?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
There are multiple in vitro and ex vivo eye irritation and corrosion test methods that are available as internationally harmonized test guidelines for regulatory use. Despite their demonstrated usefulness to a broad range of substances through inter-laboratory validation studies, they have not been widely adopted for testing agrochemical formulations due to a lack of concordance with parallel results from the traditional regulatory test method for this endpoint, the rabbit eye test. The inherent variability of the rabbit test, differences in the anatomy of the rabbit and human eyes, and differences in modelling exposures in rabbit eyes relative to human eyes contribute to this lack of concordance. Ultimately, the regulatory purpose for these tests is protection of human health, and, thus, there is a need for a testing approach based on human biology. This paper reviews the available in vivo, in vitro and ex vivo test methods with respect to their relevance to human ocular anatomy, anticipated exposure scenarios, and the mechanisms of eye irritation/corrosion in humans. Each of the in vitro and ex vivo methods described is generally appropriate for identifying non-irritants. To discriminate among eye irritants, the human three-dimensional epithelial and full thickness corneal models provide the most detailed information about the severity of irritation. Consideration of the mechanisms of eye irritation, and the strengths and limitations of the in vivo, in vitro and ex vivo test methods, show that the in vitro/ex vivo methods are as or more reflective of human biology and less variable than the currently used rabbit approach. Suggestions are made for further optimizing the most promising methods to distinguish between severe (corrosive), moderate, mild and non-irritants and provide information about the reversibility of effects. Also considered is the utility of including additional information (e.g. physical chemical properties), consistent with the Organization for Economic Cooperation and Development’s guidance document on an integrated approach to testing and assessment of potential eye irritation. Combining structural and functional information about a test substance with test results from human-relevant methods will ensure the best protection of humans following accidental eye exposure to agrochemicals.
Introduction
The human eye may be exposed to chemicals through various situations, such as accidental splashing or exposure to chemical particles, vapours or gases. Workers and consumers are usually advised to immediately wash eyes generously with water following exposure. Even without washing, it has been shown that if a substance contacts the surface of the human eye, greater than 80% of it is naturally expelled in less than 2 min [Citation1]. While efficient tear secretion (lachrymation) and drainage pathways help to protect the eye from potentially harmful substances, exposure to chemical substances may lead to irritation or corrosion [Citation2,Citation3]. Therefore, it is essential to be able to categorize the eye irritation potential of industrial and consumer agrochemicals and products so that appropriate hazard statements, handling procedures, protective equipment, and emergency response procedures can be communicated.
Eye irritation tests are conducted on agrochemical active ingredients and formulations to support their registration with regulatory authorities. The traditional regulatory test method for this purpose is the in vivo rabbit eye test [Citation4,Citation5]. In the United States (US) alone, the Environmental Protection Agency (EPA) Office of Pesticide Programs receives data on agrochemicals from more than 200 rabbit eye irritation tests each year. However, there is support for moving away from testing on animals, including a recent directive from the EPA Administrator that commits to ending funding of, and requests for, tests on mammals by 2035 and aims to reduce animal tests in the shorter term [Citation6]. Eliminating or reducing animal use is also a stated goal of many companies, investor communities, and organizations. As such, there is a strong need to establish a human-relevant, reproducible, reliable, and quantitative alternative approach that can effectively and consistently classify agrochemicals formulations. There are a large number of in vitro and ex vivo methods, many available as internationally harmonized test guidelines (TGs), which have been developed to identify the eye irritation potential of a wide range of substances. However, their widespread adoption as complete replacements for the in vivo rabbit eye test has been hindered by a lack of in vitro/ex vivo to in vivo concordance for specific ranges of eye irritation (in particular distinguishing mild and moderate irritants) and/or specific chemistries. The mechanisms leading to eye irritation/corrosion and the principle and performance of various methods are explored below to identify approaches that can be used to predict human eye irritation potential without the use of in vivo testing.
Review of existing data and prospective in vitro testing
Agrochemical formulations may be quite complex, typically composed of the active ingredient(s) as well as “inert” ingredients, and both of which may present eye irritation hazards. Accordingly, plausible test methods should be able to address the mechanisms of eye irritation of the active and inert ingredients.
Several publications have evaluated the use of in vitro and ex vivo methods for assessing the eye irritation/corrosion effects of agrochemical formulations [Citation7–10]. The results demonstrated promise in using these methods but highlighted the need for additional analyses to further understand why in vitro/ex vivo and in vivo rabbit results may not align and to further interrogate the utility of the rabbit test as a reference method for such comparisons. The exaggerated exposure conditions (as described in ) and the anatomical and physiological differences between rabbits and humans call into question the relevance of the in vivo response in rabbits to humans. Within the rabbit test, there are multiple points where subjectivity is introduced with respect to observations of reversibility and damage, therefore, results are potentially confounded both by inter-observer variation and animal variation. Further, the likelihood of achieving the same classification upon repeat testing has been shown to be <50% for substances which fall into the mild to moderate irritation range [Citation18]. Given the inherent variability of the rabbit test itself, the apparent discordance between the in vitro/ex vivo methods and the rabbit test could actually be a reflection of uncertainty in the reference classification.
Table 1. In vivo, ex vivo, and in vitro test methods to assess eye irritation and/or corrosion.
Rethinking the process of establishing confidence in new methods
While hazard categories used by regulatory agencies have been the predominant focus in developing testing approaches, the primary objective is the prediction of human responses, and therefore, a more logical approach would be the identification and optimization of approaches that reliably predict human responses based on faithful representation of relevant human biology. The concept of a 1:1 alignment with the in vivo reference classification is neither feasible nor scientifically justified considering the multiple issues associated with the rabbit eye test, which are discussed in [Citation18,Citation19]. Accordingly, there is a need to rethink how to assess the validity of new methods and to evaluate test methods based on which are most reliable and relevant to the human response. This paper outlines the available in vivo, in vitro and ex vivo test methods and, considering human ocular anatomy and physiology and mechanisms of chemically induced ocular irritation, their relevance to predicting eye effects in humans following exposure to substances (agrochemicals and formulations), with a goal of identifying those methods most appropriate for human health evaluation.
Structure of the eye across species
Commonly used methods for assessing eye irritation and corrosion are based on the human, rabbit, cow, chicken, or pig eye. To understand which method(s) are most appropriate for studying effects on the human eye, it is important to understand how the structure of the eye differs across species (). While comparisons have been made among various species, the preponderance of literature on this topic is focussed on the comparative anatomy and physiology of humans, cows, and rabbits.
Figure 1. Comparison of human, rabbit, porcine, chicken and bovine corneas. (a) Schematic depiction of the human eye (image purchased from iStock). (b) Healthy human cornea section (image courtesy of Hans E. Grossniklaus, MD), (c) rabbit cornea section (obtained after whole globe immersion in fixative), (d) dissected pig cornea section, and (e) dissected chicken cornea section. (f) Full thickness bovine cornea from the bovine corneal opacity and permeability (BCOP) assay (sterile, deionized water, 10-min exposure, 2-h post exposure). (g) Epithelium and upper stroma of a bovine cornea from the BCOP assay showing the three epithelial tissue layers, the collagen-rich acellular anterior limiting lamina between the basal lamina, and the organized stromal collagen bundles of the anterior stroma. (c, d, e, f, and g) courtesy of the Institute for In Vitro Sciences.
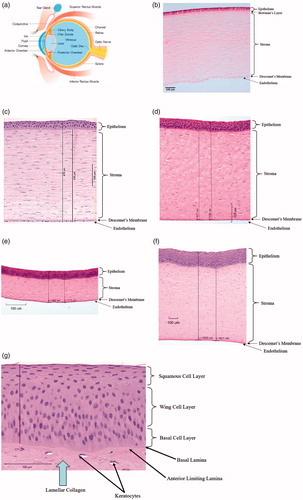
There are five layers of the human cornea: the epithelium, Bowman’s layer, stroma, Descemet’s membrane, and endothelium. Ex vivo bovine corneas (ca. 850–1000 µm [Citation74]) are thicker than those of pigs (ca. 600–700 µm [Citation75,Citation76]), humans (ca. 550 µm [Citation77,Citation78]), rabbits (ca. 400 µm [Citation77,Citation79]), or chickens (ca. 225–400 µm [Citation80,Citation81]). The thickness of corneal tissue varies depending on the age of animal and method of measurement. Of additional note, corneal thickness can increase after excision from the whole globe or from the conditions of the test method (e.g. from stromal fluid uptake in the ex vivo culture system.) Bovine corneal epithelium is thicker than that of humans, pigs, rabbits, or chickens, with 7–9 cells deep in bovine, 4–6 cells deep in humans and pigs, and 4–5 in the rabbit and chicken. In addition to having more cell layers, the cell size may vary between species [Citation82]. Similarly, the bovine corneal stroma is thicker than that of humans and pigs, which in turn is thicker than that of rabbits and chickens. These differences in the thickness of the cornea, and individual corneal layers, can impact the rate of permeation and penetration of chemicals into the cornea (and should be accounted for in test method development). Among the non-human corneas used for eye irritation studies, the porcine cornea is most similar in size and structure to the human cornea. Humans have a Bowman’s layer, an acellular collagen-rich zone just under the epithelial basement membrane, which has a unique extracellular matrix organization different from most other species. Bovine, porcine, and rabbit corneas have a functionally analogous layer called the anterior limiting lamina. The anterior limiting lamina in chickens is most similar to the Bowman’s layer in humans [Citation83]. All of the corneal layers have their own function and play a different role in the progression and recovery of corneal damage.
The conjunctival tissues are composed of an epithelium overlaying the vascular lamina propria. Conjunctival tissues are continuous from the anterior corneal–scleral limbus of the eye, where the conjunctival tissues overlaying the eye globe are referred to as the bulbar conjunctiva, to the outer edges of the eyelids, where the tissues are referred to as the palpebral conjunctiva. The palpebral conjunctiva overlays various glands including tarsal and Meibomian glands, and is populated with goblet cells [Citation84]. Humans have a smaller conjunctival cul-de-sac than rabbits and, therefore, have a notably lower likelihood of retention of a substance following exposure. Whereas the rabbit cul-de-sac is estimated to hold between 30 and 50 µL [Citation85], the human cul-de-sac is estimated to normally hold 7–10 µL of tears and potentially hold up to 25 µL volume in total [Citation86].
Many of the in vitro and ex vivo test methods model the cornea, or specific layers of the cornea. Since the superficial epithelial tissues in the cornea and conjunctiva have similar morphology, ex vivo corneal models or in vitro corneal epithelial tissue models can also be reasonable surrogates for evaluating irritation to the epithelium of the conjunctiva. To demonstrate the relevance, the loss of the squamous and upper wing cell layers in an ex vivo bovine corneal model by surfactant-based formulations coincided with mild to moderate injury to the conjunctiva of the rabbit in vivo [Citation87]. Essentially the same mechanisms that cause surfactant erosion and penetration of the stratified epithelium of the cornea also affect the stratified epithelium of the conjunctivae.
Eye effects depend on the depth and degree of injury
Ocular irritants differ from corrosive (severely irritating) substances based upon the severity of the induced injuries. Ocular irritants induce local, reversible inflammatory reactions, whereas corrosive substances irreversibly damage ocular tissues, leading to possible vision decay or blindness [Citation88]. Ocular changes and lesions in the anterior segment of the eye – including corneal swelling, corneal opacity, iritis, conjunctival redness and/or conjunctival chemosis – are caused following penetration of cell-damaging chemicals through the corneal and/or conjunctival epithelia, or by direct disruption of surface membranes. Since classification, which dictates precautionary measures, is based upon the potential adverse impacts, it is critical that human relevant models are utilized to make human relevant predictions.
The corneal and conjunctival epithelia provide effective barriers to penetration or permeation by most substances, provided those substances do not have the ability to denature, erode, dissociate or solubilize those tissues. It has been demonstrated that among ophthalmic drugs (which are presumably extremely mild to corneal tissues), lipophilic drugs are more likely to permeate the epithelium than hydrophilic drugs, due to the lipid-rich construction of the cell membranes within the epithelium. Whereas the epithelium is a lipophilic environment, the stroma is an aqueous rich environment that presents an effective barrier to permeation of hydrophobic drugs but readily allows permeation of hydrophilic drugs [Citation3]. Accordingly, chemicals that are unable to penetrate the epithelium are also unlikely to cause irritation. In contrast, those chemicals that are able to disrupt the epithelial barrier by solvent diffusion through the cellular environment, injury to the tight junctions between the squamous epithelial cells, or necrotic degradation of the epithelium may also induce cytotoxic and cytolytic effects within the cornea.
Applying the depth of injury (DOI) concept is critical to understanding ocular injuries and in selecting the appropriate test methods to model them [Citation89]. This is because injury to the cells in different regions of the eye have different impacts. In general, cell or tissue injury occurring progressively deeper into the cornea results in increasingly worse injuries. Whereas numerous attempts have been reported [Citation45,Citation46,Citation74,Citation89–92] using various histopathology methods to determine the thresholds delineating the DOI between irritation categories in ex vivo/in vitro corneal systems, not surprisingly some overlap in the DOI between categories is observed. The following summarizes generalized findings on the depth of injuries, relative to chemical irritation potentials.
Slight irritants tend only to injure cells within the superficial corneal epithelium whereas mild irritants also injure cells deeper into the epithelium. Some mild irritants may also induce injuries and changes in the uppermost stroma. Moderate irritants injure deeper into the epithelium and typically also induce injuries to the stromal keratocytes in the anterior stroma [Citation90]. In a recent study evaluating the use of live/dead staining techniques in excised rabbit eyes to determine the DOI of 16 chemicals covering the range of EPA categories (based upon Draize test results), it was demonstrated that some but not all of the EPA category III chemicals induced keratocyte injuries which were determined to impact no deeper than the upper 10% of the stroma. In comparison, the category II materials tended to induce stromal injuries notably deeper into the stroma. It should be noted that some overlap was measured in the DOI, particularly among the category II and III chemicals [Citation91]; however, this overlap is not unexpected given the variability of the rabbit response, particularly for chemicals that are classified as mild and moderate [Citation18]. Epithelial permeability increases if substances injure or destroy the epithelial barrier function initially by injuring tight junctions or desmosomes and progressively by necrosis of the epithelial layers. Substances causing eye corrosion damage cells within the epithelium, deep stroma, and often, but not always, the corneal endothelium and thus irreversibly injure the cornea [Citation3].
Whereas the depth of the injury describes which cells or tissue layers are affected, the degree of injury describes the extent of the injuries and is equally critical in eye irritation assessments [Citation90]. For example, an overall loss of stromal keratocytes in the upper stroma will have a remarkably worse outcome on corneal recoverability relative to another injury where only a few keratocytes are lost at the same depth of injury.
The depth and the degree of injury are highly dependent upon the nature, mode of action, dose, and duration of exposures of the specific chemistries. The nature of those chemical exposures dictates the potential to penetrate into the cornea, starting superficially with the initial contact with the squamous epithelium, and progressing deeper into the cornea. Dose and chemical concentration play a significant role in whether an inherently irritant chemical induces a milder, reversible injury or induces severe irreversible damage. For example, exposures to sodium hydroxide at high concentrations (≥1 N) can readily induce deep penetrating irreversible injuries into the stroma and endothelium, while low concentrations, such as those used in product formulations (e.g. to maintain a neutral pH), may have very little measurable impact on the cornea [Citation93]. In fact, since the depth and degree of corneal injuries are progressively dependent upon dose, concentration and duration of exposure, the recoverable and irreversible apical events are part of the spectrum of ocular injury responses.
Reversibility of effects
It is essential to consider reversibility when assessing the potential effects of chemicals on the eye. As presented above, the potential for reversibility of damage can occur in a dose-dependent manner. Chemicals can trigger anti-inflammatory processes and repair mechanisms, such as recruitment of phagocytes, which work to repair cellular and tissue injury. Epithelial cells have the capacity to manage low-dose exposures to irritants – on a daily and repeated basis – without injury [Citation94]. Chemical doses above a certain threshold have the potential to overwhelm anti-inflammatory mediators and repair mechanisms, demonstrating a delicate balance between inflammatory and anti-inflammatory processes [Citation95]. Significant inflammation responses in the basal epithelium may trigger matrix proteases with subsequent loss or sloughing of epithelial tissues [Citation96,Citation97]. As a general rule, corneal and conjunctival injuries limited to the epithelial tissues are likely reversible and recoverable, while deeper injuries, especially those into the basal epithelial cells and stroma, are less likely recoverable in a meaningful timeframe.
Corneal injuries that are limited to the superficial conjunctival or corneal squamous epithelium, as well as the wing cell layer of the epithelium (through the squamous epithelium and into the wing cells of the corneal epithelium), typically recover by upward cell displacement and differentiation to re-establish the squamous epithelium. Deeper corneal injuries into the basal cell layer of the epithelium may take longer for meaningful recovery, particularly when the epithelial cell sheet is lost. In such cases of epithelial cell sheet loss, the lower epithelium recovers by both horizontal epithelial sheet migration from the outer limbal tissues, and subsequent upward cell displacement and differentiation to re-establish the epithelium. Where the corneal injuries are extensive and affect the limbal tissues, recovery may be less likely in a meaningful timeframe. Although epithelial tissue injuries are generally recoverable for certain chemistries, irreversible phenotypic changes to the basal cells, or irreversible changes to the anterior limiting lamina or basal lamina, may result in incomplete recovery, or recurrent epithelial loss and erosions. An example of the latter was observed in soldiers during World War I after battlefield exposures to the chemical warfare agent, sulphur mustard, when after initial recovery, late recurrent ulcerative episodes of the cornea were reported, presumably as a result of cross-linking and denaturation of collagen [Citation98].
Damage into the stroma typically involves necrotic or apoptotic loss of keratocytes, and, depending both upon the nature and mode of chemical action and the depth and degree of stromal injury, may define whether the injury is reversible or non-reversible. Cytolytic injuries to keratocytes result in release of IL-1α, initiating recruit of inflammatory neutrophils from the limbal vasculature [Citation99]. The degree of the inflammatory response is thought to be related to the number of damaged keratocytes. Stromal opacity due to loss of keratocytes limited to the upper stroma may be reversible, particularly if the extent or frequency of keratocyte loss is minimal. Shortly after cell death, keratocytes are replaced by new keratocytes through mitosis of adjacent cells [Citation100]. Recovery is expected to take considerable time for re-establishment of the basal membrane substrate, epithelial tissue recovery, and re-establishment by keratocytes of a normal stromal collagen environment. Damage deeper into the stroma with pervasive loss of keratocytes would overwhelmingly be irreversible as significant risks of abnormal collagen deposition and fibrosis, as well as pannus and neovascularization occur with increasing loss of keratocytes, and concomitant inflammatory responses.
Damage and/or loss of the endothelial layer would almost always be associated with chemical injury superficially in the stroma and epithelium. Unlike in rabbits, the loss of endothelium in humans is not recoverable, and areas showing loss of endothelial function will result in local or diffuse stromal swelling and concomitant opacities.
The speed of recovery differs between rabbits and humans, with effects seen in rabbits more prominent than those observed in humans at comparable dose volumes [Citation49,Citation101,Citation102]. Additionally, while substances are often quickly removed by the human blinking and tearing responses [Citation1], instillation of the test substance directly into the conjunctival sac of the rabbit per the Organization for Economic Co-Operation and Development (OECD) TG contributes to the exaggerated response seen in the rabbit.
Mechanism of eye irritation
Chemicals can damage cells through multiple modes of action, including lysis of the cell membrane; promotion of coagulation or denaturation of macromolecules; solubilization of cell macromolecules; promotion of saponification of lipids; and/or covalent interaction with macromolecules [Citation103–105]. Different in vitro and ex vivo models can be used to assess these various modes of action.
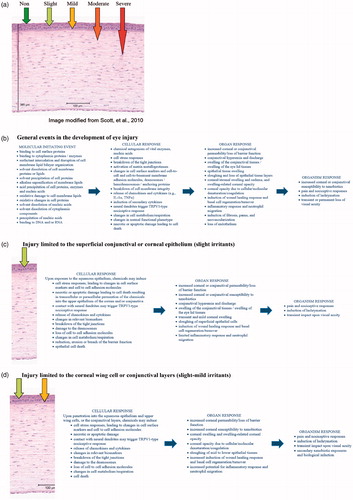
Adverse outcomes in the eye become progressively more severe with greater depth of chemical penetration and degree of toxic effects induced within each tissue layer. The adverse outcome tends to be cumulative from the initial chemical contact with the superficial outer epithelium to deep into the underlying subconjunctival scleral/tarsal tissues or corneal stromal tissues. Accordingly, the overall adverse outcomes at the organ level tend to be dictated by the prominent injuries at the deepest tissue levels. Because the adverse outcome of a toxic exposure may differ in different tissues of the eye, one can envision individual pathways outlining eye effects for each of the major corneal and conjunctival tissue layers ().
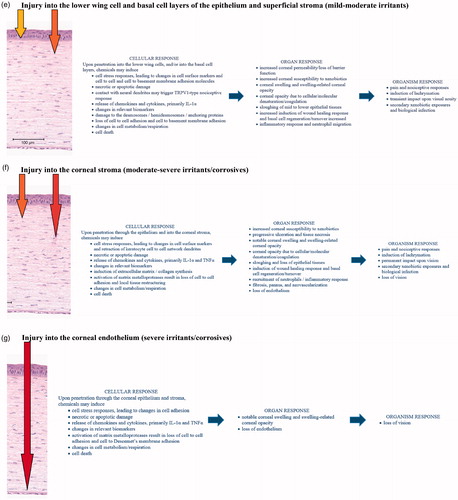
Figure 2. Mechanism of eye irritation. (a) Depth of Injury Model modified from Scott et al. [Citation105]. The depth of injury is predictive of the degree and duration of injury. (b) Shows general events that may occur in the development of eye injury, while (c) through (g) show events relevant to specific parts of the eye. Listings are intended to comprehensively present the numerous potential responses and pathways of eye injury, rather than represent linear mappings. (c) Injury limited to the superficial conjunctival or corneal squamous epithelium. This limited injury is typically reversible, and recovers by upward cell displacement and differentiation to re-establish the squamous epithelium. (d) Injury limited to the wing cell layer of the epithelium (through the squamous epithelium and into the upper wing cells of the corneal epithelium). This limited injury is typically reversible, and recovers by upward cell displacement and differentiation to re-establish the epithelium. (e) Injury into the lower wing cell and basal cell layers of the epithelium (through the corneal epithelium into the lower wing cell and basal cell layers). This progressive injury is often reversible, but may take longer for meaningful recovery. Lower epithelium recovers by both horizontal epithelial sheet migration and upward cell displacement and differentiation to re-establish the epithelium. For some chemistries, irreversible changes to the anterior limiting lamina or basal lamina, or irreversible phenotypic changes to the basal cells may result in incomplete recovery, or recurrent epithelial loss and erosions. (f) Damage into the corneal stroma (through the corneal epithelium into the corneal stroma). Damage and loss of keratocytes in the stroma often results in moderate to severe ocular irritation, depending upon the depth and degree of stromal injury. While a moderate irritant may cause cellular damage and keratocyte loss limited to the upper stroma that could be reversible, a severe irritant (corrosive) would cause damage deeper into the stroma with pervasive loss of keratocytes that is irreversible. Recovery is expected to take considerable time for re-establishment of the basal membrane substrate, epithelial tissue recovery, and re-establishment by keratocytes of a normal stromal collagen environment. Significant risks of abnormal collagen deposition and fibrosis, as well as pannus and neovascularization occur with notable stromal damage. (g) Damage into the corneal endothelium. Damage and/or loss of the endothelial layer would almost always be associated with chemical damage superficially in the stroma and epithelium. The loss of endothelium in humans is not recoverable, and areas showing loss of endothelial function will result in local or diffuse stromal swelling and concomitant opacities.
Test methods
In addition to the in vivo rabbit eye test developed in 1944, multiple in vitro and ex vivo methods have been adopted by the OECD, including the reconstructed human cornea-like epithelium (RhCE) test system, the bovine corneal opacity and permeability (BCOP) test method, the isolated chicken eye (ICE) test method, the fluorescein leakage (FL) test method, the short-time exposure (STE) test method, the vitrigel-eye irritancy (EIT) test method, and the ocular irritection® macromolecular test method. There are also several methods that are not yet OECD TGs but are being used to assess ocular effects, including the isolated rabbit eye test method, neutral red release assay (NRR), OptiSafe, porcine cornea opacity/reversibility assay (PorCORA), EYEIRR-IS®, the ex vivo eye irritation (EVEIT) test, and cytosensor microphysiometer assay. A comprehensive list of available methods and a summary of their relevance to humans can be found in .
All of the test methods presented in include (i) a test system or platform (the organism, tissues, cells, or biomolecular matrix to which test material is applied, and from which the relevant endpoints are determined), (ii) a dosing and exposure protocol, and (iii) the mechanistically based endpoint methods. In the development of many of the in vitro and ex vivo eye irritation tests, dose and exposure protocols were optimized to their respective test systems so that the range and magnitude of the endpoint response values better fit the rabbit values or rabbit-based irritation categories. Since each of the test systems differ in structure and sensitivity, the dose volumes and exposure times differ accordingly, and do not typically reflect the rabbit in vivo exposure regimen (nor necessarily model a human exposure). For example, the STE and NRR assays utilize short, 5 min exposures that are intended to model splash events and they are of sufficiently short duration to allow for a range of responses to be expressed in the sensitive cell monolayer, but they do not directly reflect any specific in vivo exposure times. Furthermore, several in vitro and ex vivo eye irritation tests require specific protocols for testing solid materials that differ from the protocols for testing liquid materials by notably increasing exposure times or doses to better reflect the rabbit data for solid materials (arguably as a consequence of abrasive injuries in the rabbit test that are inherent with solid materials, rather than solely responding to the chemical effects). Importantly, one can exercise precise control of the application and termination of the dose volumes and the duration of exposures to in vitro and ex vivo test systems, thereby greatly reducing a source of variability in the test results. Conversely, in the rabbit test, spilling and leakage of the applied dose from the cul-de-sac within seconds after application undermines reproducible, quantifiable measures of exposure and associated effects.
Given their complexity and composition, agrochemical formulations, which often include some combination of wetting agents, dispersants, solvents, and adjuvants, may fall outside of the applicability domains of some test methods [Citation8]. For example, those test methods that require preparing a dilution series in an aqueous vehicle may not be suitable for testing mixtures that are not uniformly in solution, or for testing immiscible liquids or solids that are not water-soluble. Consequently, in such cases, the bioavailability of the insoluble components is called into question, and accordingly, other test methods that do not rely upon preparation of dilutions should be used.
Some methods have been incorporated into an EPA alternate testing framework for classification of eye irritation potential of EPA pesticide products that can be used for antimicrobial cleaning products and, on a case-by-case basis, for other classes of pesticides and pesticide products, including conventional, biochemical, and other antimicrobial pesticides without cleaning claims [Citation32]. The methods are also included in an OECD guidance document on an integrated approach on testing and assessment (IATA) for serious eye damage and eye irritation that describes how physical chemical properties and existing or generated data can be integrated and used for decision making [Citation1].
Discussion
Any of the in vitro and ex vivo methods identified in as applicable to a bottom-up approach have been shown to be useful for identifying minimal or non-irritants, provided that those ingredients and formulations are compatible with the test systems (). Considering the structure of the human eye and the mechanistic pathways leading to chemically induced adverse effects in the eye, 3D reconstructed human corneal tissues and ex vivo corneal tissues (e.g. BCOP and ICE) are particularly useful models for evaluating the spectrum of eye irritation in humans. These models have the ability to most closely recapitulate certain key events in the pathways to eye irritation at the cellular (3D reconstructed human corneal tissues and ex vivo corneal tissues) and organ levels (ex vivo corneal tissues). These systems also offer the advantage of precise and well-controlled exposure protocols that increase the reliability and reproducibility of the results.
Figure 3. Schematic of a human corneal section showing which in vitro/ex vivo assays are appropriate for evaluating specific layers, with models relevant to the (a) corneal epithelium or (b) full thickness cornea.
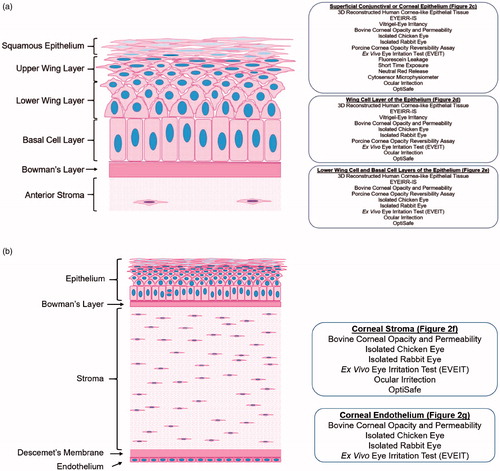
3D reconstructed human corneal tissues and ex vivo corneal tissues model the exposure and permeation kinetics of chemicals from the sites of exposure at the outermost squamous epithelium deeper into the corneal model. Only full thickness models that include the different barrier properties from corneal epithelium to endothelium fully model the potential penetration kinetics of a chemical.
The 3D reconstructed human corneal tissue models are particularly appealing because they measure a critical event in the pathway to irritation – cytotoxicity – within a stratified construct of cells from the species of interest. These tissue models can also be used to determine chemical-induced cytokine release and expression, providing further relevance to the cascade of events associated with eye irritation. Since the key adverse events typically associated with mild to moderate irritants occur within the epithelial layers, they can be used to distinguish between irritants and non-irritants and, because of the stratified epithelial construct, can provide some information about DOI within the corneal epithelium. The addition of a stromal layer would allow for further discrimination between mild and moderate irritants through DOI measurements, and more importantly provide discrimination between mild or moderate irritants and severe irritants (corrosives). Future consideration could be given to the addition of an organized stromal matrix containing stromal cells and a functional endothelium, which would further enhance the relevance of these 3D reconstructed human tissue models, allowing them to thoroughly provide corneal DOI information to better predict reversibility. Lastly, having the ability to evaluate the degree of cytotoxicity, as well as other initial key events such as cytokine release, will improve the ability to predict the severity of corneal injuries and the likelihood for meaningful recovery. Importantly, however, adapted protocols using RhCE models without a stromal or endothelial layer are currently under review at the OECD and may provide sufficient information to discriminate between severe irritants (corrosives) and moderate irritants.
Ex vivo corneal tissues provide a full thickness model (i.e. epithelial, stromal, and endothelial layers) and, therefore, when including endpoints such as histopathology can provide detailed information about DOI. Since the key adverse events typically associated with moderate to severe eye irritants (corrosives) occur within the stroma, these full thickness models uniquely provide the specific cells and structures of the stroma to model chemical permeation to the target cells, and with the inclusion of histopathology, they provide a mechanistic basis for differentiating between types of recoverable and irreversible corneal injuries. Even though there may be interspecies differences due to the use of non-human cells, the relevance of these non-human ex vivo models is justified in most cases where common chemical mechanisms of action inducing cell cytotoxic responses are observed across species.
Consistent with the OECD IATA, combining test results with information specific to the test substance (e.g. physical chemical properties) can be used to better address the range of key events that distinguish severe (corrosive), moderate, mild, or non-irritants, and to inform on the likelihood of recovery in humans. Reversibility predictions may be supplemented by utilizing in silico tools to identify those chemistries (such as protein denaturing/precipitating chemistries, reactive chemistries, alkylating agents) likely to induce irreversible phenotypic changes to the basal or limbal cells, or induce irreversible changes to the Bowman’s layer or basal lamina, without necessarily involving the stromal or endothelial elements. Furthermore, consideration of information from other endpoints can inform toxicity predictions; for example, knowing that a chemical is highly irritating to the skin or respiratory tract as a result of a cytotoxic mechanism may suggest that it is likely irritating to the eye as well. Results from multiple test methods may be combined – while conducting multiple tests instead of a single in vivo rabbit eye irritation test may be more costly in the short-term, they should produce more human-relevant results (and therefore avoid costs that would be associated with having to pull a harmful product from the market, or loss of market share due to irrelevant over-predictions) and the cost of these assays may decrease over time as they are more widely implemented. In addition, while in silico assessment of mixtures proves challenging, partnerships with industry could be leveraged to provide data for Quantitative Structure Activity Relationship (QSAR) model building for agrochemical formulations.
Various methods, alone or in combination, may be appropriate for use, depending on the circumstances; therefore, scientific justification for using a certain approach should be communicated to the regulatory agency upon submission (). Thus, there is not a single proposed testing strategy, rather various approaches may be acceptable when based on the properties of the test substance, the purpose of testing, existing data, and human-relevant in vitro/ex vivo test methods.
Figure 4. Example scenarios. There are many considerations and variables that drive the “best” test methods and testing strategy selected for each scenario. For example, the purpose of testing must be considered e.g. hazard versus risk evaluations, worker versus consumer safety, regulatory testing requirement versus product development. In addition, the type of chemical must be considered e.g. neat chemicals versus mixtures of chemicals, as well as their natures, formulations, and expected irritation levels. Practical factors must also be considered e.g. budget, timing, method availability, availability of test material-specific reference data per method, and historical data. To derive the most appropriate testing plan, a researcher should work internally or with their contract research organization to apply the best science and develop appropriate approaches. Based on integration and analysis of the information provided in this paper, the following general scenarios describe possible approaches for identifying an agrochemical formulation’s potential to cause eye irritation. GHS: Globally Harmonized System; SC: suspension concentrate; SL: soluble concentrate; EC: emulsifiable concentrate; 3D: three-dimensional; 2D: two-dimensional. GHS mixtures equation [Citation106].
![Figure 4. Example scenarios. There are many considerations and variables that drive the “best” test methods and testing strategy selected for each scenario. For example, the purpose of testing must be considered e.g. hazard versus risk evaluations, worker versus consumer safety, regulatory testing requirement versus product development. In addition, the type of chemical must be considered e.g. neat chemicals versus mixtures of chemicals, as well as their natures, formulations, and expected irritation levels. Practical factors must also be considered e.g. budget, timing, method availability, availability of test material-specific reference data per method, and historical data. To derive the most appropriate testing plan, a researcher should work internally or with their contract research organization to apply the best science and develop appropriate approaches. Based on integration and analysis of the information provided in this paper, the following general scenarios describe possible approaches for identifying an agrochemical formulation’s potential to cause eye irritation. GHS: Globally Harmonized System; SC: suspension concentrate; SL: soluble concentrate; EC: emulsifiable concentrate; 3D: three-dimensional; 2D: two-dimensional. GHS mixtures equation [Citation106].](/cms/asset/51db7c06-ca29-40b0-a95a-fa92db7b1fb3/icot_a_1910291_f0004_c.jpg)
Conclusions
A component of the EPA Administrator’s 2019 directive to staff to reduce the requests for and funding of animal studies by 2035 was the request to develop a work plan that addresses the subject of “validation to ensure that NAMs [new approach methods] are equivalent to or better than the animal tests replaced” [Citation107]. This work plan was released in June 2020 [Citation108]. Similar language is present in the amended Toxic Substances Control Act (TSCA) that requires that NAMs provide “information of equivalent or better scientific quality and relevance” than traditional animal tests. A key consideration in the evaluation of what it means to be “equal to or better” is the explicit consideration of the variability, human relevance, and predictivity of the animal data. This paper is designed to consider these factors for eye irritation.
The rabbit eye test is widely used to meet regulatory testing requirements for agrochemical formulations, and has been a requirement for pesticide registration for decades [Citation109]. However, the rabbit test involves subjective examination of ocular lesions, uses an exaggerated exposure duration, provides limited mechanistic information, has never been validated for its relevance to humans, uses live animals, and is associated with considerable variability (see for details). Furthermore, interspecies differences in structure, anatomy, and physiology exist between rabbit and human eyes, for example, rabbits have a nictitating membrane, higher pH of the eye, a larger conjunctival cul-de-sac, and are not as efficient in tear production. Thus, the rabbit test is not appropriate for use as a standard for evaluating new methods.
The in vitro and ex vivo models described herein are more human relevant than the rabbit test because they include one or more of the following properties: (a) they allow for more precise control of the application and termination of dosing, (b) they model corneal tissue barrier functions and penetration kinetics, (c) they include relevant cell types within each of the tissue layers, (d) they provide quantitative results, (e) they have been shown to be reproducible and repeatable, (f) they do not directly use live animals for testing, and/or (g) they discriminate a range of cytotoxic responses within each layer. It is not necessary for a test system to include all of these traits to be useful and relevant; a model with any one of these characteristics could be useful to address specific events in the assessment of eye irritation. Considering all available information, the in vitro/ex vivo methods presented in this paper are equivalent or scientifically superior to the use of the rabbit test.
Where discordant results exist between NAMs and the rabbit test, findings from the in vitro and ex vivo systems described herein should carry more weight than the rabbit data. The scientific validity of an in vitro/ex vivo method should be assessed by understanding the assay’s relevance to human biology and mechanisms of eye irritation. Ultimately, a replacement method that provides a model grounded in human biology will be as good as or better than the currently used rabbit test at protecting human health.
Next steps
A careful consideration of the benefits and the limitations of in vitro and ex vivo eye tests show that they can be used today to make human-relevant regulatory decisions. There are also steps that could be taken to further improve existing models, including:
Improve existing 3D reconstructed human corneal models to create a full thickness corneal model, complete with stromal and endothelial cells. Such a human cell-based method that recapitulates the distinct layers of the human cornea and provides information on the DOI following chemical exposure could be effective at distinguishing moderate and severe irritants (corrosives) and predicting reversibility [Citation110]. Since cell cytotoxicity is an early key event in eye irritation, and related to downstream consequences depending upon the tissue layers affected, the model should be able to assess cytotoxic effects throughout the full thickness structure [Citation111]. Furthermore, advanced models of a blinking human eye may be more sophisticated than needed to predict irritancy, but could provide useful information and incorporate effects of mechanical injury resulting from blinking that are not captured in other in vitro tests [Citation112]. Additional investigations could also include use of optical coherence tomography to assess full thickness injury in a non-invasive way, as is done in the EVEIT test.
Move away from high dose, longer chemical exposures in favour of more human-relevant risk-based exposure scenarios. This will better align test exposures with the common human scenario, where a substance is mostly expelled from the eye in a matter of minutes following exposure. Furthermore, multiple exposure scenarios can be more easily tested in in vitro and ex vivo systems, allowing for the assessment of a splash scenario followed by immediate rinsing as well as an intermittent exposure over a full workday.
Establish an acceptable level of variability for assays. Despite the recognized variability of the rabbit test [Citation18], particularly in the mild and moderate irritancy range, it has been considered sufficient for regulatory use and thus an acceptable level of confidence in such results has been conveyed. The level of confidence/variability deemed acceptable based on historical use of the rabbit test, in combination with the information about human biology and mechanisms described in this paper, can be used to evaluate the validity of a method. Similar analyses have been proposed to define acceptable performance of NAMs for other endpoints [Citation113–115]. Ultimately, this permits the level of variability for a new method to be held to a similar standard as that for the animal test.
Re-evaluate existing data considering the conclusions and recommendations in this paper. The US National Toxicology Program (NTP) Interagency Centre for the Evaluation of Alternative Toxicological Methods (NICEATM), EPA, the PETA Science Consortium International e.V., and a group of agrochemical companies have collaborated to conduct both retrospective and prospective assessments of available in vitro and ex vivo methods to determine their usefulness and limitations for eye irritation testing of agrochemical formulations. The prospective testing results and associated analyses have been presented [Citation13]. These results provide an opportunity to consider how best to apply these methods in regulatory decision making based on the discussions in this paper. Accordingly, additional analyses are ongoing that are intended to develop a framework for doing so.
Generate additional in vitro/ex vivo data where needed for agrochemical formulations. While there are substantial existing data for many of the in vitro and ex vivo assays (e.g. BCOP, ICE, NRR, and EpiOcular™), there are assays for which there remain limited data for agrochemical formulations. In particular, because of its use of a 3D human tissue model, additional information about the applicability of the EYEIRR-IS® assay to agrochemical formulations would be useful. In addition, since agrochemical formulations often target very specific or unique cellular processes, the genomic-based platform might reveal unique adverse outcome pathways that other test methods do not address. Transparency in the model and its associated decision criteria are essential for regulatory utility. Importantly, any future or existing in vitro/ex vivo data need to be vetted for their relevance to human biology and not to direct alignment with hazard categories derived based on rabbit data.
The above steps reiterate that opportunities to further refine existing in vitro and ex vivo models can be explored while they are being used today for decision-making. Overall, considering the variability of the currently used rabbit test and an understanding of human biology and mechanisms of eye irritation presented in this paper, to best protect human health, data from the in vitro/ex vivo methods are considered applicable for use at this time.
Disclaimer
The views expressed in this paper are those of the authors and do not necessarily represent the views or the policies of the US Environmental Protection Agency or specifically of EPA’s Office of Pesticide Programs
Acknowledgements
The authors thank Elyssa Arnold, Niladri Bhowmik, Yadvinder Bhuller, Patricia Bishop, Warren Casey, Evisabel Craig, Shelley DuTeaux, Tara Flint, Sean Gehen, Angela Gonzales, Monique Inforzato, Thomas McDonald, Edward Odenkirchen, Lindsay O’Dell, Kathryn Page, Sylvia Pelgrom, Deborah Ramsingh, Clive Roper, Natalia Ryan, Steven Snyderman, Kristie Sullivan, Neelima Verma, Elizabeth Webb, Walter Westerink, Doug Wolf, Lisa Woods, and Krystle Yozzo for their input in discussions and/or review of this paper.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Joint Meeting of the Chemicals Committee and the Working Group on Chemicals: Pesticides and Biotechnology. Guidance document on an integrated approach on testing and assessment (IATA) for serious eye damage and eye irritation series on testing assessment no. 263. Paris (France): Organisation for Economic Co-operation and Development; 2017.
- Worakul N, Robinson JR. Ocular pharmacokinetics/pharmacodynamics. Eur J Pharm Biopharm 1997;44:71–83.
- McGhee CNJ. An overview of topical ophthalmic drugs and the therapeutics of ocular infection. Ocul Ther 2008;118:1862–1867.
- US Environmental Protection Agency. Health effects test guidelines OPPTS 870.2400 acute eye irritation. Washington (DC): US Environmental Protection Agency; 1998.
- Organisation for Economic Co-operation and Development. Test no. 405: acute eye irritation/corrosion. Paris (France): OECD Publishing; 2017.
- US Environmental Protection Agency. Efforts to reduce animal testing at EPA [Internet]. Washington (DC): US Environmental Protection Agency; 2019 [cited 2020 Mar 23]. Available from: https://www.epa.gov/research/efforts-reduce-animal-testing-epa
- Kolle SN, Moreno MCR, Mayer W, et al. The EpiOcular™ Eye Irritation Test is the method of choice for the in vitro eye irritation testing of agrochemical formulations: correlation analysis of EpiOcular Eye Irritation Test and BCOP Test Data according to the UN GHS, US EPA and Brazil ANVISA classification schemes. Altern Lab Anim 2015;43:181–198.
- Kolle SN, Van Cott A, van Ravenzwaay B, et al. Lacking applicability of in vitro eye irritation methods to identify seriously eye irritating agrochemical formulations: results of bovine cornea opacity and permeability assay, isolated chicken eye test and the EpiOcular™ ET-50 method to classify according to UN GHS. Regul Toxicol Pharmacol 2017;85:33–47.
- Settivari RS, Amado RA, Corvaro M, et al. Tiered application of the neutral red release and EpiOcular™ assays for evaluating the eye irritation potential of agrochemical formulations. Regul Toxicol Pharmacol 2016;81:407–420.
- Choksi N, Daniel A, Lebrun S, et al. Performance of the OptiSafe ocular irritation assay in a three-laboratory validation study. Research Triangle Park (NC): National Toxicology Program; 2018.
- Office of Prevention Pesticides and Toxic Substances. 870.2400 Acute Eye Irritation. Washington (DC): US Environmental Protection Agency; 1998.
- Draize JH, Woodard G, Calvery HO. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exp Ther 1944;82:377–390.
- Choksi N, Clippinger AJ, Gehen S, et al. Developing a defined approach for eye irritation testing. SOT Abstr Number/Poster Board Number 2198/P556 [Internet]. 2020. Available from: https://www.piscltd.org.uk/wp-content/uploads/2020/03/SOT2020_Choksi-Clippinger-et-al.pdf
- Calabrese EJ. Principles of animal extrapolation. Boca Raton (FL): Taylor & Francis; 1991.
- Larry AM, Nemiroff B, John LU, et al. Role of the rabbit nictitating membrane in ocular irritancy testing. Cutan Ocul Toxicol 1987;6:43–56.
- Carpenter CP, Smyth HF. Chemical burns of the rabbit cornea. Am J Ophthalmol 1946;29:1363–1372.
- Huhtala A, Salminen L, Tähti H, et al. Corneal models for the toxicity testing of drugs and drug releasing materials. In: Ashammakhi N, ed. Topics in multifunctional biomaterials and devices. 2008. Available from: www.oulu.fi
- Luechtefeld T, Maertens A, Russo DP, et al. Analysis of draize eye irritation testing and its prediction by mining publicly available 2008–2014 REACH data. Altern Anim Exp 2016;33:123–134.
- Weil CS, Scala RA. Study of intra- and interlaboratory variability in the results of rabbit eye and skin irritation tests. Toxicol Appl Pharmacol 1971;19:276–360.
- Griffith JF. Freeberg Fe. Empirical and experimental bases for selecting the low volume eye irritation test as the validation standard for in vitro methods. In: Goldberg AM, ed. In Vitro Toxicology: Approaches to Validation. 1st ed. New York (NY): Mary Ann Libert; 1987:303–311.
- Adriaens E, Barroso J, Eskes C, et al. Retrospective analysis of the Draize test for serious eye damage/eye irritation: importance of understanding the in vivo endpoints under un GHS/EU CLP for the development and evaluation of in vitro test methods. Arch Toxicol 2014;88:701–723.
- Barroso J, Pfannenbecker U, Adriaens E, et al. Cosmetics Europe compilation of historical serious eye damage/eye irritation in vivo data analysed by drivers of classification to support the selection of chemicals for development and evaluation of alternative methods/strategies: the Draize eye test Reference Database (DRD). Arch Toxicol 2017;91:521–547.
- Clelatt KN. Textbook of veterinary ophthalmology. Philadelphia (PA): Lea & Febiger; 1981.
- Prince JH, Diesem CD, Eglitis I, et al. Anatomy and histology of the eye and orbit in domestic animals. 3rd ed. Springfield (IL): Charles C. Thomas; 1960.
- Swanston DW, Eye irritancy testing. In: Balls M, Riddell RJ, Worden AN, et al., eds. Animals and alternatives in toxicity testing. New York (NY): Academic Press; 1983:337–367.
- Buehler EV, Newmann EA. A comparison of eye irritation in monkeys and rabbits. Toxicol Appl Pharmacol 1964;6:701–710.
- Organisation for Economic Co-operation and Development. Test no. 492: reconstructed human cornea-like epithelium (RhCE) test method for identifying chemicals not requiring classification and labelling for eye irritation or serious eye damage. Paris (France): OECD Publishing; 2015.
- EURL ECVAM Science Advisory Committee. ESAC opinion on the EURL ECVAM eye irritation validation study (EIVS) on EpiOcularTM EIT and SkinEthicTM HCE. Ispra (Italy): European Comission; 2014.
- Van Rompay AR, Alépée N, Nardelli L, et al. CON4EI: SkinEthic™ Human Corneal Epithelium Eye Irritation Test (SkinEthic™ HCE EIT) for hazard identification and labelling of eye irritating chemicals. Toxicol in Vitro 2018;49:11–20.
- LabCyte Validation Management Team. Me-too validation report: validation study for LabCyte Cornea-Model24 eye irritation test. Kawasaki (Japan): Japanese Center for the Validation of Alternative Methods; 2017.
- Joint Meeting of the Chemicals Committee and the Working Group on Chemicals: Pesticides and Biotechnology. Report of the validation study of the MCTT human corneal-like epithelium eye irritation test model and report of the validation of the peer review. Paris (France): Organisation for Economic Co-operation and Development; 2019.
- Office of Pesticide Programs. Use of an alternative testing framework for classification of eye irritation potential of EPA pesticide products. Washington (DC): US Environmental Protection Agency; 2015.
- Kandarova H, Letasiova S, Adriaens E, et al. CON4EI: CONsortium for in vitro eye irritation testing strategy – EpiOcular™ time-to-toxicity (EpiOcular ET-50) protocols for hazard identification and labelling of eye irritating chemicals. Toxicol in Vitro 2018;49:34–52.
- Alépée N, Leblanc V, Grandidier MH, et al. Development of the SkinEthic HCE Time-to-Toxicity test method for identifying liquid chemicals not requiring classification and labelling and liquids inducing serious eye damage and eye irritation. Toxicol In Vitro 2020;69:104960.
- Cottrez F, Leblanc V, Groux H, et al. The EyeIRR-IS assay: development of an in vitro method using SkinEthic HCE model for liquid chemical eye irritation sub-categorization Protocol of the EYE IRR-IS assay for liquid products. Soc Toxicol 2019; 71:105072.
- Groux H, Cottrez F, Alepee N, et al. Method for evaluating the eye irritation potential of chemicals. Immunosearch, assignee. United States patent US 2019 300 952A1; 2019.
- Cottrez F, Leblanc V, Boitel E, et al. The EyeIRR-IS assay: development and evaluation of an in vitro assay to measure the eye irritation sub-categorization of liquid chemicals. Toxicol In Vitro 2021;71:105072.
- Organisation for Economic Co-operation and Development. Test no. 494: Vitrigel-eye irritancy test method. Paris (France): OECD Publishing; 2019.
- Srinivasan B, Kolli AR, Esch MB, et al. TEER measurement techniques for in vitro barrier model systems. J Lab Autom 2015;20:107–126.
- Organisation for Economic Co-operation and Development. Test no. 437: Bovine corneal opacity and permeability test method. Paris (France): OECD Publishing; 2017.
- Balls M, Botham PA, Bruner LH, et al. The EC/HO international validation study on alternatives to the draize eye irritation test. Toxicol Vitr 1995;9:871–929.
- Gautheron P, Giroux J, Cottin M, et al. Interlaboratory assessment of the bovine corneal opacity and permeability (BCOP) assay. Toxicol Vitr 1994;8:381–392.
- Southee J. Evaluation of the prevalidation process. Part 2, final report. Vol. 2. The bovine corneal opacity and permeability (BCOP) assay. Stirling (Scotland); 1998.
- Interagency Coordinating Committee on the Validation of Alternative Methods. Current status of in vitro test methods for identifying ocular corrosives and severe irritants: Bovine corneal opacity and permeability test method. Research Triangle Park (NC): National Institute of Environmental Health Sciences; 2006.
- Maurer JK, Parker RD, Jester JV. Extent of initial corneal injury as the mechanistic basis for ocular irritation: key findings and recommendations for the development of alternative assays. Regul Toxicol Pharmacol 2002;36:106–117.
- Furukawa M, Sakakibara T, Itoh K, et al. Histopathological evaluation of the ocular-irritation potential of shampoos, make-up removers and cleansing foams in the bovine corneal opacity and permeability assay. J Toxicol Pathol 2015;28:243–248.
- Organisation for Economic Co-operation and Development. Test no. 438: isolated chicken eye test method for identifying i) chemicals inducing serious eye damage and ii) chemicals not requiring classification for eye irritation or serious eye damage. Paris (France): OECD Publishing; 2018.
- Interagency Coordinating Committee on the Validation of Alternative Methods. ICCVAM test method evaluation report: current validation status of in vitro test methods proposed for identifying eye injury hazard potential of chemicals and products. Research Triangle Park (NC): National Toxicology Program; 2010.
- Roggeband R, York M, Pericoi M, et al. Eye irritation responses in rabbit and man after single applications of equal volumes of undiluted model liquid detergent products. Food Chem Toxicol 2000;38:727–734.
- Cazelle E, Eskes C, Hermann M, et al. Suitability of histopathology as an additional endpoint to the isolated chicken eye test for classification of non-extreme pH detergent and cleaning products. Toxicol In Vitro 2014;28:657–666.
- Burton ABG, York M, Lawrence RS. The in vitro assessment of severe eye irritants. Food Cosmet Toxicol 1981;19:471–480.
- Earl L. The rabbit enucleated eye test. Invit Protoc 1994;85:33.
- Cooper KJ, Earl LK, Harbell J, et al. Prediction of ocular irritancy of prototype shampoo formulations by the isolated rabbit eye (IRE) test and bovine corneal opacity and permeability (BCOP) assay. Toxicol In Vitro 2001;15:95–103.
- Whittle E, Basketter D, York M, et al. Findings of an interlaboratory trial of the enucleated eye method as an alternative eye irritation test. Toxicol Mech Methods 1992;2:30–41.
- York M, Wilson AP, Newsome CS. The classification of soluble silicates for eye hazard using the enucleated rabbit eye test. Toxicol In Vitro 1994;8:1265–1268.
- Piehl M, Gilotti A, Donovan A, et al. Novel cultured porcine corneal irritancy assay with reversibility endpoint. Toxicol In Vitro 2010;24:231–239.
- Piehl M, Carathers M, Soda R, et al. Porcine corneal ocular reversibility assay (PorCORA) predicts ocular damage and recovery for global regulatory agency hazard categories. Toxicol In Vitro 2011;25:1912–1918.
- Spöler F, Kray O, Kray S, et al. The ex vivo eye irritation test as an alternative test method for serious eye damage/eye irritation. Altern Lab Anim 2015;43:163–179.
- Organisation for Economic Co-operation and Development. Test no. 460: fluorescein leakage test method for identifying ocular corrosives and severe irritants. Paris (France): OECD Publishing; 2017.
- ECVAM Scientific Advisory Committee. Peer review panel report on the retrospective validation of the cytotoxicity/cell-function based in vitro assays (eye irritation). Ispra (Italy): European Commission; 2009.
- Organisation for Economic Co-operation and Development. Test no. 491: short time exposure in vitro test method. Paris (France): OECD Publishing; 2018.
- Takahashi Y, Koike M, Honda H, et al. Development of the short time exposure (STE) test: an in vitro eye irritation test using SIRC cells. Toxicol in Vitro 2008;22:760–770.
- Interagency Coordinating Committee on the Validation of Alternative Methods, National Toxicology Program (NTP) Interagency Center for the Evaluation of Alternative Toxicological Methods, National Institutes of Health, et al. Short time exposure (STE) test method summary review document. Research Triangle Park (NC): National Toxicology Program; 2013.
- Reader SJ, Blackwell V, O’Hara R. A vital dye release method for assessing the short-term cytotoxic effects of chemicals and formulations. Altern Lab Anim 1989;28–33.
- Reader SJ, Blackwell V, O’Hara R, et al. Neutral red release from pre-loaded cells as an in vitro approach to testing for eye irritancy potential. Toxicol In Vitro 1990;4:264–266.
- Zuang V. The neutral red release assay: a review. Altern Lab Anim 2001;29:575–599.
- Organisation for Economic Co-operation and Development. Draft OECD guidance for the testing of chemicals: the cytosensor microphysiometer test method: an in vitro method for identifying ocular corrosive and severe irritant chemicals as well as chemicals not classified as ocular irritants. Paris (France): OECD Publishing; 2012.
- Organisation for Economic Co-operation and Development. Test no. 496: in vitro macromolecular test method for identifying chemicals inducing serious eye damage and chemicals not requiring classification for eye irritation or serious eye damage. Paris (France): OECD Publishing; 2019.
- Eskes C, Hoffmann S, Facchini D, et al. Validation study on the Ocular Irritection assay for eye irritation testing. Toxicol In Vitro 2014;28:1046–1065.
- ECVAM Science Advisory Committee. ESAC opinion on the Ocular Irritection® test method for prediction of serious eye damage/eye irritation potential of chemicals. Ispra (Italy): European Comission; 2016.
- Organisation for Economic Co-operation and Development. Draft Performance Standards: In vitro macromolecular test method for ocular hazards [Internet]. Paris (France): OECD Publishing; 2018.
- The OptiSafe Test Method [Internet]. Anaheim (CA). Lebrun Labs LLC. [cited 2020 Apr 21]. Available from: http://www.optimethod.com
- Choksi N, Lebrun S, Nguyen M, et al. Validation of the OptiSafeTM eye irritation test. Cutan Ocul Toxicol 2020;39:180–192.
- Institute for In Vitro Sciences Inc. Guidelines for histopathological evaluation of bovine corneas as an endpoint of the Bovine Corneal Opacity and Permeability (BCOP) assay. Gaithersburg (MD); 2016.
- Leung DY, Lam DK, Yeung BY, et al. Comparison between central corneal thickness measurements by ultrasound pachymetry and optical coherence tomography. Clin Exp Ophthalmol 2006;34:751–754.
- Heichel J, Wilhelm F, Kunert KS, et al. Topographic findings of the porcine cornea. Med Hypothesis Discov Innov Ophthalmol J 2016;5:125–131.
- Li HF, Petroll WM, Møller-Pedersen T, et al. Epithelial and corneal thickness measurements by in vivo confocal microscopy through focusing (CMTF). Curr Eye Res 1997;16:214–221.
- Tognon T, Bergeron S, Mastromonaco C, et al. The use of digital microscopy to compare the thicknesses of normal Corneas and ex vivo rejected corneal grafts with a focus on the Descemet’s Membrane. J Ophthalmol 2019;2019:1–10.
- Wang X, Wu Q. Normal corneal thickness measurements in pigmented rabbits using spectral-domain anterior segment optical coherence tomography. Vet Ophthalmol 2013;16:130–134.
- Fowler WC, Chang DH, Roberts BC, et al. A new paradigm for corneal wound healing research: the white leghorn chicken (Gallus gallus domesticus). Curr Eye Res 2004;28:241–250.
- Gonçalves GC, Pérez-Merino P, Martínez-García MC, et al. Comparación de las características corneales en gallina y codorniz como modelos experimentales de cirugía refractiva. Arch Soc Esp Oftalmol 2016;91:310–315.
- Czarnoleski M, Labecka AM, Dragosz-Kluska D, et al. Concerted evolution of body mass and cell size: similar patterns among species of birds (Galliformes) and mammals (Rodentia). Biol Open 2018;7:bio029603.
- Wilson SE. Bowman’s layer in the cornea – structure and function and regeneration. Exp Eye Res 2020;195:108033.
- Martin CL, Andersen BG. Ocular anatomy. In: Gelatt KN, ed. Veterinary ophthalmology. 1st ed. Philadelphia (PA): Lea & Febiger; 1981.
- Mishima S. Clinical pharmacokinetics of the eye. Proctor lecture. Invest Ophthalmol Vis Sci 1981;21:504–541.
- Mishima S, Gasset A, Klyce SJ, et al. Determination of tear volume and tear flow. Invest Ophthalmol Vis Sci 1966;5:264–276.
- Gettings SD, Lordo RA, Hintze KL, et al. The CFTA evaluation of alternatives program: an evaluation of in vitro alternatives to the Draize primary eye irritation test. (Phase III) surfactant-based formulations. Food Chem Toxicol 1996;34:79–117.
- US Consumer Product Safety Commission. Definitions. 16 CFR 1500.3. 2015.
- Jester JV, Li L, Molai A, et al. Extent of initial corneal injury as a basis for alternative eye irritation tests. Toxicol In Vitro 2001;15:115–130.
- Jester JV, Li HF, Petroll WM, et al. Area and depth of surfactant-induced corneal injury correlates with cell death. Invest Ophthalmol Vis Sci 1998;39:922–936.
- Lebrun S, Xie Y, Chavez S, et al. An in vitro depth of injury prediction model for a histopathologic classification of EPA and GHS eye irritants. Toxicol In Vitro 2019;61:104628.
- Xie Z, Ye K, Chen SH, et al. Cellular viability and death biomarkers enables the evaluation of ocular irritation using the bovine corneal opacity and permeability assay. Toxicol Lett 2021;340:52–57.
- Kuckelkorn R, Schrage N, Keller G, et al. Emergency treatment of chemical and thermal eye burns. Acta Ophthalmol Scand 2002;80:4–10.
- European Centre for Ecotoxicology and Toxicology of Chemicals. Derivation of assessment factors for human health risk assessment technical report no. 86. Brussels (Belgium): European Centre for Ecotoxicology and Toxicology of Chemicals; 2003.
- Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol 2009;2:103–121.
- Ashby BD, Garrett Q, Willcox MD. Corneal injuries and wound healing – review of processes and therapies. Austin J Ophthalmol 2014;1:1017.
- Hemmati H, Colby K. Treating acute chemical injuries of the cornea. Am Acad Ophthalmol [Internet]. 2012;9:CD009379. Available from: www.aao.org/eyenet/article/treating-acute-chemical-injuries-of-cornea
- Pechura C, Rall D, editors. Institute of Medicine (US) Committee on the survey of the health effects of mustard gas and Lewisite. Washington (DC): National Academies Press (US); 1993.
- Marrazzo G, Bellner L, Halilovic A, et al. The role of neutrophils in corneal wound healing in HO-2 null mice. Câmara NOS, editor. PLoS One 2011;6:e21180.
- West-Mays JA, Dwivedi DJ. The keratocyte: corneal stromal cell with variable repair phenotypes. Int J Biochem Cell Biol 2006;38:1625–1631.
- Freeberg F, Nixon G, Reer P, et al. Human and rabbit eye responses to chemical insult. Fundam Appl Toxicol 1986;7:626–634.
- Freeberg F, Hooker D, Griffith J. Correlation of animal eye test data with human experience for household products: an update. J Toxicol Cutan Ocul Toxicol 1986;5:115–123.
- Fox DA, Boyes WK. Toxic responses of the ocular and visual system. In: Klaassen CD, ed. Cassaret Doull’s Toxicol basic Sci poisons. 7th ed. Withby (ON): McGraw-Hill Ryerson; 2008:665–697.
- Hackett RB, McDonald TO. Eye irritation. In: Marzulli FN, Maibach HI, eds. Advances in Modern Toxicology Dermatoxicology. 4th ed. Washington, DC: Hemisphere Publishing Corporation; 1991:749–815.
- Scott L, Eskes C, Hoffmann S, et al. A proposed eye irritation testing strategy to reduce and replace in vivo studies using bottom-up and top-down approaches. Toxicol In Vitro 2010;24:1–9.
- United Nations. Globally harmonized system of classification and labelling of chemicals (GHS). 6th ed. New York (NY); 2015.
- Wheeler AR. Memorandum: directive to prioritize efforts to reduce animal testing [Internet]. 2019. Available from: https://www.epa.gov/newsreleases/administrator-wheeler-signs-memo-reduce-animal-testing-awards-425-million-advance
- US Environmental Protection Agency. New approach methods work plan: reducing use of animals in chemical testing. 2020; Available from: https://www.epa.gov/sites/production/files/2020-06/documents/epa_nam_work_plan.pdf
- Office of Pesticide Programs. Pesticide assessment guidelines: subdivision F: hazard evaluation: human and domestic animals (revised edition). Washington, DC: US Environmental Protection Agency; 1982.
- Zorn-Kruppa M, Houdek P, Wladykowski E, et al. Determining the depth of injury in bioengineered tissue models of cornea and conjunctiva for the prediction of all three ocular GHS categories. PLoS One 2014;9:e114181.
- Griffith M, Osborne R, Hunger R, et al. Functional human corneal equivalents constructed from cell lines. Science 1999;286:2169–2172.
- Seo J, Byun WY, Alisafaei F, et al. Multiscale reverse engineering of the human ocular surface. Nat Med 2019; 25:1310–1318.
- Pham LL, Watford SM, Pradeep P, et al. Variability in in vivo studies: defining the upper limit of performance for predictions of systemic effect levels. Comput Toxicol 2020;15:100126.
- Casati S, Aschberger K, Barroso J, et al. Standardisation of defined approaches for skin sensitisation testing to support regulatory use and international adoption: position of the International Cooperation on Alternative Test Methods. Arch Toxicol 2018;92:611–617.
- Dumont C, Barroso J, Matys I, et al. Analysis of the Local Lymph Node Assay (LLNA) variability for assessing the prediction of skin sensitisation potential and potency of chemicals with non-animal approaches. Toxicol In Vitro 2016;34:220–228.