?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background: Poor ocular tolerance of sunscreens is partially responsible for poor compliance in use of sunscreens. A three-tiered approach for the testing of ocular tolerance for such products is described that includes an in vitro test for ocular irritation, an in vitro test for the activation of pain receptors, and finally a clinical study involving ocular instillation of the product under controlled conditions followed by ophthalmologic and subjective self-evaluation on a graded scale. We report the results for a new water-based facial sunscreen (SCFW) with very good ocular tolerance.
Methods: The ocular irritation potential of SCFW was determined using the EpiOcular™ human cell construct which constituted the first-tier testing. Briefly, the tissues were exposed to SCFW and appropriate positive and negative controls for 15 minutes to 24 hours. After treatment, the tissues were rinsed and cytotoxicity determined. The calculated ET50 value (time at which relative viability decreased 50%) was then used to determine the ocular irritation potential. In the second-tier testing, the sting potential of SCFW was determined by employing the NociOcular assay that measures the activation of TRPV1 (transient receptor potential cation channel subfamily V member 1) specific receptors linked to pain sensation in a neuronal model with over-expression of functional TRPV1 channels. Finally, as the third-tier testing, SCFW was tested in a clinical study with instillation of product into the ocular cul-de-sac and ocular irritation was evaluated after 30 seconds, 15 minutes, and 60 minutes by an ophthalmologist. Participating subjects were also asked to score sensation on a scale of 0 to 3 from slight prickliness to severe stinging. Assay control reference product with known good ocular tolerability (10% baby shampoo) was concurrently tested.
Results: In the in vitro topical application assay using the EpiOcular™ construct, no significant cytotoxicity was observed in the tissues exposed to SCFW, indicating minimal ocular irritation potential. In the in vitro NociOcular assay, the cells exposed to the prepared dilutions of SCFW showed minimal TRPV1 specific activity, indicating minimal ocular sting potential. In the in vivo study, no statistically significant differences were found in terms of subjective or objective eye irritation assessment between SCFW and 10% baby shampoo.
Conclusion: SCFW showed negligible ocular irritation potential in tier 1, minimal potential to activate pain receptors in tier 2, and good ocular tolerability that was comparable to 10% baby shampoo in tier 3 testing. The results suggest that SCFW has good eye tolerance and that the tiered approach can be used to evaluate facial sunscreens for ocular tolerability.
Introduction
The daily use of sunscreen as a keystone in modern dermatological practice is largely due to better understanding of photoaging and photocarcinogenesis. Use of sunscreen plays an important part in the overall approach to reduce rising numbers of ultraviolet (UV) radiation-related pathologies such as skin cancersCitation1, polymorphic light eruptionsCitation2, actinic keratosis and photoagingCitation3. Despite the recommended use and benefits of sunscreen, compliance remains alarmingly low, especially in adolescents and young adultsCitation4–6. Reasons for this low compliance include the texture and feel of sunscreens that tend to be sticky and greasy or leave a white residue on the skinCitation7. An additional reason associated with facial sunscreens may be the ocular reactions commonly reported as “stinging” or “burning” when sunscreen applied on the face inadvertently enters the eyeCitation8,Citation9. These were the key considerations that motivated us to develop a facial sunscreen product with a water-based texture, non-whitening effect on the skin after application, and optimal ocular tolerability (SafeEye TechTM).
As part of the safety assessment of personal care products and cosmetics that are designed to be used in or around the eyes, such as sunscreens, the evaluation of ocular irritation potential is of primary importance. Safety testing should include additional endpoints such as lacrimation and stinging to ensure there is a very low potential for irritation and pain associated with their use.
When developing a sunscreen product for the face, we used a three-tier testing approach to demonstrate that the product was non-irritating to the eye and did not produce a stinging or a burning sensation in the eye. The first step consisted of an in vitro ocular irritation test using a three-dimensional corneal tissue model capable of detecting if the product is a potential irritant. In the second step, we tested the potential of the product to elicit a stinging response in the in vitro NociOcular test, using a neuroblastoma cell line expressing the TRPV1 (transient receptor potential cation channel subfamily V member 1) receptors as proposed by Forsby et al.Citation10. Finally, the formulation was tested in a clinical study with ocular instillation and ophthalmological examination to determine ocular tolerability of the product under extreme conditions.
In this paper, we describe the testing strategy and the results for a new water-based facial sunscreen. While the in vitro ocular irritation test using the EpiOcular™ construct is a standard test conducted as part of the safety studies for cosmetic products, testing for “stinging” or “burning” reactions were more challenging to address in the product development phase as they were not directly related to the classical ocular irritation. A clinical study involved direct ocular instillation of the product to address a “worst case” scenario for a facial product that can be accidentally introduced in the eye as a result of over application or water-related activities such as bathing or sports.
Materials and methods
Test product
A new water-based facial sunscreen (Fotoprotector ISDIN Fusion Water, [SCFW], ISDIN, Barcelona, Spain) formulated with organic (ethylhexyl methoxycinnamate, butyl methoxydibenzoylmethane, ethylhexyl triazone) and inorganic (titanium dioxide) UV filters with very high sun protection factor and UVA protection was evaluated. For the NociOcular assay, a traditional water-in-oil base sunscreen (SC), containing both organic and inorganic filters (Fotoprotector Fusion Fluid, ISDIN, Barcelona, Spain) with poor ocular tolerance history was included in the assay as a comparator. Both products had comparable pH (7.0 ± 0.5) and viscosities (1000–3000 cps).
EpiOcular™ time-to-toxicity (ET-50) test
The EpiOcular™ tissue (OCL-200, MatTek, Ashland, MA, USA) was used to assess the ocular irritation potential of the SCFW.
Upon receipt, the EpiOcular™ tissues were stored at 2–8 °C until use. The tissues were incubated at 37 °C in a humidified atmosphere with 5% CO2 for at least 1 h to acclimatize them before treatment. One hundred microlitre of undiluted SCFW was applied on the topical surface of the tissue with uniform coverage. The exposed tissues were incubated for 4, 8, 16, and 24 h. Deionized (DI) water was used as the negative control (NC) and 0.3% Triton-X 100 as the positive control (PC). The NC was exposed for 15 min, 4, 8, and 24 h, and the PC for 15 and 45 min. Each test product and control exposure times were tested in duplicate tissues. After the appropriate exposure times, the tissues were rinsed at least three times with calcium and magnesium-free Dulbecco's phosphate buffered saline (DPBS) to remove the test product and then soaked in 5 ml of the assay medium for 10–20 min to further remove any test product absorbed into the tissue. The tissues were then incubated for 3 ± 0.1 h, exposed to a 1.0 mg/mL 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) dye solution and then placed in isopropanol solvent for 2 h at room temperature. At the end of the extraction period, the absorbance of the extract solution was measured at 550 nm (OD550).
The corrected OD550 values of the individual test product exposure times were determined by subtracting the mean OD550 value of the blank from their OD550 values. Relative tissue viability was determined for each tissue and time point by using the following formula:
The % viability of the tissues exposed to the SCFW and the positive control up to 4 h was calculated based on the mean of the 30 min and 4 h negative control. The % viability of the tissues exposed to the SCFW for 8 h was calculated based on the 8 h negative control. The % viability of the tissues exposed to the SCFW for 16 and 24 h was calculated based on the 24 h negative control. The % viability was plotted versus the exposure time. The time at which the relative viability decreased to 50% was defined as the ET50. Based on the ET50 value, the potential ocular irritation was determined.
NociOcular assay
The human neuroblastoma SH-SY5Y cell line (ECAC, 94030304) stably transfected with plasmid rat TRPV1 receptor cell cultures were used to evaluate the eye stinging potential of the SCFW by using the NociOcular Assay as described previously by Forsby et al. 2012Citation10.
The SH-SY5Y-TRPV1 cells were routinely maintained in the NociOcular Assay medium (Krebs-Ringer-HEPES Buffer (KRH, Sigma-Aldrich, containing 6 mM D-glucose) at 37 °C in a humidified atmosphere with 5% CO2. For the assay, the cells were seeded at 3 × 105 cells/mL into 96-well plates and incubated until the proper confluency of ∼70% was achieved. The cells were then incorporated with 12 uM of Fura-2; AM™ (Invitrogen), a fluorescent probe that binds free Ca2+.
One challenge with testing sunscreens in the NociOcular Assay is preparation of the test product dilutions. Considering the intended use, sunscreens in general do not mix well with aqueous solvents. SC was determined to be insoluble and the prepared dilution was unusable in the KRH buffer. In order to optimize the test product solubility, 15% solution of Johnson’s No More Tears baby shampoo (Johnson & Johnson’s, hereon referred to as “detergent”), was prepared in the KRH buffer in w/v dilution (15% or 150 mg/mL). The presence of the detergent in the solvent seemed to aid in enhancing the test product miscibility while presenting insignificant toxicity to the exposed cells. SCFW was workable in the KRH solvent and in 15% detergent. Although the KRH buffer was the primary solvent, the top stock concentration of SCFW was diluted in 15% detergent for a better comparison with SC. Only the highest stock concentration of the test products was diluted in the 15% detergent. All subsequent dilutions were in the KRH buffer. For the preliminary assay only, SCFW was also tested using KRH as the assay solvent for comparison. There were no differences in the results between SCFW diluted in 15% detergent and in the KRH.
In the definitive assays, eight selected dilutions of the test products and positive and negative controls were tested. The final concentrations ranged from 0.000728% to 2.5% (or 0.00728 to 25 mg/mL) in ∼3.2 or ½ log interval. Capsaicin (600 nM, Sigma-Aldrich) was used as the positive control and KRH buffer served as the negative control. Each test product concentration and the controls were tested in 6 wells in each assay – 3 with capsazepine and 3 without capsazepine. Half of the replicas of each treatment were exposed to 30 uM of TRPV1 channel antagonist capsazepine (Cat#C191; Sigma-Aldrich) diluted in KRH buffer so that TRPV1-specific activity related to sting sensation could be isolated and quantified. FlexStation fluorometer (Molecular Devices, Sunnyvale, CA) with SoftMax® software was configured to recognize test product dilutions and cell plates. The fluorometer was set up with excitation wavelengths of 340 nm and 380 nm and an emission wavelength of 510 nm. Following the baseline reading, contents from the test product dilutions were administered, and for all treated wells, readings were taken every 4 s for a total of 120 s.
The ratio of OD340/OD380 (dye-bound calcium/free calcium) was indicated graphically in each well, and the change in fluorescence over the reading time (area under the curve) was expressed as percentage change from the baseline.
The normalized average fluorescence of each treatment with or without capsazepine in comparison to the capsaicin response was calculated with the following equation:
The average capsaicin response was assigned to be 100% of the Ca2+ influx. The normalized value for both the SCFW and the SCFW + capsazepine treatments were processed with GraphPad Prism program. The final concentrations were transformed into logarithmic values to perform a non-linear regression curve fit, and EC50 (the Ca2+ concentration yielding 50% of the maximum capsaicin response) and Emax (maximal Ca2+ specific response of the test product) were calculated. An endpoint, Effect at 0.032%, is a part of the previously established sting potential criteria. These criteria were proposed by Forsby et al.Citation10 for surfactant-based products and are not directly applicable for sunscreens.
The prediction model to classify the surfactants is outlined in only as a reference. The sting potential of SCFW and SC was determined based on the presence and degree of TRPV1 specific activity as shown by higher Effect (Ca2+ influx) in exposed cells without capsezapine compared to identically exposed cells with capsezapine.
Figure 1. Time response curve graphs of the SCFW and the positive control. PC: positive control; SCFW: sunscreen fusion water.
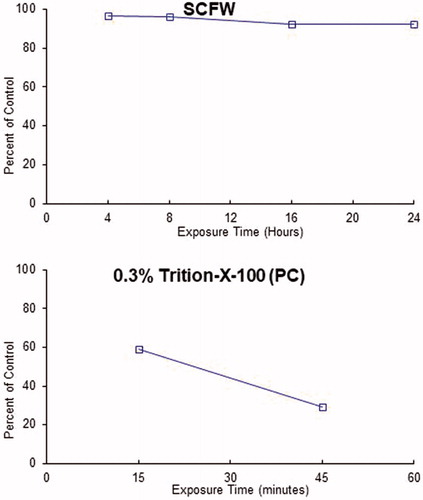
Table 1. Criteria for a product to be classified as stinging to the eye, using the NociOcular Assay for surfactants.
Ocular instillation study
Subjects
The study included 25 healthy adults, 10 men and 15 women, mean age 58.8 ± 13.6 years, with two functional eyes and no signs of relevant eye irritation or disease (e.g. acute conjunctivitis, irritation of cornea, severe sicca, keratitis, or acute allergic conjunctivitis). The study excluded subjects who were pregnant, breastfeeding, had undergone eye surgery within 3 months prior to the start of the study, had a history of allergies, sensitivity of the eye or surrounding area, or any sensation of prickling, tingling, burning, stinging and/or itching of the eye(s), or had at least moderate objective findings (lacrimation, bulbar conjunctiva, palpebral conjunctiva, scleral vessels) diagnosed by the ophthalmologist at the start of the study.
The study was conducted in a clinical research centre in October, 2017 in Germany. The study was conducted in accordance with the principles of ICH-GCP guidelines and the Declaration of Helsinki. All subjects provided written informed consent prior to study enrolment.
Study design
The study was a double-blind, randomized controlled trial. All subjects were asked not to apply any leave-on cosmetic or detergent in the test area within one hour prior to the start of the study. Subjects were also restricted from wearing any decorative cosmetics in the test area (at or near the eyes), contact lenses and/or false eyelashes on the morning of the start of the study.
Test product (15 µL of SCFW or 10% baby shampoo) was instilled into the inferior fornix of the right and left eye by the technician, according to a randomization list. Subjects were instructed to keep both eyes shut for 30 s after instillation of the last test product, unless pain was experienced which required ophthalmologist intervention. Subjects were not allowed to touch the eye area in any manner for the duration of the study.
Ophthalmological evaluation was conducted at baseline (prior to instillation of eye drops; T0) and at 30 s, 15 min, and 1 h after instillation. Subjective evaluation by the subjects was performed 30 s, 15 min, and 1 h after instillation. Immediately after the final evaluation, the subjects' eyes were thoroughly flushed with sterile 0.9% sodium chloride solution.
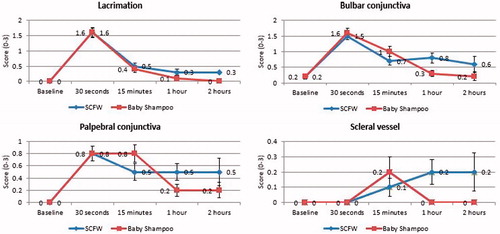
Ophthalmologist evaluation included the intensity of lacrimation, conjunctival irritation, and dilation of scleral vessels based on visual observation with a slit lamp according to the scales in . In subjective self-evaluation, subjects were asked to evaluate the intensity of sensations according to a 4 point scale (0 = None; 1 = Slight prickling, tingling, burning, stinging or itching; 2 = Moderate burning, stinging or itching; 3 = Severe burning, stinging or itching). If findings did not reach baseline level, the subject was reevaluated 2 h after instillation as per ophthalmologist criteria. All adverse reactions were recorded.
Table 2. Scales for objective ophthalmological evaluation in ocular instillation clinical study.
Statistical analysis
Descriptive statistics (mean, standard deviation, n, and percentage) were used to report results. Baseline homogeneity was assumed between the two eyes. The comparison between the SCFW and the 10% baby shampoo was tested using Wilcoxon signed-ranks test for each parameter separately. Differences were considered significant at p < .05. Statistical analysis was performed using SAS 9.4 software (SAS Institute, Inc.; Cary, NC, USA).
Results
EpiOcular time-to-toxicity (ET-50) test
The SCFW was tested undiluted at four exposure times of 4, 8, 16, and 24 h. The exposure time response curves of SCFW and the positive control are in . summarizes the ET50 results of the EpiOcular™ assay and its classification. As a part of the assay, SCFW was tested for its ability to directly reduce MTT. The results showed that there was little or no direct MTT reduction, and the MTT results were used without an adjustment. The assay was considered valid because the acceptance criteria were met. The ET50 of the positive control was 27.1 min, within the historical mean (18.5 – 39.0 min), and the corrected mean OD550 value for the negative control with 15 min exposure time (1.515) was within 20% of the corrected mean OD550 value for the negative control with 4 h exposure time (1.688). The control results indicated acceptable, consistent, and stable tissue conditions. SCFW was clearly classified as a nonirritant because the tissues exposed to SCFW up to 24 h did not show any toxicity (ET50>24 h).
Figure 2. Ca2+ influx in response to capsaicin for SCFW and SC with or without capsazepine. SC: oil-in-water sunscreen; SCFW: sunscreen fusion water.
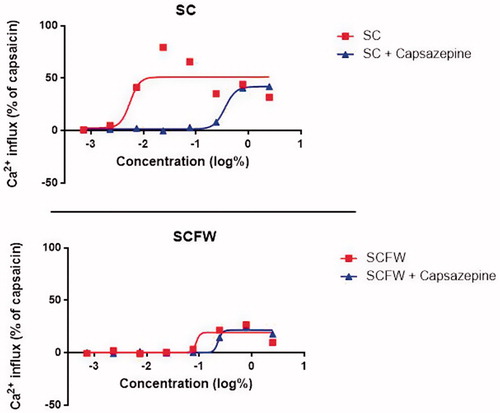
Table 3. ET50 values determined with EpiOcular time-to-toxicity (ET-50) test.
NociOcular assay
The test product SC was determined to be insoluble and 15% detergent was used as the solvent to dissolve the highest stock concentration. The highest stock concentration of SCFW was also diluted in 15% detergent for a better comparison with SC. There was no additional 15% detergent solvent control tested because only the top stock concentration was diluted in the 15% detergent and the result of the preliminary dose range finding assay of SCFW with the highest dilution prepared in both the KRH buffer and in the 15% detergent showed identical responses.
The test products were tested at eight concentrations ranging from 0.000728% to 2.5% based on results from the dose range finding assay. The negative control (KRH buffer and KRH buffer with capsazepine) confirmed no TRPV1 response and capsaicin treatment at a single concentration of 100 nM was set up as the 100% response used in the relative effect (E) calculation.
The quantitative effects were analysed as EC50 (concentration inducing 50% effect of Emax) and Emax (maximum % of capsaicin response) (), and the shape of the Ca2+ influx graphs were evaluated.
Table 4. Results of NociOcular Assay.
The SCFW induced a Ca2+ influx of 20% to 30% Effect at 3 highest concentrations of 2.5% to 0.244% both in the wells without capsazepine and wells with capsazepine (TRPV1 antagonist). Hence, the Ca2+ influx induced by these products was attributed to non-TRPV1-specific responses. Since SCFW did not induce any TRPV1 channel-specific effect, it was classified as a non-stinger (). The Ca2+ response induced by the SC was inhibited by the presence of capsazepine, indicating TRPV1 channel-specific effect, and SC was classified as a product with possible stinging potential.
Ocular instillation study
The study was completed by 25 subjects. Demographic characteristics of the subjects are presented in .
Table 5. Demographic characteristics of ocular instillation study subjects.
For all objective parameters (lacrimation, irritation of bulbar conjunctiva, irritation of palpebral conjunctiva, dilation of scleral vessels) that were scored by ophthalmologist, the average scores were similar for SCFW and 10% baby shampoo with no statistically significant differences between the groups at shorter time points (, ). Corneal assessments by the ophthalmologist showed no opacities, oedema, infiltrates, neovascularization, or epithelial defects for either of the treatment groups.
Table 6. Ophthalmological evaluation scores represented as means (SD) of individual scores.
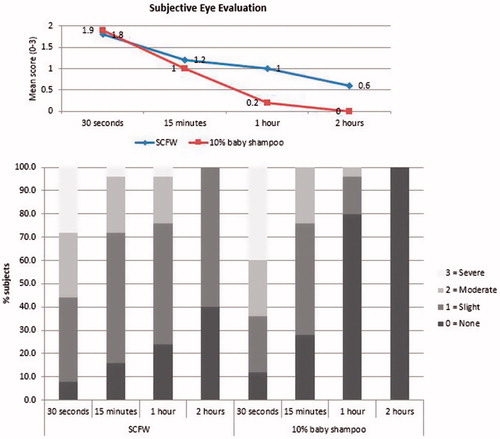
The scores of the subjective parameters (prickling, tingling, burning, stinging, and/or itching) evaluated by the subjects are presented in . The feeling of stinging, burning, itching, prickling and/or tingling was higher after treatment with the SCFW, but there was no statistically significantly difference between the SCFW and 10% baby shampoo for the sum score of subjective parameters (p = 0.052). The percentages of subjects with none, slight, moderate or severe subjective evaluation for SCFW and baby shampoo are reported in . At the shorter time points, 30 s and 15 min, the mean score of subjective parameters was comparable for the SCFW and the reference product (p values 0.479 and 0.34, respectively). After 1 h, in 40% of subjects, the results of the objective ophthalmological evaluation had not reached the level of the objective ophthalmological evaluation at baseline for SCFW treatment, therefore an additional ophthalmological evaluation was performed after 2 h. Thus, the SCFW showed a very good eye tolerance when it stayed in the eye for less than 15 min and moderate eye tolerance when it stayed for more than 15 min, which is an unlikely event in real life.
Table 7. Subjective assessment – Mean scores (SD) per assessment time and sum scores for prickling, tingling, burning, stinging, itching.
Six adverse reactions were documented in four subjects during the study. Two subjects showed dilatation of scleral vessels with lacrimation 120 min after application of the SCFW and 4 subjects showed hyperaemia of conjunctiva with lacrimation 120 min after application of SCFW. All reactions were classified as mild and all subjects recovered without sequelae.
Discussion
Most sunscreens on the market today in Europe and other highly regulated parts of the world comply with the stipulated standards of SPF value, broad spectrum protection, and water resistance testing. However, it is evident that to improve compliance, sunscreens have to be more appealing in terms of texture, good sensory feel, and comfort for the user. Fotoprotector ISDIN Fusion Water is unique in containing a combination of organic and inorganic ultraviolet filters in a formulation with an aqueous external phase. This combination translates to a very comfortable, water-like texture with almost no residue feel on skin and excellent ocular comfort with negligible ocular irritation and stinging. Described in this paper is a three-tiered approach to demonstrate the ocular tolerance of a sunscreen and report the results for this testing strategy for SCFW.
The in vitro irritation test based on reconstituted corneal epithelium with determination of ET50 value helps discriminate between products that have an ultra-mild to mild ocular irritation potential and allows for easy ranking of multiple formulations when screening potential candidates, which can be very useful in the developmental phaseCitation11. In this study, undiluted SCFW elicited negligible cytotoxicity in the corneal epithelium up to the longest prescribed exposure time of 24 h, suggesting that it would have a very low potential for ocular irritation.
Since standard in vitro assays evaluating ocular irritation do not detect stinging sensation or nociception, symptoms that are not necessarily associated with ocular irritation, it was necessary to perform an additional assay assessing nociception. TRPV1 channel activation has been reported to be a principal mechanism for eye-stinging sensation induced by personal care products as both corneal and conjunctival mucosal tissue are rich in C-fibre innervationsCitation12, which express TRPV1 channelsCitation13. As such, the in vitro NociOcular test was performed as a tier-two test to define ocular tolerance. The NociOcular test for rinse-off products was modified to include a diluent (15% Johnson’s baby shampoo) that could improve the solubility of otherwise insoluble formulations. Such diluent was shown not to produce a TRPV1-specific response by itself. By using capsazepine, an antagonist of the TRPV1 receptor, it was shown that SCFW did not activate the TRPV1 receptor in a significant manner and was classified as a non-stinger. Although SCFW was seen to induce the Ca2+ influx at higher concentrations in this assay, this influx can be attributed to one of many reasons for seeing Ca influx, many of which are unrelated to the TRPV1 activity, for example cytotoxicity, or external stimuli, among others. Since the influx was observed in the wells with and the wells without capsazepine (TRPV1 antagonist) exposed to these higher concentrations of the test product, the influx was assessed as non-TRPV1 specific and not counted.
The results of the NociOcular study were strengthened with concurrent testing of SC, a reference material with unacceptable ocular tolerance history. By comparison, SC showed a significant TRPV1 activity indicating sting potential and confirming acceptable sensitivity of the NociOcular assay for sunscreens.
The two in vitro studies allowed us to be reasonably confident that the ocular instillation of the product would have a low potential for inducing appreciable discomfort in the clinical study. Previously, Gao et al. looked at the ocular irritation of several classes of products by ocular instillation. The evaluation of ocular discomfort combined the objective evaluation of irritation and subjective self-evaluationCitation14. A 2008 paper reported results from ocular testing for a sunscreen product after instillation compared to the same marketed baby shampoo that we have used in our study combining objective and subjective evaluationsCitation15. Prior to the development of the NociOcular test to detect the stinging response in vitro, clinical testing was the only option to test for stinging.
The results of the clinical study involving ocular instillation of the undiluted SCFW compared with diluted baby shampoo demonstrated that up to 15-min post-dose, the stinging response for SCFW was comparable to 10% baby shampoo, confirming the non-stinging character of the product. No statistically significant differences were found in terms of subjective and/or objective eye irritation assessments between the SCFW and the baby shampoo. Despite the rather low p values (0.052) for the comparison between SCFW and baby shampoo groups for subjective scoring, which may suggest the difference between these groups might reach significance with a larger sample size, a closer look at the values at each time point suggests that greater differences appear only at the longer assessment time points, confirming that at shorter time points, up to 15 min, the response was similar between two products tested. The decision to keep the eyes shut for 30 s after installation was done to ensure good contact of the product with ocular tissue. However, this may have inhibited the normal clearance of the product via a natural blinking response, thus artificially maximizing the irritation response in our study. For future studies, a more real-life approach of exposure of eyes to products may be considered.
Conclusion
To improve compliance for daily use facial sunscreens, these products should meet stricter criteria for ocular safety and comfort. A three-tiered approach evaluating a sunscreen’s potential for ocular tolerance is proposed, where the first tier is represented by in vitro ocular irritation test, the second tier is represented by in vitro NociOcular test to detect a stinging response, and the third tier is a clinical study to confirm ocular safety and comfort that includes either direct product instillation or a periocular application of the product, depending on the expected use of the product. In this study, the facial sunscreen SCFW was shown to be non-irritating, non-stinging and have very good comfort and irritation scores that were comparable to the marketed baby shampoo up to 15 min post-administration.
Disclosure statement
M. Narda, D. Ramos-Lopez, P. Valderas-Martinez and C. Granger are employees of ISDIN S.A. who sponsored the study.
References
- Elmets CA, Anderson CY. Sunscreens and photocarcinogenesis: An objective assessment. Photochem Photobiol 1996;63:435–440.
- Ling TC, Gibbs NK, Rhodes LE. Treatment of polymorphic light eruption. Photoderm Photoimm Photomed 2003;19:217–227.
- Bens G. Sunscreens. Adv Exp Med Biol 2014;810:429–463.
- Spradlin K, Bass M, Hyman W, Keathley R. Skin cancer: knowledge, behaviors, and attitudes of college students. South Med J 2010;103:999–1003.
- Peacey V, Steptoe A, Sanderman R, Wardle J. Ten-year changes in sun protection behaviors and beliefs of young adults in 13 European countries. Prev Med 2006;43:460–465.
- Buller DB, Cokkinides V, Hall HI, et al. Prevalence of sunburn, sun protection, and indoor tanning behaviors among Americans: review from national surveys and case studies of 3 states. J Am Acad Dermatol 2011;65:S114–S123.
- Wang SQ, Xu H, Dusza SW, et al. Improving compliance of daily sunscreen application by changing accessibility. Photodermatol Photoimmunol Photomed 2017;33:112–113.
- Draelos ZD. Compliance and sunscreens. Dermatol Clin 2006;24:101–104.
- Solky BA, Phillips PK, Christenson LJ, et al. Patient preferences for facial sunscreens: a split-face, randomized, blinded trial. J Am Acad Dermatol 2007;57:67–72.
- Forsby A, Norman KG, El Andaloussi-Lilja J, et al. Using novel in vitro NociOcular assay based on TRPV1 channel activation for prediction of eye sting potential of baby shampoos. Toxicol Sci 2012;129:325–331.
- Landin WE, Mun GC, Nims RW, Harbell JW. Use of the cytosensor microphysiometer to predict results of a 21-day cumulative irritation patch test in humans. Toxicol In Vitro 2007;21:1165–1173.
- Müller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res 2003;76:521–542.
- Nakamura A, Hayakawa T, Kuwahara S, et al. Morphological and immunohistochemical characterization of the trigeminal ganglion neurons innervating the cornea and upper eyelid of the rat. J. Chem. Neuroanat 2007;34:95–101.
- Gao Y, Kanengiser BE. Categorical evaluation of the ocular irritancy of cosmetic and consumer products by human ocular instillation procedures. J Cosmet Sci 2004;55:317–325.
- Yan XS, Riccardi G, Meola M, et al. A tear-free, SPF50 sunscreen product. Cutan Ocul Toxicol 2008;27:231–239.