper T, Krohn M, Haustedt LO, et al. Inhibition of TRPV1 for the treatment of sensitive skin. Exp Dermatol 2010;19:980–986
- Sulzberger M, Worthmann AC, Holtzmann U, et al. Effective treatment for sensitive skin: 4-t-butylcyclohexanol and licochalcone A. J Eur Acad Dermatol Venereol 2016;30(Suppl 1):9–17
- Ehnis-Pérez A, Torres-Álvarez B, Cortés-García D, et al. Relationship between transient receptor potential vanilloid-1 expression and the intensity of sensitive skin symptoms. J Cosmet Dermatol 2016;15:231–237
- Sun L, Wang X, Zhang Y, et al. The evaluation of neural and vascular hyper-reactivity for sensitive skin. Skin Res Technol 2016;22:381–387
- Farage MA, Mandl CP, Berardesca E, et al. Sensitive skin in China. J Cosmetic Dermatol Sci Appl 2012;2:184–195
- Xu F, Yan S, Wu M, et al. Self-declared sensitive skin in China: a community-based study in three top metropolises. J Eur Acad Dermatol Venereol 2013;27:370–375
- Hernández-Blanco D, Castanedo-Cázares JP, Ehnis-Pérez A, et al. Prevalence of sensitive skin and its biophysical response in a Mexican population. World J Dermatol 2013;2:1–7
- Kamide R, Misery L, Perez-Cullell N, et al. Sensitive skin evaluation in the Japanese population. J Dermatol 2013;40:177–181
- Taieb C, Auges M, Georgescu V, et al. Sensitive skin in Brazil and Russia: an epidemiological and comparative approach. Eur J Dermatol 2014;24:372–376
- Misery L, Myon E, Martin N, et al. Sensitive skin: psychological effects and seasonal changes. J Eur Acad Dermatol Venereol 2007;21:620–628
- Misery L, Boussetta S, Nocera T, et al. Sensitive skin in Europe. J Eur Acad Dermatol Venereol 2009;23:376–381
- Kwon S, Holland D, Kern P. Skin safety evaluation of laundry detergent products. J Toxicol Environ Health Part A 2009;72:1369–1379
- Robinson MK, Perkins MA. A strategy for skin irritation testing. Am J Contact Derm 2002;13:21–29
- Farage MA, Stadler A, Elsner P, et al. Safety evaluation of modern hygiene pads: two decades of use. Female Patient 2004;29:23–30
- Scientific Committee on Consumer Safety. Opinion on fragrance allergens in cosmetic products. 2012: SCCS/1459/11. Available from: http://ec.europa.eu/health/scientific_committees/consumer_safety/index_en.htm [Accessed on May 23 2016]
- Schalock PC, Dunnick CA, Nedorost S, et al. American contact dermatitis society core allergen series. Dermatitis 2013;24:7–9
- Stotts J. Planning, conduct, and interpretation of human predictive sensitization patch tests. In: Drill val, P, ed. Current concepts in cutaneous toxicity. New York: Academic Press; 1980: 41–53
- ASTM International. ASTM D6355-07: Standard test method for human repeat insult patch testing of medical gloves. 2008. Available at: www.astm.org [last accessed 8 Jul 2011]
- Berger RS, Bowman JP. A reappraisal of the 21-day cumulative irritation test in man. J Toxicol Cutan Ocul Toxicol 1982;1:109–115
- Bannan EA, Griffith JF, Nusair TL, et al. Skin testing of laundered fabrics in the dermal safety assessment of enzyme-containing detergents. Cutan Ocular Toxicol 1992;11:327–339
- Frosch PJ, Kligman AM. A method for appraising the stinging capacity of topically applied substances. J Soc Cosmet Chem 1977;28:197–209
- Farage MA. Does sensitive skin differ between men and women? Cutan Ocul Toxicol 2010;29:153–163.
- American Cleaning Institute. Consumer Product Ingredient Safety: Exposure and Risk Screening Methods for Consumer Product Ingredients, 2nd ed. 2010. Available from: http://www.aciscience.org/docs/Consumer_Product_Ingredient_Safety_v2.0.pdf [last accessed 27 May 2016]
