Abstract
Objective
To explore the toxicity of methanol and its metabolite, formic acid on αB-crystallin(CRYB), aldehyde dehydrogenase (ALDH2), and ATPsynthase (ATP5A1) of rat retinal ganglion cells (RGCs).
Methods
RGCs are cultured in vitro in a toxic environment with 15/30/60 mM methanol or formic acid, respectively. Then, the morphological changes of RGCs and protein and mRNA levels of ALDH2, ATP5A1, and CRYB in rat RGCs were evaluated.
Results
1) Compared to the toxicity of 15 mM formic acid on RGCs, 30 mM of formic acid environment significantly promoted apoptosis, and cell death occurred in the 60-mM formic acid group 24 h later. The toxicity of methanol for inducing apoptosis was not as obvious as formic acid. 2) In the 15-mM group, the level of CRYB protein was down-regulated after stimulating with both methanol and formic acid for 48 h, and ATP5A1 protein level decreased significantly with formic but not methanol. No change in ALDH2 was observed in methanol or formic acid. With a prolonged duration (>7 d) or high concentration (>30 mM) stimulation, cells treated with both methanol and formic acid showed severe apoptosis, rendering it challenging to collect a sufficient number of cells for protein detection. 3) In the 48-h group, no significant effect was detected on the mRNA of CRYB, ATP5A1, and ALDH2 by both 15/30 mM formic acid and 15 mM methanol. Conversely, 30 mM methanol had a significant up-regulation effect on the expression of the three genes, while no significant effect was observed in the 7-d groups.
Conclusions
Formic acid exerted stronger toxicity on CRYB, ATP5A1, and ALDH2 than methanol and played a regulatory role at the translation level, while the effect of methanol is still uncertain, needing additional investigation.
1. Introduction
Methanol intoxication causes various health problems, such as optic neuropathy, gastrointestinal problems, and metabolic acidosis leading to cell deathCitation1,Citation2. As it provokes different degrees of visual defects, from blurred vision to blindness depending on the severity of the exposure, methanol is considered a unique neurotoxin to the retina function. Formic acid, the metabolite of methanol, acts as a direct retinal toxin in methanol explorationCitation3,Citation4.
Our previous studies have shown that methanol was completely metabolized in blood after 7 d of intoxication, while formic acid could be detected in both 3- and 7-d intoxication groups, indicating that formic acid might have a more persistent toxic effect than methanol on retinal structure and functionCitation5. In addition, proteomics found that ALDH2, ATP5A1, and CRYB occupy the core position in the signal transduction network in methanol intoxication modelsCitation6. On the other hand, the effects of methanol and formic acid on the expression of ALDH2, ATP5A1, and CRYB are yet to be elucidated.
2. Materials and methods
2.1. RGC model of methanol and formic acid
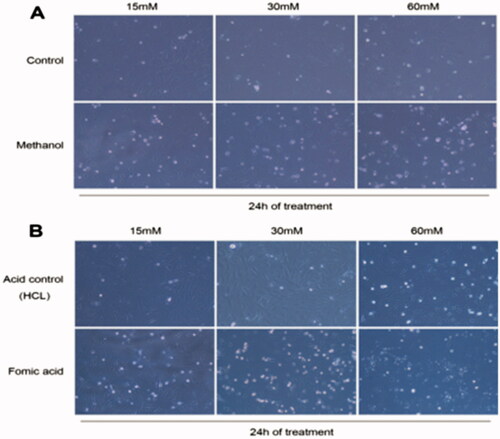
Primary cultured rat retinal ganglion cells (RGCs) were plated in two 6-well plates at a density of 4 × 105 cells/well. The medium was changed on day 1. On day 2, it was changed to serum-free medium at 4:00 p.m., and then the cells were starved overnight. On day 3, 15/30/60 mM methanol or formic acid was added, respectively, at 9 a.m., similar to the control. The morphology of RGCs was observed and recorded after 24 h. The images were acquired at 100× magnification.
2.2. Quantitative analysis of CRYB, ATP5A1, and ALDH2 proteins on RGCs
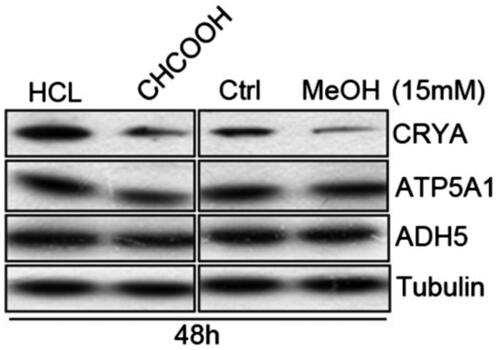
RGC grouping is described in . RGCs were seeded in 6-well plates. After reaching 80% confluency, the cells were serum-starved overnight. The next morning, 15/30 mM methanol or formic acid was added to the cells. The samples were collected at 48 h and after 7 d for protein detection. An appropriate volume of RIPA buffer was added to the samples for cell lysis. An equivalent of 50 µg total protein of each sample was separated by 10% SDS-PAGE and transferred to the PVDF membrane. Subsequently, the membrane was blocked with 5% skimmed milk in TBST on a shaker at room temperature for 1 h and probed with CRAB, ATP5A1, and ALDH5 (1:1000 dilution) primary antibodies at 4 °C, overnight, followed by incubation with the corresponding HRP-conjugated secondary antibody (1:30 000) at room temperature for 1 h. Finally, the immunoreactive bands were developed using enhanced chemiluminescence (ECL) substrate solution and X-ray film exposure.
Table 1. RGCs group and deal with formic acid and methanol.
2.3. mRNA level determination of CRYB, ATP5A1, and ALDH2 on RGCs
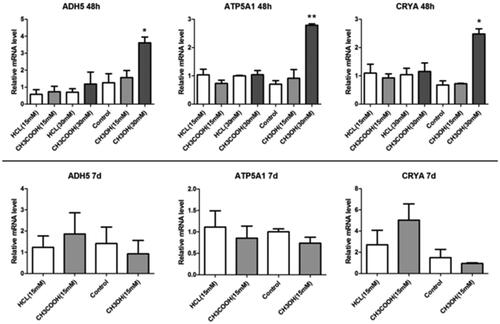
Cell treatment: RGCs were seeded in 6-well plates at a destiny of 1 × 105 cells/well. At 80% confluency, the cells were starved overnight in serum-free medium. On day 2, 15 or 30 mM methanol or formic acid was added for cell stimulation. RNA samples were collected after 48 h and 7 d, respectively.
RNA extraction: An equivalent of 1 mL TRIzol was added to each well in a 6-well plate and homogenized. Then, 200 µL chloroform was added, and the mixture was incubated at room temperature for 5 min, followed by centrifugation at 12 000 rpm, 4 °C for 15 min. The mixture was transferred to a new 1.5-mL Eppendorf microtube and 400 µL of isopropanol was added to the upper water phase (about 400 µL) and incubated at room temperature for 10 min. Subsequently, the supernatant obtained after centrifugation at 12 000 rpm, 4 °C for 10 min was discarded, and the precipitate was washed three times with precooled 70% anhydrous ethanol. After drying, the precipitate was solubilized in 20 µL DEPC-treated with water, and the RNA yield was determined on a Spectrophotometer (Shanghai Sunyuheng Scientific Instrument Co., Ltd., Shanghai, China).
RT-PCR: An equivalent amount of RNA was withdrawn from each sample for reverse transcription on the PCR instrument (EDC-810, Dongsheng innovation Biotechnology Co., Ltd, Beijing, China); the protocol is described in .
Table 2. Composition and procedure of RT-PCR.
3. Results
3.1. Morphological changes of RGCs with methanol and formic acid
A subset of RGCs stimulated with 15 mM formic acid showed apoptosis; 30 mM formic acid significantly promoted apoptosis after 24 h, while 60 mM caused cell death. Although cells in the 30-mM methanol group underwent apoptosis, the toxic effect was not as obvious as that of formic acid ().
3.2. Expression of CRYB, ATP5A1, and ALDH2 proteins in RGCs
The levels of CRYB protein were downregulated 48 h after stimulation with 15 mM of methanol and formic acid. Similarly, ATP5A1 protein was significantly decreased after stimulation with 15 mM formic acid, while toxic effect was not observed at the same concentration of methanol, and no change was detected in the expression of ALDH2 by methanol or formic acid. After 7 d or high concentration (>30 mM) stimulation with methanol or formic, cells were significantly apoptotic, making it difficult to collect a sufficient number for protein detection ().
3.3. mRNA level of CRYB, ATP5A1, and ALDH2 in RGCs
In 48-h groups, no significant effect was observed on the mRNA of CRYB, ATP5A1, and ALDH2 by 15 or 30 mM formic acid; also, the toxic effect of 15 mM methanol was not obvious. Conversely, the mRNA levels of CRYB, ATP5A1, and ALDH2 were significantly upregulated with 30 mM methanol. In the 7-d groups, no significant effect was exerted by either formic or methanol ().
4. Discussion
RGCs are located in the innermost layer of the retina and constitute a crucial path for transmitting visual information. Several diseases can cause damage and death of RGCs, leading to permanent blindness or retinal dysfunction, such as glaucoma, diabetic retinopathy, demyelinating diseases, tumours, optic neuropathy, ischaemia, and traumaCitation7,Citation8.
The analyses of the effects of methanol and formic acid on RGC morphology and the expression of CRYB, ATP5A1, and ALDH2 mRNAs revealed that a small amount of formic acid and methanol could produce toxicity in 24 h for the primary cultured RGCs, promoting significant apoptosis of cells. Compared to methanol, formic acid had a stronger toxic effect on RGCs. The concentration and effect time (30 mM or 24 h, respectively) were favourable to establish a short-term, acute toxic in vitro stimulation model to evaluate the toxicity of methanol and formic acid to retinal cells. However, if the toxicity of long-term stimulation to cells needs to be studied (e.g. >2D), 30 mM concentration is extremely high; thus, ≤15 mm was utilized. Herein, we used formic acid or methanol to stimulate the RGCs for 48 h or 7 d, and the results showed that the cells at 48 h (15 mM) were in good condition, while those stimulated with 30 mM or 7 d were severely apoptotic, such that a sufficient number of cells could not be collected for protein detection.
In terms of morphology, although the cells treated with 15 mM of methanol or formic acid were in relatively good condition, CRYB protein was significantly downregulated, and ATP5A1 protein level was significantly downregulated under formic acid but not methanol stimulation. At the gene level, 48 h of formic acid or 15 mM of methanol stimulation did not have any significant effect on the expression of the three genes. However 30 mM of methanol significantly upregulated the effect on the gene expression of CRYB, ATP5A1, and ALDH2, and 7 d of formic acid or methanol stimulation had no significant effect on the expression of the three genes. Combined with the analysis of protein and mRNA expression, it can be preliminarily determined that the regulatory effect of 15 mM formic acid on these three genes is at the translational and not transcriptional level, which might promote the degradation process of CRYB, ALDH2, and ATP5A1 and avoid any change in the mRNA level. On the other hand, 15 mM methanol only promoted the degradation of CRYB protein, while 30 mM methanol only promoted the mRNA expression of the three genes.
This study showed that ATP5A1 and CRYB expression decreased significantly under the stimulation of 15 mM formic acid, while the effect of methanol was still uncertain because the inhibition effect occurred at the low concentration of 15 mM and the promotion effect at the high concentration of 30 mM.
Generally, the expression of CRYB, ATP5A1, and ALDH2 was sensitive to the concentration and the time of methanol exposure, and the prolonged toxic stimulus led to degradation of some key proteins in the retina, and then led to the damage of retinal functionCitation5,Citation6,Citation9,Citation10. In the future, we will carry out further exploration according to the findings and uncertainty in this study.
5. Conclusions
A small amount of formic acid and methanol exerted toxicity on RGCs by inducing apoptosis in a short period, and the toxicity of formic acid was stronger than that of methanol. The translational regulation of CRYB and ATP5A1 by formic acid was decreased with 15 mM formic acid. On the other hand, the effect of methanol itself on CRYB, ATP5A1, and ALDH2 was uncertain.
Disclosure statement
The authors declare that they have no competing interests.
Additional information
Funding
References
- Tanrivermis Sayit A, Aslan K, Elmali M, et al. Methanol-induced toxic optic neuropathy with diffusion weighted MRI findings. J Toxicol Cutaneous Ocular Toxicol 2016;35:337–340.
- Johlin FC, Fortman CS, Nghiem DD, et al. Studies on the role of folic acid and folate-dependent enzymes in human methanol poisoning. Mol Pharmacol 1987;31:557–561.
- Eells JT, Salzman M, Lewandowski MF, et al. Formate-induced alterations in retinal function in methanol-intoxicated rats. Toxicol Appl Pharmacol 1996;140:58–69.
- Seme MT, Summerfelt P, Henry MM, et al. Formate-induced inhibition of photoreceptor function in methanol intoxication. J Pharmacol Exp Ther 1999;289:361.
- Dong-Mei L, Shu Z, Jie-Min C, et al. The intoxication effects of methanol and formic acid on rat retina function. J Ophthalmol 2016;2016;4087096.
- Chen JM, Zhu GY, Xia WT, et al. Proteomic analysis of rat retina after methanol intoxication. Toxicology 2012;293:89–96.
- Shatz CJ, Oleary DD. Repair and replacement to restore sight. Report from the panel on ganglion cell/connectivity. Arch Ophthalmol 1993;111:472–477.
- Wu JH, Zhang SH, Nickerson JM, et al. Cumulative mtDNA damage and mutations contribute to the progressive loss of RGCs in a rat model of glaucoma.Neurobiol Dis 2015;74:167–179.
- Dong-Mei L, Shu Z, Jie-Min C, et al. Changes of oscillatory potentials of Electroretinogram after methanol intoxication in rats. J Forensic Med 2014;30:178–180.
- Jie-Min C, Guang-You Z, Zi-Qin Z, et al. Electroretinogram and histopathologic changes of the retina after methanol intoxication. J Forensic Med 2013;29:5–11.