Abstract
Objective
To investigate the effect of topical sinomenine (SIN) on ocular surface damage in experimental dry eye in mice.
Material and methods
Experimental dry eye was created using scopolamine hydrobromide in female C57BL/6 mice. Eye drops consisting of 0.05%, or 0.1% SIN or phosphate-buffered saline (PBS) were applied to the experimental dry eye in mice. Tear product and corneal staining scores were measured at 7 and 14 days after treatment. Interleukin (IL)-1β and tumour necrosis factor (TNF)-α levels in the SIN groups at 14 days after treatment were compared with those of other groups.
Results
Mice treated with 0.05% or 0.1% SIN showed a significant improvement in tear product and corneal irregularity compared to the control and PBS-treated groups. A significant decrease in the levels of IL-1βand TNF-α was observed in the 0.05% and 0.01% SIN-treated groups.
Conclusions
Topical SIN eye drop application can effectively improve clinical signs and decrease inflammation in the ocular surface, and alleviate ocular surface damage in dry eye.
Keywords:
Introduction
Dry eye disease is a frequently chronic and progressive condition of the tear fluid and ocular surface affecting 11–17% in the general population and rates of up to 29% in clinical optometry practices depending on the diagnostic criteria applied, the age of the population studied and geographic locationCitation1–4. The symptoms of dry eye syndrome include discomfort, burning or foreign body sensation, irritation, itchy or painful eyes, visual disturbance and, in some cases, even to blindness. Dry eye syndrome is caused by insufficient tear production or increased evaporation of the tears, hyperosmolarity, and inflammatory damageCitation5. Recent studies have shown that dry eye syndrome is an inflammatory disease with many features in common with autoimmune diseaseCitation5–7. Chronic inflammation stimulated by the activation of innate immune in the ocular surface, instability of the tear film, and the hyperosmolar tears plays a key role in the immuno-pathogenic mechanism of dry eyeCitation1–3,Citation8. Whatever the initial aetiology of dry eye, once it has developed, inflammation becomes the key mechanism of ocular surface damageCitation3,Citation8.
The management of dry eye includes environmental modification, the use of artificial tears and ointments, punctual plugs, anti-inflammatory agents, serum eye drops, contact lenses, behaviour modifications, and surgery according to disease severityCitation9,Citation10. Currently, based on the concept that inflammation is a major factor in the pathogenesis of dry eye, inhibition of inflammation in the lacrimal functional unit is the mainstream choice for treating dry eye among the various treatment optionsCitation11. It has been well documented that treatment aimed at suppressing the ocular surface inflammation could effectively improve tear film stability, normalize tear osmolality, sustain ocular surface homeostasis, and alleviate ocular discomfort.
Sinomenine (SIN) (7,8-didehydro-4-hydroxy3,7-dimethoxy-17-methyl-α, 13α, 14α -morphinan-6-one) is a quinoline alkaloid and a morphinan derivative extracted from the stems and roots of Sinomenium acutum (Thunb.) Rehd. Et Wils and S. acutum (Thunb.) Rehd. et Wils var. cinereum Rehd. et Wils. Since the main active component of S. acutum, SIN was isolated by Ishiwari from this medicinal plant in the 1920sCitation12, it has been used extensively in China, Japan, and other Asian countries to treat immune-related disorders in pharmacologically experimental animal models and in some clinical applications, such as rheumatoid arthritis, arrhythmia, and neuralgiaCitation13. It has been demonstrated that SIN possesses anti-inflammatory and immunoregulatory properties. It could enhance macrophage phagocytosis by downregulating the expression of IL-6 and TNF-αin the macrophagesCitation14. Uveitis and dry eye syndrome are the two common ocular inflammatory diseases in which the autoimmunity and inflammatory are the main pathophysiological mechanism as well as with significant differences between the two diseases. A previous study showed that topical administration of SIN in situ gel to rats could inhibit autoimmune uveitisCitation15. However, to our knowledge, there is no study to explore the application of SIN on dry eye. Therefore, the purpose of this study was to investigate the effect of SIN on the experimental dry eye in mice.
Materials and methods
Reagents
Scopolamine hydrobromide was purchased from Sigma-Aldrich (St. Louis, MO), and dissolved in 0.9% sterilized NaCl solution at 6 mg/ml prior to injections. SIN (analytical reagent, Xi’an Guanyu Biological Engineering Co. Ltd, Shaanxi, China) was diluted to two concentrations (0.05% and 1%) with phosphate-buffered saline (PBS). The diluted solutions were then filtered through a 0.22-um membrane (Shine, Qiongdao, China) and stored at 4 °C.
Mice
The procedures used in this research were approved by the Experimental Animal Ethics Committee of Shanghai Jiaotong University Affiliated Sixth People’s Hospital and it conformed to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Female C57BL/6 mice (8–11 weeks, Shanghai SLAC Laboratory Animal Centre, Shanghai, China) were housed under relatively constant temperature(25 ± 1 °C) and humidity conditions (40 ± 4%) in a 12 h:12 h light-dark illumination cycle (8 am to 8 pm). Prior to the beginning of the study, all animals were acclimated to the study environment at least 1 week and were fed with food and water ad libitum.
Murine dye eye model
Scopolamine hydrobromide was used to induce experimental dry eye (EDE) in mice. Mice received 0.1 ml scopolamine hydrobromide by subcutaneous injection of 4 times a day (8:00, 11:00, 15:00, 18:00) and exposure to an air draft and less than 30% ambient humidity for 5 daysCitation16,Citation17. Control group of age and gender-matched mice received an equal volume of PBS as a non-treated (NT) group.
Experimental procedure
The mice with EDE were evaluated with tear product and cornea fluorescein staining score as a EDE baseline condition, then were randomly assigned to 4 groups with each group of eight mice according to topical treatment administered as follows: (1) EDE control group mice did not receive any eye drop treatment; (2) EDE + PBS group mice were treated with PBS solution; (3) EDE + 0.05%SIN group mice were treated with 0.05% preservative-free SIN hydrochloride eye drops; (4) EDE + 1%SIN group mice were treated with 1% preservative-free SIN hydrochloride eye drops. SIN hydrochloride eye drops were made by diluting SIN hydrochloride dry powder with PBS solution and pH value is adjusted to 6.8–7.2. Then it was filtered by microporous membrane filtration. The whole process of the pharmaceutical was completed.in the cell culture room. (5) NT group mice were treated with PBS solution without induction of EDE. All groups received 2 µl eye drops by topical application four times daily in two eyes. The eye drops were administered by one investigator throughout the study. Tear product and cornea fluorescein staining score were measured under slit lamp microscope at 7 and 14 days after treatment. Data were collected from two eyes of the mice in each group. The treatments were encoded, and the group allocation was blinded to the technician administering the treatment, and to the researcher assessing the outcome of the experiment. Group identification was uncovered at the end of the analysis.
Tear product
The amount of tears was measured with phenol red thread tear test using cotton threads (Zone-Quick; Yokota, Tokyo, Japan) on D7 and D14 at the same time point (8 AM) as previously describedCitation18. The subjects received no sedatives, anaesthetics, or any other agent. The eyelid was pulled down slightly and the thread was placed on the lower conjunctival fornix by using forceps at approximately one-third of the lower eyelid distance from the lateral canthus for 20 s. The wetted red portion of the thread is measured in millimetres under a slit-lamp biomicroscope (XL1, Shin-Nippon, Niigata, Japan) to maximize accuracy.
Corneal fluorescein staining
Corneal fluorescein staining was used to evaluate corneal epithelial damage. A dose of 1 µL of 2.5% sodium fluorescein (Sigma, St. Louis, MO) was administered into the inferior conjunctival sac using a micropipette. Three minutes later, the corneal epithelial staining was graded using a slit-lamp microscope (Kowa, Tokyo, Japan) under cobalt blue filter light (Topcon Medical Systems Inc., Oakland, NJ). The cornea was divided into four quadrants, and the damage in each quadrant was scored, respectively, as follows: 0 = absent; 1 = slightly punctate staining with less than 30 spots, 2= punctate staining with more than 30 spots, but without diffuse staining, 3= severe and diffuse staining but without positive plaque; 4 = positive fluorescein plaque. The scores of each quadrant were added to represent the damage on each cornea, ranging from 0 to 16Citation19.
Inflammatory responses
Murine cornea was surgically excised, and total RNA was isolated from the cornea by a PicoPure RNA isolation kit (Catalogue no. KIT0204; Arcturus, Mountain View, CA). qRT-PCR was used for the detection of the expression of IL-1β, TNF-α and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Total RNA from corneas was extracted and pooled from two eyes of the same experimental group and the same compound administration schedule was used with the RNA isolation kit according to the manufacturer’s instructions (PicoPure RNA isolation kit; Applied Biosystem, Foster City, CA). cDNA was synthesized using a reverse transcription kit (catalog no. RR047A; TaKaRa, Shiga, Japan) from 1 μg total RNA using random primers and Moloney Murine Leukaemia Virus (M-MuLV). The primer sequences for qRT-PCR detection of the inflammatory mediators of interest are listed in . The RNA concentration was measured at 260 nm and stored at −80 °C before use. qRT-PCR analysis was performed by using the SYBR Green PCR Core Reagents System (Applied Biosystems, Paisley, UK) and Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems). The amplification program included an initial denaturation step at 95 °C for 10 min, followed by 40 cycles of 95 °C for 10 s, and 60 for 30 s, after which a melt curve analysis was conducted to check amplification specificity. Assays were performed in duplicate and repeated three times. The qRT-PCR results were analyzed using the comparative threshold cycle method and normalized with GAPDH as an endogenous reference.
Table 1. The primer sequences used for qRT-PCR.
Statistical analysis
All data were expressed as mean ± SD. Statistical analyses were performed using Statistical Program for Social Sciences 20.0 (IBM SPSS Inc., Chicago, IL). The data were analyzed by Two-way ANOVA followed by post hoc analysis. A p < 0.05 was considered significant.
Results
Induction of the dry eye
EDE was induced in the test group by subcutaneous injection of scopolamine hydrobromide 0.1 ml four times per day. The mice were harboured in controlled-environment chamber at a humidity less than 30%. After inducing EDE, 1 ml 0.5% fluorescein was applied into mouse conjunctival sac, and corneal fluorescein staining was examined using slit-lamp microscopy. We observed erosion above grade 3 in most of the test group mice, confirming the effective induction of EDE using the study protocol.
The effects of topical application of SIN on tear production
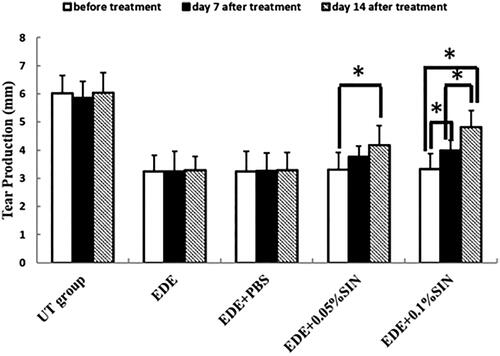
The phenol red thread test was used to assess the effect of SIN eye drops on tear production in EDE-induced murine in our study (). The tear production before eye drops application in the NT group, EDE group, EDE + PBS group, EDE + 0.05%SIN group and EDE + 0.1%SIN group is 4.71–6.62 mm, 2.46–4.3 mm, 2.18–4.25 mm, 2.46–4.12 mm and 2.25–4.03 mm, respectively. The tear production on 7 days after eye drops application in the NT group, EDE group, EDE + PBS group, EDE + 0.05%SIN group and EDE + 0.1%SIN group is 4.74–6.51 mm, 2.18–4.56 mm, 2.43–4.34 mm, 3.17–4.27 mm and 3.57–4.81 mm, respectively. The tear production on 14 days after eye drops application in the NT group, EDE group, EDE + PBS group, EDE + 0.05%SIN group and EDE + 0.1%SIN group is 4.63–6.92 mm, 2.34–4.03 mm, 2.24–4.02 mm, 3.29–5.30 mm and 3.98–5.97 mm, respectively. Two-way ANOVA was used to analyze the effect of group and time on the tear production. The statistical results showed that the effects of both factors were significant. Post hoc analysis showed that the tear production in the EDE group (3.25 ± 0.57 mm), EDE + PBS group (3.24 ± 0.73), EDE + 0.05%SIN group (3.31 ± 0.60) and EDE + 0.1%SIN group (3.33 ± 0.56) decreased significantly compared to the UT group (6.03 ± 0.63 mm) with all the p values smaller than 0.05. After 7 days and 14 days of treatment, the tear production in the EDE and EDE + PBS groups didn’t change compared with that before treatment (3.25 ± 0.57 mm, 3.24 ± 0.73 mm and 3.28 ± 0.48 mm for EDE group before treatment, 7 days and 14 days after treatment respectively, p > 0.05; 3.25 ± 0.73 mm, 3.26 ± 0.64 mm and 3.30 ± 0.62 mm for EDE + PBS group before treatment, 7 days and 14 days after treatment respectively, p > 0.05). After 7 days and 14 days of treatment, the tear production in the EDE + 0.05% SIN group and the EDE + 0.1% SIN group gradually increased compared with that before treatment. In the EDE + 0.05%SIN group, the difference between 14 days after treatment and that before treatment was significantly (4.18 ± 0.67 mm versus 3.31 ± 0.60 mm, p < 0.05), but not between 7 days after treatment and before treatment (3.75 ± 0.40 mm versus 3.31 ± 0.60 mm, p > 0.05), and not between 14 days after treatment and 7 days after treatment (4.18 ± 0.67 mm versus 3.75 ± 0.40 mm, p > 0.05). In the EDE + 0.1%SIN group, the differences between any two time points were statistically significant (4.81 ± 0.60 mm versus 3.99 ± 0.37 mm and 3.33 ± 0.56 mm for 14 days after treatment, 7 days after treatment and before treatment, all p < 0.05).
The effects of topical application of SIN on corneal fluorescein staining scores
Fluorescein staining was used to assess the effect of SIN eye drops on the changes in corneal epithelial integrity in NT, EDE, EDE + PBS, EDE + 0.05%SIN and EDE + 1%SIN groups in this study ().
Figure 2. Mean corneal fluorescein staining scores in the untreated (UT) control, experimental dry eye (EDE), 0.05% sinomenine (SIN) treated, and 0.1% SIN-treated groups before treatment and 7 and 14 days after treatment (8 mice, 16 eyes in each group). Data shown as mean ± SD. * p < 0.05.
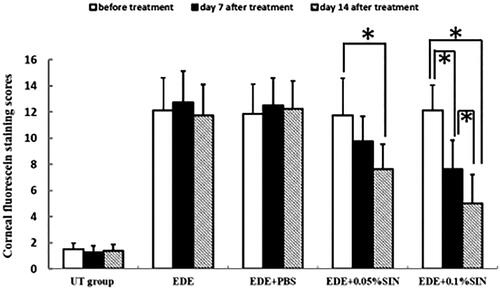
The corneal fluorescein staining scores before eye drops application in the NT group, EDE group, EDE + PBS group, EDE + 0.05%SIN group and EDE + 0.1%SIN group is 1–2, 9–15, 8–14, 8–14 and 10–15, respectively. The corneal fluorescein staining scores on 7 days after eye drops application in the NT group, EDE group, EDE + PBS group, EDE + 0.05%SIN group and EDE + 0.1%SIN group is 1–2, 8–16, 9–15, 6–13 and 4–10, respectively. The corneal fluorescein staining scores on 14 days after eye drops application in the NT group, EDE group, EDE + PBS group, EDE + 0.05%SIN group and EDE + 0.1%SIN group is 1–2, 8–15, 9–15, 5–10 and 2–8, respectively. Two-way ANOVA was used to analyze the effect of group and time on the tear production. The statistical results showed that the effects of both factors were significant. Post hoc analysis showed that the corneal staining scores were significantly higher in EDE (12.13 ± 2.17), EDE + PBS (11.88 ± 1.81), EDE + 0.05%SIN (11.75 ± 2.05) and EDE + 1%SIN group (12.13 ± 1.64) compared with that of NT group (1.5 ± 0.53) with all the p values smaller than 0.05. After 14 days and 7 days of drop application, the corneal staining scores in the EDE and EDE + PBS groups did not change compared with that before treatment (12.13 ± 2.17, 12.75 ± 2.49 and 11.75 ± 2.38 for EDE group before treatment, 7 days and 14 days after treatment, respectively, p > 0.05; 11.88 ± 1.81, 12.50 ± 2.27 and 12.25 ± 2.12 for EDE + PBS group before treatment, 7 days and 14 days after treatment respectively, p > 0.05). After 7 days and 14 days of treatment, the staining scores in the EDE + 0.05%SIN group and the EDE + 0.1% SIN group gradually decreased compared with that before treatment. In the EDE + 0.05%SIN group, the difference between 14 days after treatment and that before treatment was significantly (7.63 ± 1.92 versus 11.75 ± 2.05, p < 0.05), but not between 7 days after treatment and before treatment (9.75 ± 2.82 versus 11.75 ± 2.05, p > 0.05), and not between 14 days after treatment and 7 days after treatment (7.63 ± 1.92 versus 9.75 ± 2.82, p > 0.05). In the EDE + 0.1%SIN group, the differences between any two time points were statistically significant (5.00 ± 2.20 versus 7.63 ± 1.92 and 12.13 ± 1.64 for 14 days after treatment, 7 days after treatment and before treatment respectively, all p < 0.05).
Changes in cytokine levels in cornea after SIN treatment
Inflammatory cytokines in corneas were determined by qRT-PCR on day 14 after treatment. The IL-1β relative mRNA levels in the corneas before treatment and 14 days after treatment are shown in . The IL-1β relative mRNA levels in the corneas before eye drops application in the NT group, EDE group, EDE + PBS group, EDE + 0.05%SIN group and EDE + 0.1%SIN group is 0.90–1.1, 7.03–11.33, 7.22–11.23, 7.17–12.05 and 7.15–11.03, respectively. The IL-1β relative mRNA levels in the corneas on 14 days after eye drops application in the NT group, EDE group, EDE + PBS group, EDE + 0.05%SIN group and EDE + 0.1%SIN group is 0.81–1.16, 7.37–11.32, 7.32–11.74, 4.13–7.17 and 3.13–7.83, respectively. The TNF-α relative mRNA levels in the corneas before treatment and 14 days after treatment are shown in . The TNF-α relative mRNA levels in the corneas before eye drops application in the NT group, EDE group, EDE + PBS group, EDE + 0.05%SIN group and EDE + 0.1%SIN group is 0.8–1.1, 10.5–14.1, 9.3–14.7, 8.5–14.9 and 9.5–14.4, respectively. The TNF-α relative mRNA levels in the corneas on 14 days after eye drops application in the NT group, EDE group, EDE + PBS group, EDE + 0.05%SIN group and EDE + 0.1%SIN group is 0.9–1.16, 9.5–14.1, 8.6–14.3, 8.4–13.9 and 4.1–9.6, respectively.
Figure 3. Real-time PCR analysis of IL-1β expression in mice (8 mice,16 eyes in each group). *Significant difference when compared with pre-treatment values (p < 0.05). Error bars represent SD.
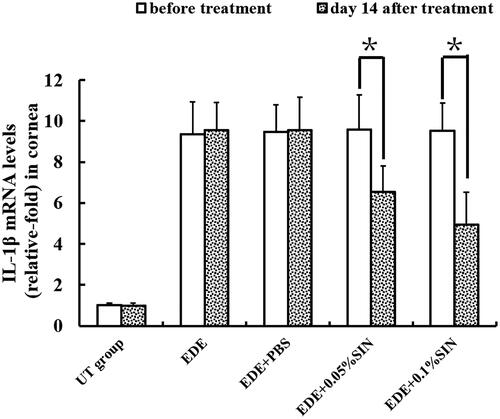
Figure 4. Real-time PCR analysis of TNF-α expression in mice (8 mice,16 eyes in each group). *Significant difference when compared with pre-treatment values (p < 0.05, n = 16 eyes). Error bars represent SD.
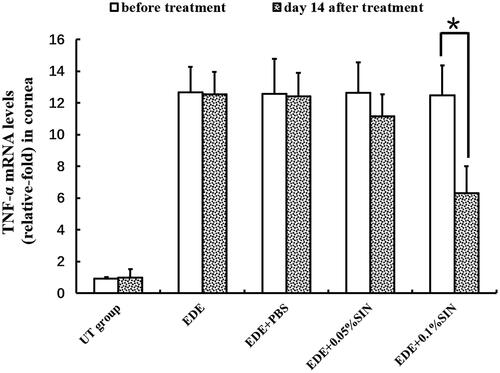
The mRNA relative expressions levels of IL-1β and TNF-α on the ocular surface in EDE + 0.05% SIN group and in EDE + 1% SIN group decreased in a dose-dependent manner compared to that before treatment. However, statistical analysis showed that the levels of IL-1β in the mice treated with two doses of SIN decreased significantly. The levels of TNF-α decreased only in the mice treated with 0.1% SIN eye drops but not for 0.05% SIN-treated mice.
Discussion
Dry eye is a chronic ocular surface disease, and a serious dry eye will cause a visual disorder that affects quality of life. Dry eye is compounded by destabilization of the tear film that leads to a decrease in tear production, which results from immoderate vaporization of tears on the ocular surfaceCitation20. The continued imbalance of the tear film and dry eye condition will result in gradual dysfunction and destruction of the lacrimal glands and impairment of the corneal epithelial cells and loss of the conjunctival goblet cellsCitation11,Citation21–24. Long-term dry eye has been reported with increased inflammatory response in the ocular surface of dry eye, increased expression of immune activation and apoptosis markers, adhesion molecules, matrix metalloproteinases, inflammatory cytokines such as tumour necrosis factor-alpha (TNF), matrix metalloproteinase, intercellular adhesion molecule-1, vascular cell adhesion molecule-1Citation25–29, chemokines and their receptors, and CD4+ T cellsCitation30–33 in the ocular surface.
Topical anti-inflammatory agents such as corticosteroids and cyclosporine A improve symptoms, as well as tear film and ocular surface parameters, by inhibiting inflammation in the ocular surface and lacrimal glandCitation34–36. Recently, many researchers tried several anti-inflammatory eyes drops on EDE animals and gained hopeful results.
SIN is a new anti-inflammatory and immune suppression agent that has been reported to treat rheumatoid arthritis, arrhythmiaCitation37,Citation38 and chronic glomerulonephritis and achieved good clinical efficacy. The pharmacological effect of SIN was inhibiting the secretion of inflammatory mediators, reducing the generation of immune complexes; therefore, the systemic or local abnormal immune responses were alleviated or inhibited. For example, the alkaloid reduces the mRNA expression of proinflammatory cytokines including TNF and IL-1 in rats with adjuvant arthritisCitation39. SN was also found to reduce the synthesis of prostaglandin E3, leukotriene C4, nitric oxide, and TNF-α by lipopolysaccharide-treated macrophages in culture in vitro and in vivoCitation40.
In dry eye pathology, the lacrimal functional unit, which is composed of the ocular surface epithelium and lacrimal land, can become the target of the immune system and show signs of inflammationCitation41. Several studies have shown that IL-6, IL-17, interferon-γ, TNF-α, chemokine ligand 2, and MMPs have been implicated in lacrimal gland tissuesCitation42,Citation43. These cytokines can impair lacrimal gland secretion via direct inhibition of neural activity or neurotransmitter releaseCitation44, and then were involved in immune and inflammatory response. Inhibition of these cytokines could improve DED were reported previouslyCitation45.
In the present study, we have shown herein that tear production was higher and cornel surface irregularity scores were lower, in 0.05% and 0.1% SIN-treated mice compared with EDE control and PBS-treated mice. It means topical application of SIN eye drops could increase the tear production and decrease the corneal staining scores in the EDE mice with dose-dependent manner. IL-1β and TNF-α expression in the cornea were significantly lower in 0.05% and 0.1% SIN-treated mice than control mice. The mechanism of SIN eye drops on EDE mice maybe inhibit the inflammation in the ocular surface.
Dry eye is a chronic inflammatory disease with frequent recurrence and needs long-term treatment. It has been confirmed that no obvious side effect was noted after systemic application of SIN for 2 weeks in vivo. Future research is necessary to investigate the safety of long-term use of SIN eye drops on the ocular surface.
In conclusion, this study provides evidence that the application of 0.05% and 0.1% SIN eye drops can improve tear production and effectively alleviate ocular surface irregularities, decrease inflammatory cytokines on the ocular surface by suppressing inflammatory cytokine expression in mouse EDE model. Our results suggest that the topical application of SIN may be useful for the treatment of dry eye disease.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Brewitt H, Sistani F. Dry eye disease: the scale of the problem. Surv Ophthalmol 2001;45:S199–S202.
- Schein OD, Hochberg MC, Munoz B, et al. Dry eye and dry mouth in the elderly: a population-based assessment [Research Support, U.S. Gov't, P.H.S.]. Arch Intern Med 1999;159:1359–1363.
- Moss SE, Klein R, Klein BE. Prevalence of and risk factors for dry eye syndrome [Research Support, Non-U.S. Gov't Research Support, U.S.]. Arch Ophthalmol 2000;118:1264–1268.
- Albietz JM. Prevalence of dry eye subtypes in clinical optometry practice [Comparative Study Research Support, Non-U.S. Gov't]. Optom Vis Sci: off Publ Am Acad Optom 2000;77:357–363.
- Stevenson W, Chauhan SK, Dana R. Dry eye disease: an immune-mediated ocular surface disorder [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Review]. Arch Ophthalmol 2012;130:90–100.
- Stern ME, Schaumburg CS, Pflugfelder SC. Dry eye as a mucosal autoimmune disease [Review]. Int Rev Immunol 2013;32:19–41.
- Goyal S, Chauhan SK, El Annan J, et al. Evidence of corneal lymphangiogenesis in dry eye disease: a potential link to adaptive immunity? Arch Ophthalmol 2010;128:819–824.
- Bandeen-Roche K, Munoz B, Tielsch JM, et al. Self-reported assessment of dry eye in a population-based setting [Research Support, U.S. Gov't, P.H.S.]. Invest Ophthalmol Visual Sci 1997;38:2469–2475.
- Behrens A, Doyle JJ, Stern L, et al. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations [Consensus Development Conference Research Support, Non-U.S. Gov't]. Cornea 2006;25:900–907.
- Gupta H, Jain S, Mathur R, et al. Sustained ocular drug delivery from a temperature and pH triggered novel in situ gel system. Drug Deliv 2007;14:507–515.
- Oh HN, Kim CE, Lee JH, et al. Effects of quercetin in a mouse model of experimental dry eye [Research Support, Non-U.S. Gov't]. Cornea 2015;34:1130–1136.
- Yamasaki H. Pharmacology of sinomenine, an anti-rheumatic alkaloid from Sinomenium acutum. Acta Med Okayama 1976;30:1–20.
- Xu M, Liu L, Qi C, et al. Sinomenine versus NSAIDs for the treatment of rheumatoid arthritis: a systematic review and meta-analysis [Meta-Analysis Research Support, Non-U.S. Gov't Review]. Planta Med 2008;74:1423–1429.
- Wang AL, Li Z, Yuan M, et al. Sinomenine inhibits activation of rat retinal microglia induced by advanced glycation end products [Research Support, Non-U.S. Gov't]. Int Immunopharmacol 2007;7:1552–1558.
- Song J, Bi H, Xie X, et al. Preparation and evaluation of sinomenine hydrochloride in situ gel for uveitis treatment [Research Support, Non-U.S. Gov't]. Int Immunopharmacol 2013;17:99–107.
- Li Z, Choi JH, Oh HJ, et al. Effects of eye drops containing a mixture of omega-3 essential fatty acids and hyaluronic acid on the ocular surface in desiccating stress-induced murine dry eye. Curr Eye Res 2014;39:871–878.
- Choi JH, Kim JH, Li Z, et al. Efficacy of the mineral oil and hyaluronic acid mixture eye drops in murine dry eye. Korean J Ophthalmol 2015;29:131–137.
- Villareal AL, Farley W, Pflugfelder SC. Effect of topical ophthalmic epinastine and olopatadine on tear volume in mice. Eye Contact Lens 2006;32:272–276.
- Sung MS, Li Z, Cui L, et al. Effect of topical 5-aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside in a mouse model of experimental dry eye. Invest Ophthalmol Vis Sci 2015;56:3149–3158.
- Lemp MA. Report of the National Eye Institute/Industry workshop on clinical trials in dry eyes [Consensus Development Conference Consensus Development Conference, NIH Review]. CLAO J 1995;21:221–232.
- Situ P, Simpson TL, Fonn D, et al. Conjunctival and corneal pneumatic sensitivity is associated with signs and symptoms of ocular dryness [Research Support, Non-U.S. Gov't]. Invest Ophthalmol Vis Sci 2008;49:2971–2976.
- Rolando M, Zierhut M. The ocular surface and tear film and their dysfunction in dry eye disease. Surv Ophthalmol 2001;45:S203–S210.
- Kim CE, Oh HN, Lee JH, et al. Effects of chondrocyte-derived extracellular matrix in a dry eye mouse model [Research Support, Non-U.S. Gov't]. Mol Vis 2015;21:1210–1223.
- Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Deutsches Arzteblatt Int 2015;112:71–81.
- Calonge M, Enriquez-de-Salamanca A, Diebold Y, et al. Dry eye disease as an inflammatory disorder. Ocul Immunol Inflamm 2010;18:244–253. ].
- Enriquez-de-Salamanca A, Castellanos E, Stern ME, et al. Tear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye disease [Research Support, Non-U.S. Gov't]. Mol Vis 2010;16:862–873.
- Galbis-Estrada C, Pinazo-Duran MD, Cantu-Dibildox J, et al. Patients undergoing long-term treatment with antihypertensive eye drops responded positively with respect to their ocular surface disorder to oral supplementation with antioxidants and essential fatty acids [Randomized Controlled Trial]. Clin Interv Aging 2013;8:711–719.
- Pinazo-Duran MD, Galbis-Estrada C, Pons-Vazquez S, et al. Effects of a nutraceutical formulation based on the combination of antioxidants and omega-3 essential fatty acids in the expression of inflammation and immune response mediators in tears from patients with dry eye disorders [Randomized Controlled Trial Research Support, Non-U.S. Gov't]. Clin Interv Aging 2013;8:139–148.
- Brignole F, Pisella PJ, Goldschild M, et al. Flow cytometric analysis of inflammatory markers in conjunctival epithelial cells of patients with dry eyes [Clinical Trial Multicenter Study Randomized Controlled Trial]. Invest Ophthalmol Vis Sci 2000;41:1356–1363. ].
- Pflugfelder SC, de Paiva CS, Li DQ, et al. Epithelial-immune cell interaction in dry eye [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Review]. Cornea 2008;27:S9–S11.
- Yoon KC, De Paiva CS, Qi H, et al. Expression of Th-1 chemokines and chemokine receptors on the ocular surface of C57BL/6 mice: effects of desiccating stress [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]. Invest Ophthalmol Vis Sci 2007;48:2561–2569.
- Yoon KC, Park CS, You IC, et al. Expression of CXCL9, -10, -11, and CXCR3 in the tear film and ocular surface of patients with dry eye syndrome [Clinical Trial]. Invest Ophthalmol Vis Sci 2010;51:643–650.
- Stern ME, Pflugfelder SC. Inflammation in dry eye. Ocul Surf 2004;2:124–130.
- Palmer SL, Bowen PA, 2nd, Green K. Tear flow in cyclosporine recipients [Clinical Trial Comparative Study Controlled Clinical Trial Research Support, Non-U.S. Gov't]. Ophthalmology 1995;102:118–121.
- Matsuda S, Koyasu S. Mechanisms of action of cyclosporine. Immunopharmacology 2000;47:119–125.
- Pflugfelder SC. Antiinflammatory therapy for dry eye [Editorial Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S. Review]. Am J Ophthalmol 2004;137:337–342.
- Liu L, Buchner E, Beitze D, et al. Amelioration of rat experimental arthritides by treatment with the alkaloid sinomenine [Research Support, Non-U.S. Gov't]. Int J Immunopharmacol 1996;18:529–543.
- Wang Y, Wang PX, Li XJ. The effect of sinomenine on cyclooxygenase activity and the expression of COX-1 and COX-2 mRNA in human peripheral monocytes. Zhongguo Zhong Yao za Zhi = Zhongguo Zhongyao Zazhi = China Journal of Chinese Materia Medica 2003;28:352–355.
- Wang Y, Fang Y, Huang W, et al. Effect of sinomenine on cytokine expression of macrophages and synoviocytes in adjuvant arthritis rats [Research Support, Non-U.S. Gov't]. J Ethnopharmacol 2005;98:37–43.
- Liu L, Riese J, Resch K, et al. Impairment of macrophage eicosanoid and nitric oxide production by an alkaloid from Sinomenium acutum [Research Support, Non-U.S. Gov't]. Arzneimittel-Forschung 1994;44:1223–1226.
- Stern ME, Gao J, Siemasko KF, et al. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res 2004;78:409–416.
- Pitcher JD, 3rd, De Paiva CS, Pelegrino FS, et al. Pharmacological cholinergic blockade stimulates inflammatory cytokine production and lymphocytic infiltration in the mouse lacrimal gland [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]. Invest Ophthalmol Vis Sci 2011;52:3221–3227.
- Luo L, Li DQ, Doshi A, et al. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]. Invest Ophthalmol Vis Sci 2004;45:4293–4301.
- Song XJ, Li DQ, Farley W, et al. Neurturin-deficient mice develop dry eye and keratoconjunctivitis sicca [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]. Invest Ophthalmol Vis Sci 2003;44:4223–4229.
- Choi W, Noh H, Yeo A, et al. The effect of TNF-α blocker HL036337 and its best concentration to inhibit dry eye inflammation . Korean J Ophthalmol 2016;30:302–308.