Abstract
Thiol chemistry is an efficient tool to manipulate the microstructure of aliphatic polyesters and open the way to different applications. Synthetic strategies that aim to synthesize thiol-functionalized aliphatic polyesters are reviewed herein. The introduction of thiol-editable groups on aliphatic polyesters can occur both at chain ends and along the chains, enabling diverse modifications of the polymeric chains and imparting new properties and functions. The use of thiol chemistry for postpolymerization modification of this class of polymers and the (co)polymerizations of monomers bearing thiol groups has also been described herein.
1. Introduction
The advancements in the use of degradable polymers in the biomedical field rely on the possibility of customizing the properties of the device to match the application needs. From the polymer chemistry standpoint, this customization means the designing of efficient synthetic approaches to the manipulation of the polymer structure, modulating the physical properties and integrating active tags that can foster cellular interactions.[Citation1,Citation2]
Among the degradable polymer classes utilized in the biomedical field, the aliphatic polyesters have a leading position.[Citation3] However, while their physical properties can be tailored via copolymerization, a major limitation toward application in new areas results from the lack of readily accessible side-chain functionalities.[Citation4] The synthesis of polyesters susceptible to further modifications is highly desired since the presence of functional groups could allow for tuning the physical and mechanical properties, for example, the rate of hydrolysis, degree of crystallinity, melting point, glass transition temperature, and processability, as well as to improve hydrophilicity and biocompatibility.[Citation5–7] Indeed, for applications in the biomedical field, such as tissue engineering and drug delivery, a functional group could provide an anchoring site for biologically active ligands, thus improving cell adhesion and function, or allow the preparation of degradable particles and polymeric networks with controlled dimension and function.[Citation8] Therefore, methods to integrate amino, carboxyl, hydroxyl, allyl and many other functionalities onto aliphatic polyester chains for fine-tuning of their physical and biological properties have been sought, and review papers are available in the literature.[Citation2,Citation9–11]
However, because of the reactivity of the polyester backbone, which can undergo hydrolytic degradation with a consequent drop in the molecular weight and change of the physical properties of the material, the controlled introduction of functional moieties requires some synthetic effort. Therefore, a good functional group, once added, should allow for a wide range of modifications and open the way to diverse applications: the great versatility of the thiol chemistry can ensure this flexibility.
Indeed, thiol chemistry plays an important role in polymer science. The number of publications reporting the use of thiol groups or related to the synthesis and postpolymerization modification of polymers and materials with specific and advanced features is increasing rapidly. The most used reaction among the plethora of the thiol chemistry reactions in polymer science is the thiol-ene reaction, which is also considered a click reaction. Comprehensive review papers about thiol-click chemistry[Citation12,Citation13] and its feasibility in polymer synthesis and postpolymerization modification,[Citation14–16] as well as the exploitation of click reactions to design biomaterials, have been reported in the literature,[Citation17,Citation18] while the key role of the disulfide bonds in the preparation of smart materials has also been highlighted recently.[Citation19]
When thiol groups are introduced in aliphatic polyester chains, the sensitiveness of these materials due to the reactivity of the ester bonds complicates the functionalization; however, polymer chemists have developed diverse approaches that allow for the functionalization either at the chain end or throughout the polymeric chains by using protecting groups or other expedients.
Herein, the aim was to provide an overview of the synthetic strategies that have been developed to allow for the combination of the outstanding degradability and biocompatibility of aliphatic polyesters with the broad versatility of the thiol chemistry, allowing for the fabrication of nanoparticles, elastomers, hydrogels, and functionalized scaffolds for tissue regeneration. Indeed, due to the plethora of reactions that the thiol groups or related groups can undergo, the introduction of these reactive moieties into aliphatic polyester main chains enhances the possibility of manipulation of the macromolecular chains, thereby endowing the polymer with new functions and properties. When these polymers are intended for biomedical applications, such editable groups can be used, for example, to link active tags to enhance the cell-material interactions. Due to nature of disulfide and thioether bond materials, which can receive stimuli from the body and induce changes in the shape and function of the polymeric device, smart materials can also be engineered.
The approaches involving the use of thiol chemistry for the synthesis and/or postpolymerization of functionalized poly(ester)s are herein compared according to modification and polymerization strategies.
2. Chain-end thiol functionalization
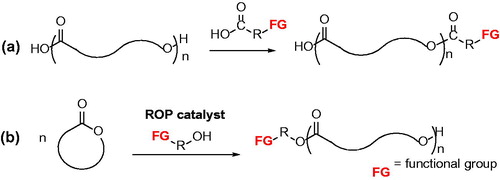
According to their structure, the chain ends of linear aliphatic polyesters can be utilized to introduce functional groups (FGs). Aliphatic polyesters with reactive end groups can be used as macromonomers for further postpolymerization modification, copolymerization, or cross-linking reactions, functioning in some cases as telechelic polymers. In general, this functionalization strategy has been accomplished through end-capping reactions () and/or by using functional initiators for the polymerization ().
Both approaches have been exploited to obtain chain end thiol-functionalized polyesters. End-capping reactions have been performed with carboxylic acids possessing a protected thiol.[Citation20,Citation21] The first example of end-functionalized poly(ε-caprolactone), PCL, was prepared by reacting the hydroxyl-terminated polymer with 2-((2,4-dinitrophenyl)thio)acetic acid in the presence of diisopropyl azodicarboxylate (DIAD) and triphenylphosphine (TPP). The polymer with the free thiol group was obtained after the cleavage of the protecting group, the 2,4-dinitrofluorebenzene, by reaction with a large excess of mercaptoethanol and triethylamine ().[Citation20]
Figure 2. Postpolymerization end-functionalization of poly(ε-caprolactone).[Citation20]
![Figure 2. Postpolymerization end-functionalization of poly(ε-caprolactone).[Citation20]](/cms/asset/e2291b2e-099c-4654-bb08-9db3c46dd737/lmsc_a_1625059_f0002_c.jpg)
Another approach, that is, the incorporation of a thiol functionality into polyesters by utilizing hydroxyl initiators bearing a protected thiol moiety, has also been developed.[Citation21] The end-functionalization of PCL was achieved by ring-opening polymerization (ROP) of ε-caprolactone, CL, in the presence of aluminum triisopropoxide using a functional alcohol initiator, the 2-((2,4-dinitrophenyl)thio)ethan-1-ol; the deprotection reaction performed after polymerization gave the free thiol end ().[Citation21]
Figure 3. End-functionalization of poly(ε-caprolactone) by using a functional initiator.[Citation21]
![Figure 3. End-functionalization of poly(ε-caprolactone) by using a functional initiator.[Citation21]](/cms/asset/f48fc780-74f6-48fa-9839-c8e3e2ab51ad/lmsc_a_1625059_f0003_c.jpg)
A similar strategy was recently exploited to prepare analogous thiol-end capped poly(lactide), PLA.[Citation22] Well-defined PCL macroinitiators capped with protected thiols have been utilized for the engineering of stable and aggregation-free gold nanoparticles with size of 9 nm, which have potential to be used as drug carriers in bladder cancer therapy.[Citation23]
The above examples required a protecting/deprotecting strategy for the end-functionalization of polyesters. The protecting groups were necessary to prevent undesired reactions of the thiol group that can, for instance, compete with hydroxyl groups to initiate ROP. Therefore, chemoselective catalysts are required to promote the direct synthesis of thiol-end functionalized polyesters and to avoid the use of protecting groups.
Direct routes to thiol-functionalized PCL were developed by exploiting the chemoselectivity of the lipase enzymes toward hydroxyl groups. Candida Antarctica lipase B (CALB) was used as the enzymatic catalyst to introduce thiol group by end-capping of preformed PCL by reaction with thiobutyrolactone () or with 3-mercapto propionic acid ().[Citation24]
Figure 4. End-functionalization of poly(ε-caprolactone) by chemoselective enzymatic catalyst.[Citation24]
![Figure 4. End-functionalization of poly(ε-caprolactone) by chemoselective enzymatic catalyst.[Citation24]](/cms/asset/bdbda22f-199f-484b-a074-61f44d4c4fc1/lmsc_a_1625059_f0004_c.jpg)
CALB was also tested as a chemoselective catalyst for promoting the ROP of CL in presence of 2-mercapto ethanol used as the initiator (); the product contained 70% of the thiol-end functionalized PCL, as disclosed by NMR characterization of the end groups.[Citation24] However, the molecular weight (Mn) that was achieved was quite low but sufficient if the polymers are intended to be used as macroinitiators in a further polymerization step, and the dispersity (Ð) was quite broad.
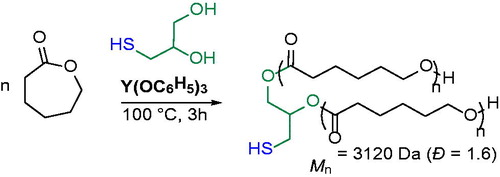
CALB was later used as a chemoselective and efficient catalyst to promote the ROP of a strainless 16-member ring macrolactone, the pentadecalactone (PDL), in presence of 6-mercapto-1-hexanol and 11-mercapto-1-undecanoic acid or 6-mercapto-1-hexanol and vinyl acrylate; the polymers with dithiol end groups or the polymers with thiol-acrylate end groups were prepared.[Citation25] Such a strategy represented an efficient route for simultaneous ROP and end-capping reactions with high chemoselectivity and end-group fidelity up to 96%.
Higher chemoselectivity, up to 99% of thiol end group fidelity, was later achieved by using Candida sp. 99-125 as catalyst and 6-mercapto-1-hexanol as initiator for the ROP of CL. However, regardless of the high selectivity, the molecular weight was still low, and the dispersity was broad, with Mn up to 4,700 Da and Ð up to 3.0, respectively.[Citation26] The same research group also developed the direct synthesis of thiol-end functionalized PCL by using rare earth phenolate-based compounds as catalysts.[Citation27] Such a strategy, which was allowed to avoid the protecting/deprotecting strategy and the use of expensive enzymatic catalysts, afforded the polymer with higher molecular weight and narrower dispersity. The chemoselectivity of the metal catalysts was attributed to the preferred exchange of the phenolate ligands with the hydroxyl group rather than the thiol group of the 6-mercaptohexanol or the 2-mercaptohexanol used as initiators. The polymerization was then claimed to proceed by a coordination-insertion mechanism, affording the polymer with the free thiol groups up to 87% fidelity.[Citation27] Following an analogous approach, 3-mercapto propane-1,2-diol has been exploited as a cheap and commercially available initiator for the synthesis of branched thiol-functionalized PCL ().[Citation28] The ROP, catalyzed by yttrium trisphenolate, gave the branched polymer with a thiol selectivity up to 80%.
A deeper comprehension of the kinetics and mechanism of CL polymerization in the presence of alcohol initiators bearing free-thiol groups and catalyzed by rare earth phenolate complexes has recently been provided.[Citation29] The understanding of the reaction allowed for the optimization of the reaction conditions and synthesis of thiol-terminated PCL with higher molecular weight, Mn up to 20,000 Da, and better chemoselectivity under mild conditions.
In addition to CALB and rare earth phenolates, Sn(OTf)2 has also been reported to be a chemoselective catalyst for the direct synthesis of thiol-terminated PCL using 6-mercapto-1-hexanol as initiator. The polymerization was carried out in continuous flow achieving quantitative chemoselectivity, a fast polymerization rate and good control over chain growth, 1.1 < Ð > 1.3.[Citation30]
Direct nonenzymatic synthesis for the preparation of thiol-functionalized PCL and poly(valerolactone), PVL, and related copolymers was described using unprotected 2-mercaptoethanol as the initiator and HCl or methanesulfonic acid to promote the cationic ROP.[Citation31] The ROP proceeded by an activated monomer mechanism leading to 85–100% of thiol-functionalized polymer but with low molecular weight. The byproduct was the α-carbonyl-ω-chloro-functionalized PCL.
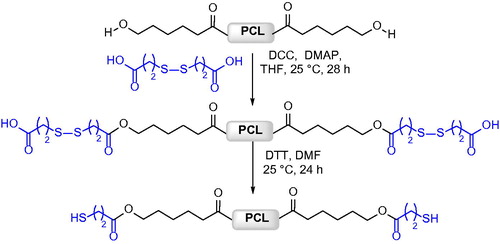
Telechelic[Citation32] and start-shaped[Citation33] PCL with thiol end groups utilized as a macroinitiator for block copolymer synthesis intended for nanocarriers and micelle engineering was prepared by condensation of hydroxyl-terminated PCL, obtained by using a di- or tetrafunctional initiator, with 3,3-dithiobis(propionic acid), and by the following reduction of the disulfide groups ().
Such a strategy is one of the most efficient for the synthesis of aliphatic polyesters bearing thiol groups at all chain ends, which can be exploited as macroinitiators for further polymerization, and the strategy involves the introduction and reduction of disulfide bonds. Multiblock copolymers containing disulfide bonds could also be prepared by polycondensation between analogous di-hydroxyl terminated PCL and 3,3-dithiobis(propionic acid).[Citation34]
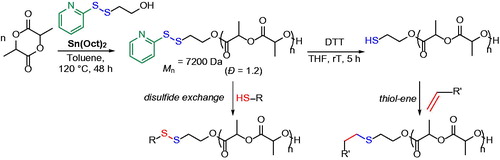
Although disulfide bonds are less explored than thiols in polymer synthesis and modification, their role in polymer science is emerging since they can be exploited for the attachment of biologically active tags to polymeric materials and to fabricate cross-linked redox-responsive carries, which are usually stable in blood and present degradability inside the cells.[Citation19] Disulfide end-functionalized poly(lactide), PLA, has been synthesized by performing the ROP of lactide monomer, LA, using an initiator-bearing pyridyl disulfide group and tin octanoate, Sn(Oct)2, as catalyst ().[Citation35] Despite the high polymerization temperature and the long reaction time, the pyridyl disulfide groups were not affected and could be exploited for further chain-end modifications. Pyridyl disulfide exchange reactions were performed with thiol-bearing molecules such as glucose tetraacetate and the chromophore naphthalene diimide. The author reported another approach for modification that involved the free thiol groups obtained after reduction of the disulfide bond in the presence of 1,4-dithioerythritol, DTT, which could give thiol-ene reactions with a library of acrylate-terminated molecules or polyethylene oxide, as well as with molecules bearing the maleimide moiety ().[Citation35]
Figure 7. Synthesis of pyridyl disulfide chain-end functionalized PLA and postpolymerization modification via disulfide exchange reaction and thiol-ene addition.
The opposite functionalization strategy, that is, the synthesis of “clickable” PLA containing unsaturated end-groups, prepared by a metal-free approach exploiting allyl- or propargyl-alcohol as initiators, and the following coupling reaction with thiols, after the polymerization, has also been reported.[Citation36]
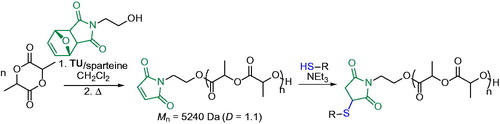
Because of chemical versatility that the maleimide group represents,[Citation37] the maleimide-thiol coupling reaction has also been disclosed to be an effective strategy for the synthesis of end-functionalized PLA ().[Citation38] The organocatalyzed ROP of LA by the thiourea (TU)/sparteine system was performed using a furan-protected alcohol functional maleimide as initiator. The removal of the furan ring, used to prevent undesired reaction during the polymerization, afforded the PLA with free maleimide moieties, whose reactivity was explored in presence of a library of thiol-bearing molecules (). The reaction occurred under mild conditions with a range of different substrates and solvents without observing any degradation of the polymeric chain.[Citation38] Furthermore, the Michael addition of a dithiol such as 1,2-ethanedithiol to maleimide groups at both ends of PLA allowed for the polymer cyclization.[Citation39]
3. Polyesters with pendant thiol groups along the chains
Chain-end modification provides a simple method to introduce reactive groups into aliphatic polyesters. However, more functionalities are often required to meet the demand of a greater manipulation throughout the macromolecular structure. Extensive efforts are currently devoted to the preparation of tailor-made functionalized aliphatic polyesters that represent promising platforms for different applications.[Citation7,Citation10] The functionalization of aliphatic polyesters is particularly challenging compared to that of nondegradable polymers. Any reaction condition that might cleave the ester bonds and be responsible for premature polymer degradation should be avoided during polyester modification.
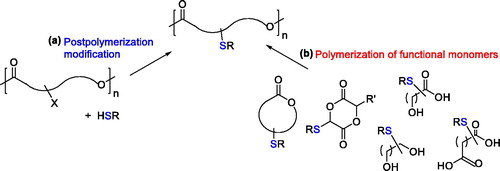
The two main strategies generally used for the synthesis of functionalized polyesters have also been proposed for the synthesis of aliphatic polyesters bearing thiol functionalities incorporated as side groups: postpolymerization modification of preformed polymers or polymerization of monomers bearing functional groups ().
Examples of both synthetic strategies reported for the synthesis of polyesters with pendant thiol (or their derivatives) groups are herein described. The concept of postpolymerization modification is generally intended as a modification of the polymeric chains occurring after the polymerization on nonfunctionalized polyesters, such as PLA or PCL.[Citation40,Citation41] However, for sake of clarity, the postpolymerization approaches are herein also intended as synthetic strategies that lead to the introduction of the thiol or related groups on a preformed polymeric chain, which can also result from the polymerization of a functional monomer (). For the latter strategy, both step-growth polycondensation and ring-opening polymerization approaches have been exploited ().
3.1. Postpolymerization modification strategies involving thiol chemistry
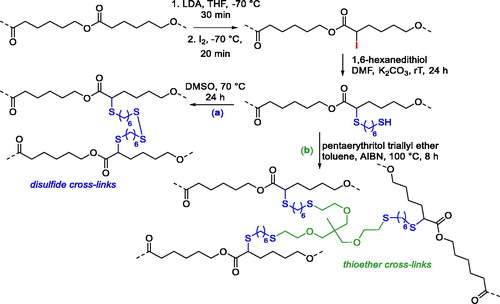
Postpolymerization strategies on preformed polyesters, such as PCL or PLA, are convenient strategies for the introduction of editable groups since they do not require tedious and/or several synthetic steps; however, in most of cases, chain cleavage can occur with a consequent change of the properties of the material.[Citation42] This strategy is, however, suitable when the functional groups that are introduced are exploited to functionalize the surface without affecting the bulk properties or for the preparation of networks and cross-linked systems. For example, pendant mercapto groups were introduced into commercial PCL by anionic chemical modification of the polymer. The introduction of pendant and free thiol groups occurred in two steps. The former step was the abstraction of the α-proton by reaction with lithium diisopropylamide (LDA) and introduction of iodine that led to a decrease of approximately 70% in the molecular weight. The latter step was the nucleophilic substitution of the iodine in the presence of 1,6-hexanedithiol ().[Citation43] Approximately 6.5% of the CL monomeric units became functionalized, and the as-modified PCL was exploited for the preparation of elastomeric materials. By simple oxidation of the thiol groups, the corresponding disulfide () was prepared, and the reversibility of the disulfide bonds into thiols was also evaluated. A thiol-ene click reaction thermally activated by azobisisobutyronitrile (AIBN) was also performed in the presence of pentaerythritol triallyl ether (), which led to the formation of an elastomeric material with thioether cross-links, having a higher Young’s modulus and elastic deformation than the starting PCL.[Citation43] Analogous degradable networks were developed later by the same research group introducing alkyne groups on PCL chains activated by LDA, which were later exploited for the UV-activated thiol-yne reaction with a cross-linker having four thiol groups, the pentaerythritol tetrakis(3-mercaptopropionate).[Citation44]
Indeed, most of the postpolymerization modification strategies of polyester chains involving thiol chemistry have occurred on unsaturated polyesters having pendant double or triple bonds. In other cases, the double bond can also be exo to the polymer chain or part of it. These polymers are often prepared by (co)polymerization of editable halogenated or unsaturated monomers.
Figure 10. Anionic postpolymerization modification of PCL to introduce mercapto groups and formation of elastomeric material with disulfide (a) and thioether (b) cross-links.
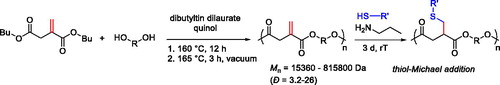
The thiol-ene reaction was exploited to graft the perfluoroctyl chain and poly(ethylene glycol) (PEG) oligomers sequentially to poly(3-hydroxyalkanoate) bearing pendant double bonds. The thiol-ene reaction occurred with a radical mechanism, using AIBN as a thermally activated initiator; thus, functionalized polymers were utilized to prepare multicompartment micelles by nanoprecipitation.[Citation45] Linear unsaturated and semicrystalline polyesters were also prepared by melt trans-esterification polymerization of dibutyl itaconate, a diester derivative of the renewable itaconic acid, and different diols, resulting in periodic distribution of exo-double bonds along the polymeric chains, which were not affected by the condensation strategy ().[Citation46]
The polymerization strategy did not allow for good control of the molecular weight distribution. However, the double bonds were conjugated to the ester carbonyl and could serve as a substrate to give nearly quantitative Michael addition of thiol bearing compounds using n-propyl amine as the catalyst, generating a library of functionalized polyesters. Derivatives with pendant hydroxyl groups, aromatic, oligoether, and cysteine moieties could be prepared.[Citation46] A scalable synthesis of unsaturated polyesters was developed based on the step-growth polymerization between trimethylolpropane allyl ether with diacid chlorides, and the pendant double bond was then exploited in postpolymerization reaction modification to prepare a library of functionalized polyesters, also involving thiol-ene click approaches.[Citation47]
Copolylactide with exo double bonds along the polymeric chains were alternatively prepared by ring-opening copolymerization of 95 mol% of L-lactide (L-LA) and 5 mol% of chlorolactide, with the latter prepared by cyclization of two molecules of 3-chlorolactic acid, which could in turn be obtained by oxidation of 3-chloropropane-1,2-diol.[Citation48] The elimination of the equivalent of hydrochloric acid under basic conditions yielded the unsaturated polymer chain having two exo and double bonds. Therefore, two α,β–unsaturated ester groups appeared in the same monomeric unit, without affecting the degree of polymerization. The addition of thiols to the unsaturated ester units was explored both by a radical mechanism and by the base-catalyzed thiol Michael addition, leading to 40% addition to the double bond in the case of the radical approach and nearly quantitative addition for the latter approach. The method was feasible for a variety of thiols as well.[Citation48]
Michael addition of thiol to pendant acryloyl groups has also been exploited to quantitatively link a variety of thiol-containing compounds, as well as a peptide sequence containing a cysteine unit to poly(ester-cocarbonates) obtained by ROP of caprolactone and carbonate cyclic monomers having the pendant unsaturated groups.[Citation49] A carbonate monomer with a latent maleimide pendant group was also reported and copolymerized with L-LA. The feasibility of the thiol addition to the maleimide moieties without affecting the polymeric backbone was also presented in the contribution.[Citation50]
In addition to carbonates, cyclic diester monomers bearing pendant double bonds have also been prepared. Lactide-type monomers having a pendant allyl group were reported as building blocks to prepare well-defined nanoparticles and nanocapsules through photosynthesis with a dithiol cross linker using a microemulsion as the synthetic template ().[Citation51]
Figure 12. Preparation of nanoparticle and nanocapsules by thiol-ene photosynthetic reaction on allyl-functionalized PLA.
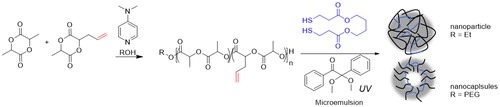
The thiol-ene reaction proceeded in the transparent microemulsion between the allyl groups and the thiol functionalities of the 1,4-butanediol bis(mercaptopropionate) using 2,2-dimethoxy-2-phenylacetophenone as the photoinitiator, achieving well-controlled and cross-linked nanostructures. Cyclic lactones bearing pendant double and triple bonds, valerolactone- and caprolactone-type monomers, were also described for the preparation of cross-linked polyester-based nanoparticles through the thiol-ene reaction[Citation52] and for the synthesis of cationic polyesters with pendant amino groups.[Citation53] Polyesters with pendant norbornene moieties susceptible to thiol-ene modification were also prepared by ring-opening alternated copolymerization of anhydrides and epoxides.[Citation54]
The above examples reported the synthesis of unsaturated monomers, whose preparation required a multistep synthetic procedure. A more straightforward approach to unsaturated polyesters is represented by the ROP of commercially available unsaturated macrolactones; however, their reactivity in ROP is much lower compared to small-ring lactones.[Citation55] Despite the lower reactivity of the internal double bond, unsaturated poly(macrolactones) are able to give thiol-ene reactions. Heise et al. reported a first example of this approach.[Citation56] An unsaturated polyester, with Mn up to 16,000 Da, by ROP of globalide, a 16-member ring macrolactone, catalyzed by CALB, was prepared, and the feasibility of the thiol-ene click reaction on the internal double bond of the linear polymer was assessed in the presence of three different thiol-bearing molecules ().
The reaction with mercapto-2-hexanol, butyl-3-mercaptopropionate or N-acetylcysteamine, thermally promoted by AIBN, gave the corresponding polyesters with pendant ester, primary alcohol, or amine, respectively. The efficiency of the coupling was more than 95% in the presence of mercapto-2-hexanol and N-acetylcysteamine, while it was lower for the butyl-3-mercaptopropionate, at 75%.[Citation56] The poly(globalide) was semicrystalline with a melting temperature, Tm, of 48 °C, but the introduction of the pendant moieties resulted in amorphous polymers. The thermally induced thiol-ene addition of the 6-mercapto-1-hexanol on the double bonds along the chains of poly(ω-6-hexadecenlactone), an unsaturated polymacrolactone obtained by ROP of the renewable ω-6-hexadecenlactone, has also been reported as a postpolymerization approach to modulate the structural and thermal properties of the native polymer.[Citation57] The thermal thiol-ene addition has also been reported on unsaturated macrolactones, which undergo the ROP in the next step.[Citation58]
Functional films consisting of cross-linked poly(globalide) were also prepared by exploiting thiol-ene reactions.[Citation59] The copolymer of globalide and CL prepared by enzymatic ring-opening copolymerization was utilized to prepare thermoset via the photoinduced thiol-ene reaction.[Citation60] The most recent contribution in this field reported the functionally analogous copolymer with N-acetylcysteine through the thiol-ene click reaction and the antioxidant character from the functional moiety provides materials that could combat the cellular oxidative stress.[Citation61] Direct UV-triggered thiol-ene has recently been reported as an in situ method to cross-link electrospun fibers from unsaturated poly(macrolactones), which have been claimed to have potential biomedical application as load-bearing scaffolds.[Citation62]
A polyester-bearing internal triple bond, suitable as a substrate in the thiol Michael reaction, can also be prepared by condensation of acetylenedicarboxylic acid and diols in the presence of p-toluenesulfonic acid.[Citation63]
3.2. Polymerization of monomers bearing thiol, thioether, and disulfide pendant groups
3.2.1. Polycondensation strategies
The first example for the direct synthesis of polyesters by using monomers bearing thiol groups was a polycondensation reaction between mercaptosuccinic acid and ethylene glycol or oligo(ethylene glycol). The polymerization reactions were performed at 120 °C using concentrated HCl (C = 12 M) as catalyst (). Notably, the polyesters were obtained with high molecular weight and narrow dispersity.[Citation64] Aiming at preparation of materials for the topical release of nitric oxide (NO), the as-prepared polyesters were allowed to react with a gaseous NO/O2 mixture to cause the S-nitrosation of the thiol group.[Citation64]
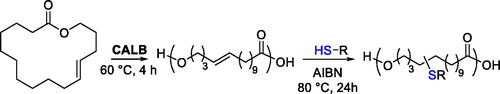
The direct synthesis of aliphatic polyesters bearing free thiol groups was also achieved under milder conditions by enzymatic selective polycondensation of diols and ester derivatives of dicarboxylic acids bearing pendant free thiol groups.[Citation65] After screening of a few enzymatic catalysts and substrates, the authors found that the polymers with the highest molecular weight and narrowest dispersity were obtained by condensation of hexane-1,6-diol and dimethyl 2-mercaptosuccinate in the presence of CALB carried out at 70 °C for 48 hr ().
Figure 14. Polycondensation of ethylene glycol or oligo(ethylene glycol) with mercaptosuccinic acid catalyzed by HCl.
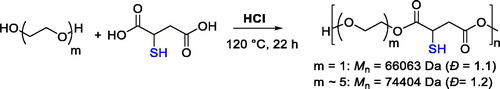
Figure 15. Lipase-catalyzed synthesis of polyesters with free pendant thiol groups and network formation by oxidation of the thiol into disulfide groups.
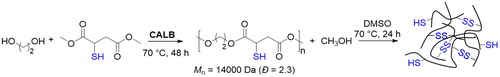
The formation of a relatively high molecular weight polymer in a chemoselective way was ascribed to the bulkiness of the thiol group compared to the hydroxyl groups and to the reaction temperature, which favors the elimination of the side MeOH formed as a product of the condensation and the formation of the polymer. Indeed, the reaction temperature was 70 °C, which is higher than methanol boiling point. The free thiol groups could also be oxidized in the corresponding disulfide with consequent network formation.[Citation65] The strategy was later extended and polyester-bearing free mercapto groups with higher molecular weight could be prepared using different diols,[Citation66] confirming that CALB could be used both for promoting both the chemoselective synthesis of thiol-terminated polyesters and polyesters with appended thiol groups. In addition to the enzymatic catalyst, Sc(OTf)3 has also been used for the chemoselective condensation of the thiomalic acid and diols; however, the molecular weights achieved were lower compared to the enzymatic approach.[Citation67] Recently, the possibility of chemoselective synthesis of thiolated polyesters by condensation of thiomalic acid in the absence of catalyst has also been demonstrated.[Citation68]
A synthetic approach that enabled the synthesis of functionalized poly(lactic acid) bearing thiol-protected groups along the polymeric chain by condensation of related functional hydroxyl acids and lactic acid was also developed ().[Citation69]
Figure 16. Thiol-functionalized a-hydroxy acid synthesis and polycondensation with lactic acid catalyzed by Sn(Oct)2.
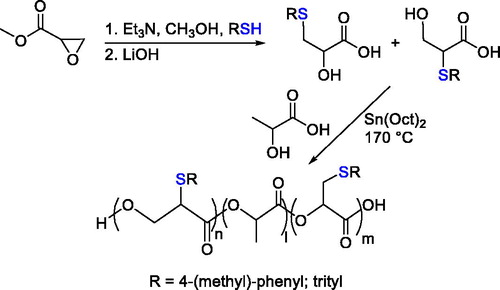
The functionalized hydroxyl acids were obtained by reaction of the desired thiols with the methyl oxirane-2-carboxylate. However, the polycondensation approach led to low molecular weight polymers and it required harsh conditions to favor the formation of the product. Moreover, when a polycondensation is carried out, the formation of side products such as water or alcohol should be considered.
The thiol-ene click reaction represents an efficient and easy step-growth polymerization method which does not lead to the formation of side products, and it has, for example, been exploited to yield polyester chains or networks containing both ester and thioether bonds by reaction between di-thiol-bearing compounds and di- or multi-functional acrylates.[Citation70–72] Both thiol-yne and thiol-ene reaction strategies have been exploited for the preparation of bio-based hydroxyl fatty acid that could later undergo condensation polymerization.[Citation73]
3.2.2. Ring-opening polymerization strategies
Although step-growth polymerization can be used to introduce functional groups along the polymeric chains easily, polycondensation methods generate low control over molecular weight and dispersity and, in some cases, necessitate expensive enzymatic catalysts. In contrast, ring-opening polymerization (ROP) is a well-established strategy for the synthesis of aliphatic polyesters that allows for good control over the chain growth and composition, affording polymers with high molecular weight and narrow dispersity. A precise control of the polymer properties is indeed crucial when aliphatic polyesters are used for sophisticated biomedical applications.[Citation74,Citation75]
However, the introduction of functional groups along polyester chains by ROP of suitable monomers remains highly challenging. The monomer preparation often requires multistep synthetic pathways and the product is obtained in low yield, especially when a cyclization step is required. All these problems limit the scale and chemical scope of the polymer production. Furthermore, reactions involved during the modification of lactones and cyclic diesters should not affect the cycles, and thereafter, the functional groups should be inert to the polymerization conditions; if necessary, protecting groups must be used to avoid side reactions or catalyst poisoning.[Citation9,Citation10] Indeed, the nucleophilic character and the oxidative instability of the thiol group could interfere with the ROP. Therefore, for all the functionalized monomers developed, the thiol group is usually incorporated as a thioether moiety. When the free thiol group is to be exposed after the polymerization, a monomer with a protecting group that should withstand the ROP conditions and could be cleaved without affecting the polyester backbone should be designed.
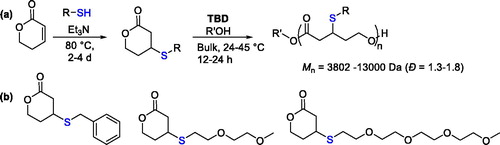
3-Mercaptovalerolactones were prepared by a straight one-step synthetic approach that involved the Michael addition of thiol-bearing compounds to the endocyclic commercially available α,β-unsaturated δ-valerolactone ().[Citation76]
Three monomers bearing different thioether side groups were prepared in good yield, 76–44%, bearing benzyl and oligo(ethylene glycol) side chains (). Their ability to undergo ROP was assessed in the presence of 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD) used as the organocatalyst and an alcohol as initiator. Good conversion was achieved in bulk and homo- and co-polymers with CL were prepared with molecular weight up to 13,000 Da (). However, the concomitant retro-Michael addition occurred during the polymerization, leading to lower molecular weight than the expected molecular weight based on the monomer conversion.[Citation76]
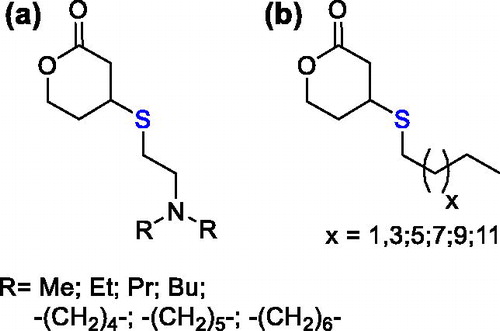
An analogous procedure was later followed for the synthesis of a library of monomers bearing tertiary amine () and alkyl side moieties (); their ROP promoted by methyl lithium led to lipocationic polyesters with promising application for siRNA delivery.[Citation77]
Figure 18. Functionalized six-member lactones with tertiary amine and alkyl side moieties, prepared by the thiol Michael addition on the endocyclic α,β-unsaturated δ-valerolactone.
A functional six-member monomer was obtained by Michael addition of 2-mercaptoethanol on the exocyclic α,β-unsaturated δ-valerolactone, which is not commercially available, and it was prepared by a two-step procedure (). A seven-member monomer was also prepared by thiol addition of the 2-mercaptoethanol in the presence of UV light on an allyl-functionalized ε-caprolactone ().[Citation78]
Figure 19. Synthesis of six- (a) and seven-member (b) unsaturated lactones and their thiol-ene click reaction.
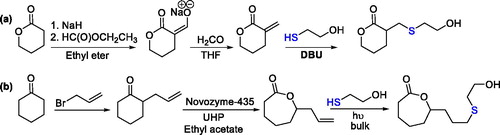
The thiol addition on exocyclic double bonds gave monomers with 91 and 85% yield.[Citation78] Thus, thiol addition on exocyclic double bonds was more efficient than the reaction previously performed on the endocyclic unsaturated α,β-unsaturated δ-valerolactone.[Citation76] The as-prepared monomers were then polymerized in bulk in the presence of Sn(Oct)2 as catalyst, and the free hydroxyl group on monomers initiated the ROP, leading to hyperbranched polyesters.[Citation78]
The thiol Michael addition to exocyclic α,β-unsaturated δ-valerolactone was also used for the synthesis of analogous monomers previously obtained, bearing oligo(ethylene glycol) (),[Citation79] tertiary amine (), and alkyl side moieties ().[Citation80]
Figure 20. Functionalized six-member lactones, prepared by the thiol Michael addition on the exocyclic α,β-unsaturated δ-valerolactone.
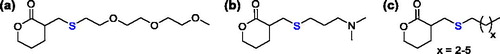
The major stability of the thiol-ene adducts on the exocyclic unsaturated substrate with respect to the endocyclic unsaturated substrate was pointed out, and both the monomer synthesis and their ROP were overall more efficient.[Citation79] The polymerization of such monomers was exploited for the preparation of poly(ethylene glycol)-like polyesters as materials for antifouling applications[Citation79] and for the synthesis of block and random copolyesters suitable for gene delivery.[Citation80]
Despite the feasibility of δ-valerolactone to give functionalized polyesters, biomedical devices made of poly(δ-valerolactone) have not been commercialized. Other aliphatic polyesters have found more interest for application in the biomedical field, among them PCL, PLA and related copolymers with glycolide (GA) have a leading position, and medical devices made of these materials have also been approved by the FDA.[Citation81]
PCL-bearing pendant thioether groups at the α position could be synthesized by ROP of the related functionalized monomers. The CL-type monomers were prepared by a three-step synthetic strategy that uses cyclohexanone as the starting material and involves the nucleophilic substitution of a thiol-bearing compound on the α-brominated CL ().[Citation82]
Figure 21. Synthesis and ring-opening polymerization of α-ethylthiocaprolactone and α-phenylthiocaprolactone to give ROS-responsive PCL (a) and oxidation pathways of the related polymers in the presence of H2O2 (b,c).
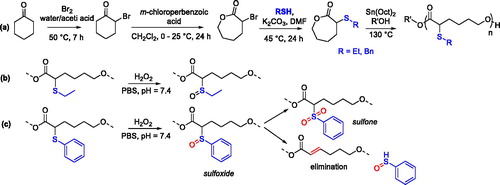
The ROP of the as-prepared monomers was catalyzed by Sn(Oct)2 using either benzyl alcohol or PEG as initiator or macroinitiator, respectively. Regardless of the high temperature, 130 °C, the polymerization reaction allowed for good control over the chain growth. Polymers with very narrow dispersity were obtained (Ð = 1.02–1.05), and the Mn was in the range of 14–23 kDa, although the polymerization kinetics were slower compared to the CL, probably because of the steric hindrance at the α–position. Moreover, by using PEG as macroinitiator, amphiphilic block-copolymers could be prepared. The authors also assessed the reactive oxygen species (ROS) responsiveness capability of the two polymers, finding that while the oxidation of the ethylthioether in the presence of H2O2 led only to the formation of the sulfoxide (), the phenylsulfoxide could be further oxidized to sulfone or undergo elimination of sulfenic acid and formation of the conjugated polymer (). This synthetic approach represents an efficient and controlled strategy to combine the degradability and biocompatibility of PCL with the ROS responsiveness.[Citation82]
Extensive research has also been devoted to the synthesis of functionalized lactide- and glycolide-type monomers, bearing, for example, amino, hydroxyl and carboxylic groups.[Citation10] However, only a few examples of a lactide-type monomer having pendant thiol groups have been reported.[Citation83–85]
The allyl lactide was used as the starting material to prepare a functionalized monomer bearing a triethylene glycol pendant unit terminated with an azide group and linked to the diester cycle through a thioether bond () by radical UV-initiated thiol-ene reaction.[Citation83]
Figure 22. Synthesis of GRGDS-functionalized PLA by ROP of a lactide-type monomer with thioether pendant groups.
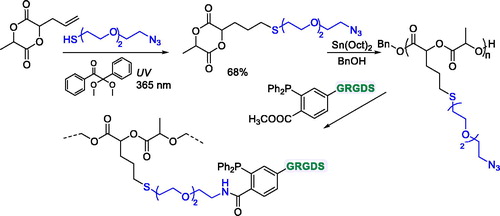
The monomer was readily polymerized in the presence of Sn(Oct)2 using benzyl alcohol as initiator to give a low molecular weight chain where Mn was 6,000 Da. A GRGDS peptide sequence (glycine-arginine-glycine-aspartic acid-serine) was linked to the polymer prepared through a Staudinger ligation reaction (). This strategy allowed for obtaining peptide-polymer conjugates in only two steps. However, the monomer synthesis requires several steps: the preparation of allyl lactide, which proceeded in four steps,[Citation86] the preparation of the HS-triethylene glycol-N3 linker by a 2-step procedure, and then the coupling between them.
Thiol-substituted copolylactide, which could further undergo postpolymerization modification exploiting the thiol functionalities such as thiol-ene click reactions were prepared by ring-opening copolymerization of L-lactide and 3,6-bis(4-methoxy-benzylthiomethyl)-1,4-dioxane-2,5-dione, a lactide type monomer prepared by cyclization of the corresponding α-hydroxyacid in the presence of hydroxybenzotriazole (HOBt) and N,N'-dicyclohexylcarbodiimide (DCC), which could be obtained by a two-step procedure using 3-chloro-1,2-propanediol as the starting material ().[Citation87]
Figure 23. Synthesis, ring opening copolymerization of 3,6-bis(4-methoxy-benzylthiomethyl)-1,4-dioxane-2,5-dione and postpolymerization cleavage of the methoxybenzyl protecting groups.
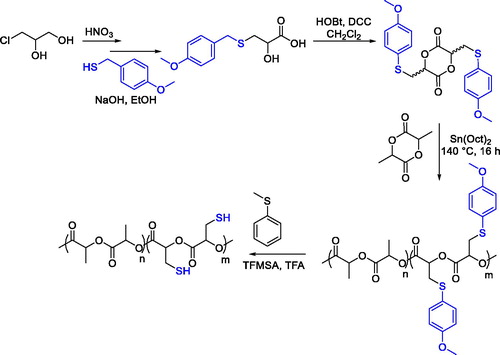
The latent thiol groups were incorporated as 4-methoxybenzylthioether, and the protecting groups could withstand the copolymerization conditions while being removed in presence of thioanisole under acidic conditions, using trifluoromethanesulfonic acid (TFMSA) and trifluoroacetic acid (TFA).
Another strategy that has been developed involves the addition of a thiol-containing compound on the exomethylene lactide,[Citation84,Citation85] which can be prepared by a two-step pathway,[Citation88] that is, bromination-elimination, using L-LA as the starting material (). Such an approach does not involve a cyclization step, thereby preventing the formation of a linear oligomer that lowers the yield of the cyclic monomer and renders its purification difficult. Indeed, the yield of monomer formation (i.e., the last step) is usually approximately 65–70% when a thiol is added on the exomethylene lactide[Citation84,Citation85] against a yield of 30–40% achieved in the best case for a cyclization step.[Citation10]
Figure 24. Synthesis of a lactide-type monomer having a thiol pendant group by thiol addition to the exomethylene lactide.
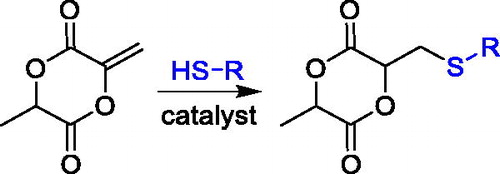
Moreover, the cyclization approach requires several steps, and in most cases, the monomer preparation is quite lengthy and with variable yield, and the desired functional group is usually incorporated in the early steps. While the use of the exomethylene lactide as the precursor cyclic monomer is beneficial since the addition of the functionality occurs in the last reaction step and therefore, it can serve as the substrate for the introduction of different functionalities, at the same time avoiding a low-yield cyclization step. A first example of so-prepared monomer was a lactide functionalized with 4-hydroxythiobenzamide (). The monomer was obtained through seven synthetic steps and used, in copolymerization with L-LA, to prepare polymers and microparticles able to release thiobenzamide slowly and therefore H2S.[Citation84]
Figure 25. Lactide-type monomer functionalized with 4-hydroxythiobenzamide.[Citation84]
![Figure 25. Lactide-type monomer functionalized with 4-hydroxythiobenzamide.[Citation84]](/cms/asset/1c52e1a6-1545-4e05-a8ee-e79a43862937/lmsc_a_1625059_f0025_c.jpg)
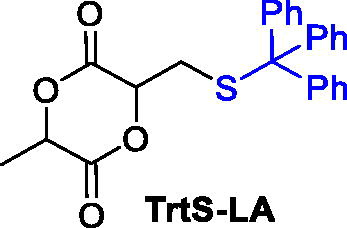
An analogous strategy for the development of a monomer able to combine the degradability and biocompatibility of PLA with the versatility of the thiol chemistry, the 3-methyl-6-((tritylthio)methyl)-1,4-dioxane-2,5-dione (TrtS-LA), was independently reported.[Citation85] The TrtS-LA was prepared by the Michael addition of the triphenylmethanethiol to the exomethylene lactide, , resulting in a lactide-type monomer having the latent thiol group protected as the trityl thioether. The same monomer could also be prepared by cyclization of 2-hydroxy3-(S − triphenylmethyl)-thiopropanoic acid with a much lower yield (30%) than the Michael addition approach that afforded the product with 68% of yield.
The trityl moiety was a suitable protecting group that could be cleaved under mildly acidic conditions once incorporated along the polymer chain, offering a wide range of possibilities for the fabrication of functional materials.[Citation85]
The TrtS-LA could be prepared by a three-step synthesis and was revealed to be a useful building block for the preparation of functionalized aliphatic polyesters. The monomer was copolymerized with commercially available monomers such as LA, and CL in the presence of different catalytic systems able to promote the ring-opening copolymerization and achieved good control over the chain growth ().[Citation85,Citation89]
Figure 27. Ring-opening copolymerization of TrtS-LA with LA and CL and postpolymerization modification strategies to engineer (a) RGDC biomimetic functionalized polyester scaffolds; (b) PLA biomimetic surfaces grafted with oligopeptide sequences; and (c) PLA-PEG disulfide cross-linked redox-responsive nanoparticles.
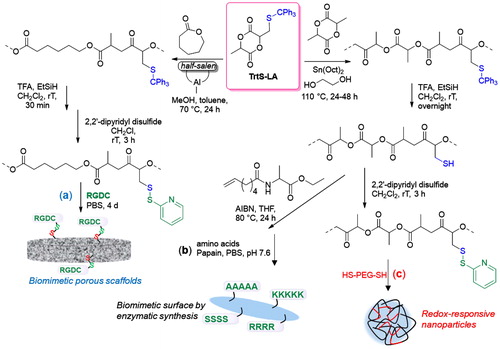
The latent thiol groups along the polymeric chains could be quantitatively deprotected by cleavage of the trityl moieties without affecting the polymeric chains. Such thiolated polyesters could serve as substrates for the thiol-ene click reaction. Moreover, the thiol groups were modified into pyridyl disulfide functionalities, and an as-functionalized copolymer obtained by copolymerization of TrtS-LA and CL was used to fabricate editable porous scaffolds (). A cysteine-terminated RGD peptide (RGDC) was covalently attached to the porous scaffolds by a disulfide-exchange reaction in aqueous solution.[Citation85] The potential applications in the biomedical field of analogous functional PLA-bearing disulfide moieties used to prepare RGDC-functionalized porous scaffolds have been further evaluated.[Citation90] The free thiol groups along the chain can also serve as an anchoring site to attach short peptide sequences prepared in situ by enzymatic synthesis ().[Citation91] Furthermore, redox-responsive PEG-PLA-based nanoparticles by reaction of pyridyl disulfide-functionalized polyesters with a telechelic PEG having thiol groups at both ends were prepared (). Such networks were assembled in flower-like particles in water solution and their size was in the range 167–300 nm, which is suitable for drug delivery. The nanoparticles were stable over long periods of time, up to six months, in aqueous solution and their size was not affected by dilution, while they quickly disassembled in the presence of cellular concentrations of glutathione, proving that they could be used as a drug carrier with controlled and targeted release.[Citation89]
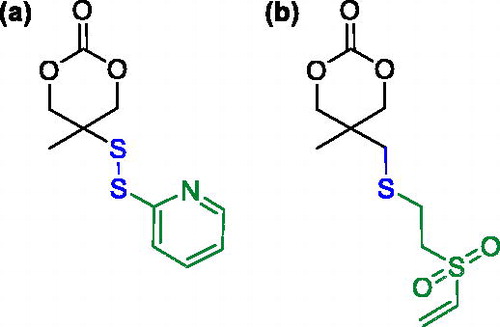
Along with aliphatic polyesters, aliphatic polycarbonates, such as poly(trimethylene carbonate), and poly(ester carbonate) copolymers find also application in biomedical field.[Citation49,Citation92] The copolymerization of cyclic esters with functionalized cyclic carbonates represents an interesting strategy to prepare functional degradable polymers.[Citation93] For example, poly(ester carbonate)s bearing pyridyl disulfide pendant groups were prepared by ring-opening copolymerization of pyridyl disulfide carbonate monomer (), obtained by a four-step synthetic strategy, with CL.[Citation94,Citation95]
The disulfide exchange reaction between the pyridyl groups along the copolymer chain and a thiolated PEG was exploited to prepare amphiphilic copolymers that could assemble into redox-responsive degradable micelles with nanometric dimensions.[Citation94] While the disulfide exchange reaction between the same functionalized copolymer and thiolated lactobionic acid was reported by same author to fabricate glycol-nanoparticles having sheddable saccharide shells.[Citation95] Both systems could mediate the efficient intracellular delivery of anticancer drugs, such as doxorubicin.[Citation94,Citation95]
Vinyl sulfone functionalized PCL and PLA were also prepared by copolymerization of CL or LA with an analogous functionalized cyclic carbonate monomer bearing the vinyl sulfone moiety (). Such side-chain functionalities could undergo thiol Michael addition with a variety of thiol-containing molecules and polymers at room temperature and in absence of any catalyst to afford functionalized and degradable polymers or degradable coatings.[Citation96]
4. Conclusions and outlook
Herein, we have presented an overview of the broad variety of pathways to introduce thiol groups on aliphatic polyester chains that endow such polymer classes with diverse and new functions and applications.
The thiol groups represent a valid option to combine the possibility of a great manipulation of the polyesters with their degradability. Indeed, if, on the one hand, the postpolymerization approaches permit several reactions starting from the same polyester chains, they can favor chain degradation, while on the other hand, the polymerization of functionalized monomers ensures more control of the polymer properties but often requires several synthetic steps. Hence, the best combination is the controlled polymerization of monomers bearing versatile functional groups such as thiol groups, which, once incorporated along the polymeric chain, could represent a platform for a broad range of postpolymerization modifications.
The incorporation of thiol or related functional moieties, being both at the chain end and along the chains, can be achieved by several strategies and occur at different stages of the synthetic pathway. From the different strategies, it is evident that the most controlled route to prepare high molecular weight polymers in a controlled way is the ring-opening polymerization. However, there is a need to develop new chemoselective approaches that could avoid the protection/deprotection strategies and permit the incorporation of free thiol groups, thus reducing the synthetic steps. Moreover, monomers bearing readily reactive side groups toward postpolymerization modifications but that could withstand the polymerization procedure should also be designed. Another possible strategy to be implemented could be the use of versatile precursor monomers that allow for the introduction of several functional groups in a fast and easy way as a last synthetic step, right before the polymerization.
These approaches would give quick access to multifunctional aliphatic polyesters with customized though controlled properties with potential broad range functions and roles. The thiol chemistry should play a key role to introduce functionalities along the polyester chains, especially if stimuli-responsive materials and degradable substrates with an enhanced cell-material interaction are intended.
Acknowledgments
The authors acknowledge the financial support from the Swedish Foundation for Strategic Research (RMA15-0010).
References
- Shoichet, M. S. Polymer Scaffolds for Biomaterials Applications. Macromolecules 2010, 43, 581–591. DOI: 10.1021/ma901530r.
- Tian, H.; Tang, Z.; Zhuang, X.; Chen, X.; Jing, X. Biodegradable Synthetic Polymers: Preparation, Functionalization and Biomedical Application. Prog. Polym. Sci. 2012, 37, 237–280. DOI: 10.1016/j.progpolymsci.2011.06.004.
- Nair, L. S.; Laurencin, C. T. Biodegradable Polymers as Biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. DOI: 10.1016/j.progpolymsci.2007.05.017.
- Vert, M. Aliphatic Polyesters: Great Degradable Polymers that Cannot Do Everything. Biomacromolecules 2005, 6, 538–546. DOI: 10.1021/bm0494702.
- Seyednejad, H.; Ghassemi, A. H.; van Nostrum, C. F.; Vermonden, T.; Hennink, W. E. Functional Aliphatic Polyesters for Biomedical and Pharmaceutical Applications. J. Control. Release 2011, 152, 168–176. DOI: 10.1016/j.jconrel.2010.12.016.
- Williams, C. K. Synthesis of Functionalized Biodegradable Polyesters. Chem. Soc. Rev. 2007, 36, 1573–1580. DOI: 10.1039/b614342n.
- Heiny, M.; Wurth, J. J.; V. P. Chapter, S. 9 - Progress in functionalized biodegradable polyesters A2. In Natural and Synthetic Biomedical Polymers, Kumbar, S., Laurencin, C., Deng, M., Eds; Elsevier: Oxford, 2014; pp 167–180.
- Winnacker, M. Covalent Polyester–Biomolecule Conjugates: Advances in Their Synthesis and Applications in Biomedicine and Nanotechnology. Polym. Int. 2017, 66, 1747–1755. DOI: 10.1002/pi.5459.
- Pounder, R. J.; Dove, A. P. Towards Poly(Ester) Nanoparticles: Recent Advances in the Synthesis of Functional Poly(Ester)s by Ring-Opening Polymerization. Polym. Chem. 2010, 1, 260–271. DOI: 10.1039/b9py00327d.
- Yu, Y.; Zou, J.; Cheng, C. Synthesis and Biomedical Applications of Functional Poly([Small Alpha]-Hydroxyl Acid)s. Polym. Chem. 2014, 5, 5854–5872. DOI: 10.1039/C4PY00667D.
- Tong, R. New Chemistry in Functional Aliphatic Polyesters. Ind. Eng. Chem. Res. 2017, 56, 4207–4219. DOI: 10.1021/acs.iecr.7b00524.
- Dondoni, A. The Emergence of Thiol–Ene Coupling as a Click Process for Materials and Bioorganic Chemistry. Angew. Chem. Int. Ed. 2008, 47, 8995–8997. DOI: 10.1002/anie.200802516.
- Hoyle, C. E.; Lowe, A. B.; Bowman, C. N. Thiol-Click Chemistry: A Multifaceted Toolbox for Small Molecule and Polymer Synthesis. Chem. Soc. Rev. 2010, 39, 1355–1387. DOI: 10.1039/b901979k.
- Lowe, A. B. Thiol-Ene “Click” Reactions and Recent Applications in Polymer and Materials Synthesis. Polym. Chem. 2010, 1, 17–36. DOI: 10.1039/B9PY00216B.
- Lowe, A. B. Thiol-Ene “Click” Reactions and Recent Applications in Polymer and Materials Synthesis: A First Update. Polym. Chem. 2014, 5, 4820–4870. DOI: 10.1039/C4PY00339J.
- Lowe, A. B. Thiol-Yne ‘Click’/Coupling Chemistry and Recent Applications in Polymer and Materials Synthesis and Modification. Polymer 2014, 55, 5517–5549. DOI: 10.1016/j.polymer.2014.08.015.
- Hvilsted, S. Facile Design of Biomaterials by ‘Click’ Chemistry. Polym. Int. 2012, 61, 485–494. DOI: 10.1002/pi.4135.
- Zou, Y.; Zhang, L.; Yang, L.; Zhu, F.; Ding, M.; Lin, F.; Wang, Z.; Li, Y. Click” Chemistry in Polymeric Scaffolds: bioactive Materials for Tissue Engineering. J. Control. Release 2018, 273, 160–179. DOI: DOI: 10.1016/j.jconrel.2018.01.023.
- Schäfer, O.; Barz, M. Of Thiols and Disulfides: Methods for Chemoselective Formation of Asymmetric Disulfides in Synthetic Peptides and Polymers. Chem. Eur. J. 2018, 24, 12131–12142. DOI: 10.1002/chem.201800681.
- Trollsås, M.; Hawker, C. J.; Hedrick, J. L.; Carrot, G.; Hilborn, J. A Mild and Versatile Synthesis for the Preparation of Thiol-Functionalized Polymers. Macromolecules 1998, 31, 5960–5963. DOI: 10.1021/ma980775g.
- Carrot, G.; Hilborn, J. G.; Trollsås, M.; Hedrick, J. L. Two General Methods for the Synthesis of Thiol-Functional Polycaprolactones. Macromolecules 1999, 32, 5264–5269. DOI: 10.1021/ma990198b.
- Qiu, H.; Rieger, J.; Gilbert, B.; Jérôme, R.; Jérôme, C. PLA-Coated Gold Nanoparticles for the Labeling of PLA Biocarriers. Chem. Mater. 2004, 16, 850–856. DOI: 10.1021/cm034519g.
- Javakhishvili, I.; Hvilsted, S. Gold Nanoparticles Protected with Thiol-Derivatized Amphiphilic Poly(ϵ-Caprolactone)-b-Poly(Acrylic Acid). Biomacromolecules 2009, 10, 74–81. DOI: 10.1021/bm800860t.
- Hedfors, C.; Östmark, E.; Malmström, E.; Hult, K.; Martinelle, M. Thiol End-Functionalization of Poly(ε-Caprolactone), Catalyzed by Candida antarctica Lipase B. Macromolecules 2005, 38, 647–649. DOI: 10.1021/ma048056r.
- Takwa, M.; Hult, K.; Martinelle, M. Single-Step, Solvent-Free Enzymatic Route to α,ω-Functionalized Polypentadecalactone Macromonomers. Macromolecules 2008, 41, 5230–5236. DOI: 10.1021/ma800074a.
- Zhu, N.; Zhang, Z.-L.; He, W.; Geng, X.-C.; Fang, Z.; Li, X.; Li, Z.-J.; Guo, K. Highly Chemoselective Lipase from Candida sp. 99-125 Catalyzed Ring-Opening Polymerization for Direct Synthesis of Thiol-Terminated Poly(ɛ-Caprolactone). Chin. Chem. Lett. 2015, 26, 361–364. DOI: 10.1016/j.cclet.2014.11.016.
- Zhu, N.; Ling, J.; Zhu, Y.; Sun, W.; Shen, Z. Novel Direct Synthetic Approach to Thiol-Functionalized Poly(ε-Caprolactone) by Highly Chemselective and Low Costly Rare Earth Phenolate Catalysts. J. Polym. Sci. A Polym. Chem. 2010, 48, 4366–4369. DOI: 10.1002/pola.24233.
- Zhu, N.; Feng, W.; Zhang, Z.; Fang, Z.; Li, Z.; Guo, K. Thiol-Functionalized Branched and Linear Poly(ε-Caprolactone): Direct Synthesis, Characterization and Application in Stabilizing Silver Nanoparticles. Polymer 2015, 80, 88–94. DOI: 10.1016/j.polymer.2015.10.053.
- Liu, Y.; Huang, W.; Zhu, N.; Guo, K. Direct Synthesis of Thiol-Terminated Poly(ε-Caprolactone): A Study on Polymerization Kinetics, Mechanism and Rare Earth Phenolates' Structure-Activity Relationship. RSC Adv. 2017, 7, 37412–37418. DOI: 10.1039/C7RA06781J.
- Zhu, N.; Liu, Y.; Feng, W.; Huang, W.; Zhang, Z.; Hu, X.; Fang, Z.; Li, Z.; Guo, K. Continuous Flow Protecting-Group-Free Synthetic Approach to Thiol-Terminated Poly(ε-Caprolactone). Eur. Polym. J 2016, 80, 234–239. DOI: DOI: 10.1016/j.eurpolymj.2016.04.010.
- Kryuchkov, M. A.; Qi, Y. H.; Perepichka, I. I.; Pelletier, C.; Regnaud, A.; Song, Z.; Varshney, S. K. Challenging Activated Monomer Ring-Opening Polymerization for Direct Synthesis of Thiol End-Functionalized Polyesters. React. Funct. Polym. 2015, 90, 1–6. DOI: DOI: 10.1016/j.reactfunctpolym.2015.03.005.
- Lele, B. S.; Leroux, J. C. Synthesis and Micellar Characterization of Novel Amphiphilic a − B−a Triblock Copolymers of N-(2-Hydroxypropyl)Methacrylamide or N-Vinyl-2-Pyrrolidone with Poly(ε-Caprolactone). Macromolecules 2002, 35, 6714–6723. DOI: 10.1021/ma020433h.
- Lele, B. S.; Leroux, J. C. Synthesis of Novel Amphiphilic Star-Shaped Poly(ε-Caprolactone)-Block-Poly(N-(2-Hydroxypropyl)Methacrylamide) by Combination of Ring-Opening and Chain Transfer Polymerization. Polymer 2002, 43, 5595–5606. DOI: 10.1016/S0032-3861(02)00435-4.
- Takasu, A.; Tsuruta, H.; Narukawa, Y.; Shibata, Y.; Oshimura, M.; Hirabayashi, T. Dual Catalytic System for Combination of Chain and Step Polymerizations: ring-Opening Polymerization of ϵ-Caprolactone and Successive Dehydration Polycondensation with Dicarboxylic Acid Using the Same Catalyst. Macromolecules 2008, 41, 4688–4693. DOI: 10.1021/ma8005944.
- Molla, M. R.; Ghosh, S. Exploring Versatile Sulfhydryl Chemistry in the Chain End of a Synthetic Polylactide. Macromolecules 2012, 45, 8561–8570. DOI: 10.1021/ma302130f.
- Bednarek, M. Coupling Reaction with Thiols as the Efficient Method of Functionalization of ‘‘Clickable” Polylactide. React. Funct. Polym. 2013, 73, 1130–1136. DOI: DOI: 10.1016/j.reactfunctpolym.2013.04.001.
- Hall, D. J.; Van Den Berghe, H. M.; Dove, A. P. Synthesis and Post-Polymerization Modification of Maleimide-Containing Polymers by ‘Thiol-Ene’ Click and Diels–Alder Chemistries. Polym. Int. 2011, 60, 1149–1157. DOI: 10.1002/pi.3121.
- Pounder, R. J.; Stanford, M. J.; Brooks, P.; Richards, S. P.; Dove, A. P. Metal Free Thiol-Maleimide 'Click' Reaction as a Mild Functionalisation Strategy for Degradable Polymers. Chem. Commun. 2008, 5158–5160. DOI: 10.1039/b809167f.
- Stanford, M. J.; Pflughaupt, R. L.; Dove, A. P. Synthesis of Stereoregular Cyclic Poly(Lactide)s via “Thiol − Ene” Click Chemistry. Macromolecules 2010, 43, 6538–6541. DOI: 10.1021/ma101291v.
- El Habnouni, S.; Darcos, V.; Garric, X.; Lavigne, J.-P.; Nottelet, B.; Coudane, J. Mild Methodology for the Versatile Chemical Modification of Polylactide Surfaces: Original Combination of Anionic and Click Chemistry for Biomedical Applications. Adv. Funct. Mater. 2011, 21, 3321–3330. DOI: 10.1002/adfm.201100412.
- Zhu, Y.; Mao, Z.; Gao, C. Aminolysis-Based Surface Modification of Polyesters for Biomedical Applications. RSC Adv. 2013, 3, 2509–2519. DOI: 10.1039/C2RA22358A.
- Ponsart, S.; Coudane, J.; Vert, M. A Novel Route to Poly(ε-Caprolactone)-Based Copolymers via Anionic Derivatization. Biomacromolecules 2000, 1, 275–281. DOI: 10.1021/bm005521t.
- Nottelet, B.; Tambutet, G.; Bakkour, Y.; Coudane, J. Redox and Thiol-Ene Cross-Linking of Mercapto Poly(ε-Caprolactone) for the Preparation of Reversible Degradable Elastomeric Materials. Polym. Chem. 2012, 3, 2956–2963. DOI: 10.1039/c2py20436c.
- Leroy, A.; Al Samad, A.; Garric, X.; Hunger, S.; Noel, D.; Coudane, J.; Nottelet, B. Biodegradable Networks for Soft Tissue Engineering by Thiol-Yne Photo Cross-Linking of Multifunctional Polyesters. RSC Adv. 2014, 4, 32017–32023. DOI: 10.1039/C4RA03665D.
- Babinot, J.; Renard, E.; Le Droumaguet, B.; Guigner, J.-M.; Mura, S.; Nicolas, J.; Couvreur, P.; Langlois, V. Facile Synthesis of Multicompartment Micelles Based on Biocompatible Poly(3-Hydroxyalkanoate). Macromol. Rapid Commun. 2013, 34, 362–368. DOI: 10.1002/marc.201200692.
- Chanda, S.; Ramakrishnan, S. Poly(Alkylene Itaconate)s - An Interesting Class of Polyesters with Periodically Located Exo-Chain Double Bonds Susceptible to Michael Addition. Polym. Chem. 2015, 6, 2108–2114. DOI: 10.1039/C4PY01613K.
- Yan, Y.; Siegwart, D. J. Scalable Synthesis and Derivation of Functional Polyesters Bearing Ene and Epoxide Side Chains. Polym. Chem. 2014, 5, 1362–1371. DOI: 10.1039/C3PY01474F.
- Kalelkar, P. P.; Alas, G. R.; Collard, D. M. Synthesis of an Alkene-Containing Copolylactide and Its Facile Modification by the Addition of Thiols. Macromolecules 2016, 49, 2609–2617. DOI: 10.1021/acs.macromol.5b02431.
- Chen, W.; Yang, H.; Wang, R.; Cheng, R.; Meng, F.; Wei, W.; Zhong, Z. Versatile Synthesis of Functional Biodegradable Polymers by Combining Ring-Opening Polymerization and Postpolymerization Modification via Michael-Type Addition Reaction. Macromolecules 2010, 43, 201–207. DOI: 10.1021/ma901897y.
- Onbulak, S.; Tempelaar, S.; Pounder, R. J.; Gok, O.; Sanyal, R.; Dove, A. P.; Sanyal, A. Synthesis and Functionalization of Thiol-Reactive Biodegradable Polymers. Macromolecules 2012, 45, 1715–1722. DOI: 10.1021/ma2019528.
- Zou, J.; Hew, C. C.; Themistou, E.; Li, Y.; Chen, C.-K.; Alexandridis, P.; Cheng, C. Clicking Well-Defined Biodegradable Nanoparticles and Nanocapsules by UV-Induced Thiol-Ene Cross-Linking in Transparent Miniemulsions. Adv. Mater. 2011, 23, 4274–4277. DOI: 10.1002/adma.201101646.
- Silvers, A. L.; Chang, C.-C.; Emrick, T. Functional Aliphatic Polyesters and Nanoparticles Prepared by Organocatalysis and Orthogonal Grafting Chemistry. J. Polym. Sci. A Polym. Chem. 2012, 50, 3517–3529. DOI: 10.1002/pola.26114.
- Darcos, V.; Antoniacomi, S.; Paniagua, C.; Coudane, J. Cationic Polyesters Bearing Pendent Amino Groups Prepared by Thiol–Ene Chemistry. Polym. Chem. 2012, 3, 362–368. DOI: 10.1039/C1PY00414J.
- Baumgartner, R.; Song, Z.; Zhang, Y.; Cheng, J. Functional Polyesters Derived from Alternating Copolymerization of Norbornene Anhydride and Epoxides. Polym. Chem. 2015, 6, 3586–3590. DOI: 10.1039/C5PY00119F.
- Strandman, S.; Gautrot, J. E.; Zhu, X. X. Recent Advances in Entropy-Driven Ring-Opening Polymerizations. Polym. Chem. 2011, 2, 791–799. DOI: 10.1039/C0PY00328J.
- Ates, Z.; Thornton, P. D.; Heise, A. Side-Chain Functionalisation of Unsaturated Polyesters from Ring-Opening Polymerisation of Macrolactones by Thiol-Ene Click Chemistry. Polym. Chem. 2011, 2, 309–312. DOI: 10.1039/C0PY00294A.
- Fuoco, T.; Meduri, A.; Lamberti, M.; Venditto, V.; Pellecchia, C.; Pappalardo, D. Ring-Opening Polymerization of ω-6-Hexadecenlactone by a Salicylaldiminato Aluminum Complex: A Route to Semicrystalline and Functional Poly(Ester)s. Polym. Chem. 2015, 6, 1727–1740. DOI: 10.1039/C4PY01445F.
- Pepels, M. P. F.; Koeken, R. A. C.; van der Linden, S. J. J.; Heise, A.; Duchateau, R. Mimicking (Linear) Low-Density Polyethylenes Using Modified Polymacrolactones. Macromolecules 2015, 48, 4779–4792. DOI: 10.1039/C4PY01445F.
- Ates, Z.; Heise, A. Functional Films from Unsaturated Poly(Macrolactones) by Thiol-Ene Cross-Linking and Functionalisation. Polym. Chem. 2014, 5, 2936–2941. DOI: 10.1039/c3py01679j.
- Claudino, M.; van der Meulen, I.; Trey, S.; Jonsson, M.; Heise, A.; Johansson, M. Photoinduced Thiol–Ene Crosslinking of Globalide/ε-Caprolactone Copolymers: Curing Performance and Resulting Thermoset Properties. J. Polym. Sci. A Polym. Chem. 2012, 50, 16–24. DOI: 10.1002/pola.24940.
- Guindani, C.; Dozoretz, P.; Araújo, P. H. H.; Ferreira, S. R. S.; de Oliveira, D. N-Acetylcysteine Side-Chain Functionalization of Poly(Globalide-co-ε-Caprolactone) through Thiol-Ene Reaction. Mater. Sci. Eng., C 2019, 94, 477–483. DOI: DOI: 10.1016/j.msec.2018.09.060.
- de Oliveira, F. C. S.; Olvera, D.; Sawkins, M. J.; Cryan, S.-A.; Kimmins, S. D.; da Silva, T. E.; Kelly, D. J.; Duffy, G. P.; Kearney, C.; Heise, A. Direct UV-Triggered Thiol–Ene Cross-Linking of Electrospun Polyester Fibers from Unsaturated Poly(Macrolactone)s and Their Drug Loading by Solvent Swelling. Biomacromolecules 2017, 18, 4292–4298. DOI: 10.1021/acs.biomac.7b01335.
- Gunay, U. S.; Cetin, M.; Daglar, O.; Hizal, G.; Tunca, U.; Durmaz, H. Ultrafast and Efficient Aza- and thiol-Michael Reactions on a Polyester Scaffold with Internal Electron Deficient Triple Bonds. Polym. Chem. 2018, 9, 3037–3054. DOI: 10.1039/C8PY00485D.
- Seabra, A. B.; da Silva, R.; de Oliveira, M. G. Polynitrosated Polyesters: Preparation, Characterization, and Potential Use for Topical Nitric Oxide Release. Biomacromolecules 2005, 6, 2512–2520. DOI: 10.1021/bm050216z.
- Kato, M.; Toshima, K.; Matsumura, S. Direct Enzymatic Synthesis of a Polyester with Free Pendant Mercapto Groups. Biomacromolecules 2009, 10, 366–373. DOI: 10.1021/bm801132d.
- Tanaka, A.; Kohri, M.; Takiguchi, T.; Kato, M.; Matsumura, S. Enzymatic Synthesis of Reversibly Crosslinkable Polyesters with Pendant Mercapto Groups. Polym. Degrad. Stab. 2012, 97, 1415–1422. DOI: DOI: 10.1016/j.polymdegradstab.2012.05.016.
- Yamamoto, K.; Takasu, A. Preparation of Gelatinous Reversible Addition − Fragmentation Chain Transfer Agents “RAFT Gel” via Chemoselective Polycondensations of a Dicarboxylic Acid Containing a Mercapto Group and Diols. Macromolecules 2010, 43, 8519–8523. DOI: 10.1021/ma101501r.
- Yapor, J. P.; Neufeld, B. H.; Tapia, J. B.; Reynolds, M. M. Biodegradable Crosslinked Polyesters Derived from Thiomalic Acid and S-Nitrosothiol Analogues for Nitric Oxide Release. J. Mater. Chem. B 2018, 6, 4071–4081. DOI: 10.1039/C8TB00566D.
- Pappalardo, D.; Målberg, S.; Finne-Winstrad, A.; Albertsson, A.-C. Synthetic Pathways Enables the Design of Functionalized Poly(Lactic Acid) with Pendant Mercapto Groups. J. Polym. Sci. A Polym. Chem. 2012, 50, 792–800. DOI: 10.1002/pola.25834.
- Wang, N.; Dong, A.; Radosz, M.; Shen, Y. Thermoresponsive Degradable Poly(Ethylene Glycol) Analogues. J. Biomed. Mater. Res. Part 2008, 84A, 148–157. DOI: 10.1002/jbm.a.31466.
- Jasinski, F.; Rannée, A.; Schweitzer, J.; Fischer, D.; Lobry, E.; Croutxé-Barghorn, C.; Schmutz, M.; Le Nouen, D.; Criqui, A.; Chemtob, A. Thiol–Ene Linear Step-Growth Photopolymerization in Miniemulsion: Fast Rates, Redox-Responsive Particles, and Semicrystalline Films. Macromolecules 2016, 49, 1143–1153. DOI: 10.1021/acs.macromol.5b02512.
- Hong, S. H.; Patel, T.; Ip, S.; Garg, S.; Oh, J. K. Microfluidic Assembly to Synthesize Dual Enzyme/Oxidation-Responsive Polyester-Based Nanoparticulates with Controlled Sizes for Drug Delivery. Langmuir 2018, 34, 3316–3325. DOI: 10.1021/acs.langmuir.8b00338.
- Beyazkilic, Z.; Lligadas, G.; Ronda, J. C.; Galià, M.; Cádiz, V. Vinylsulfide-Containing Polyesters and Copolyesters from Fatty Acids: Thiol-Yne Monomer Synthesis and Thiol-Ene Functionalization. Macromol. Chem. Phys. 2014, 215, 2248–2259. DOI: 10.1002/macp.201400191.
- Albertsson, A.-C.; Varma, I. K. Recent Developments in Ring Opening Polymerization of Lactones for Biomedical Applications. Biomacromolecules 2003, 4, 1466–1486. DOI: 10.1021/bm034247a.
- Dechy-Cabaret, O.; Martin-Vaca, B.; Bourissou, D. Controlled Ring-Opening Polymerization of Lactide and Glycolide. Chem. Rev. 2004, 104, 6147–6176. DOI: 10.1021/cr040002s.
- Kim, H.; Olsson, J. V.; Hedrick, J. L.; Waymouth, R. M. Facile Synthesis of Functionalized Lactones and Organocatalytic Ring-Opening Polymerization. ACS Macro Lett. 2012, 1, 845–847. DOI: 10.1021/mz3001397.
- Hao, J.; Kos, P.; Zhou, K.; Miller, J. B.; Xue, L.; Yan, Y.; Xiong, H.; Elkassih, S.; Siegwart, D. J. Rapid Synthesis of a Lipocationic Polyester Library via Ring-Opening Polymerization of Functional Valerolactones for Efficacious siRNA Delivery. J. Am. Chem. Soc. 2015, 137, 9206–9209. DOI: 10.1021/jacs.5b03429.
- Stöhr, O.; Ritter, H. Hyperbranched Polyesters Based on Hydroxyalkyl-Lactones via Thiol-Ene Click Reaction. Polym. Int. 2015, 64, 37–41. DOI: 10.1002/pi.4825.
- Li, X.; Li, H.; Zhao, Y.; Tang, X.; Ma, S.; Gong, B.; Li, M. Facile Synthesis of Well-Defined Hydrophilic Polyesters as Degradable Poly(Ethylene Glycol)-like Biomaterials. Polym. Chem. 2015, 6, 6452–6456. DOI: 10.1039/C5PY00762C.
- Song, L.; Ding, A.-X.; Zhang, K.-X.; Gong, B.; Lu, Z.-L.; He, L. Degradable Polyesters via Ring-Opening Polymerization of Functional Valerolactones for Efficient Gene Delivery. Org. Biomol. Chem. 2017, 15, 6567–6574. DOI: 10.1039/C7OB00822H.
- Middleton, J. C.; Tipton, A. J. Synthetic Biodegradable Polymers as Orthopedic Devices. Biomaterials 2000, 21, 2335–2346. DOI: 10.1016/S0142-9612(00)00101-0.
- Yu, L.; Zhang, M.; Du, F.-S.; Li, Z.-C. ROS-Responsive Poly(ε-Caprolactone) with Pendent Thioether and Selenide Motifs. Polym. Chem. 2018, 9, 3762–3773. DOI: 10.1039/C8PY00620B.
- Borchmann, D. E.; ten Brummelhuis, N.; Weck, M. GRGDS-Functionalized Poly(Lactide)-Graft-Poly(Ethylene Glycol) Copolymers: combining Thiol–Ene Chemistry with Staudinger Ligation. Macromolecules 2013, 46, 4426–4431. DOI: 10.1021/ma4005633.
- Long, T. R.; Wongrakpanich, A.; Do, A.-V.; Salem, A. K.; Bowden, N. B. Long-Term Release of a Thiobenzamide from a Backbone Functionalized Poly(Lactic Acid). Polym. Chem. 2015, 6, 7188–7195. DOI: 10.1039/C5PY01059D.
- Fuoco, T.; Finne-Wistrand, A.; Pappalardo, D. A Route to Aliphatic Poly(Ester)s with Thiol Pendant Groups: From Monomer Design to Editable Porous Scaffolds. Biomacromolecules 2016, 17, 1383–1394. DOI: 10.1021/acs.biomac.6b00005.
- Leemhuis, M.; Akeroyd, N.; Kruijtzer, J. A. W.; van Nostrum, C. F.; Hennink, W. E. Synthesis and Characterization of Allyl Functionalized Poly(α-Hydroxy)Acids and Their Further Dihydroxylation and Epoxidation. Eur. Polym. J. 2008, 44, 308–317. DOI: DOI: 10.1016/j.eurpolymj.2007.12.004.
- Kalelkar, P. P.; Collard, D. M. Thiol-Substituted Copolylactide: synthesis, Characterization and Post-Polymerization Modification Using Thiol–Ene Chemistry. Polym. Chem. 2018, 9, 1022–1031. DOI: 10.1039/C7PY01930K.
- Jing, F.; Hillmyer, M. A. A Bifunctional Monomer Derived from Lactide for Toughening Polylactide. J. Am. Chem. Soc. 2008, 130, 13826–13827. DOI: 10.1021/ja804357u.
- Fuoco, T.; Pappalardo, D.; Finne-Wistrand, A. Redox-Responsive Disulfide cross-Linked PLA–PEG Nanoparticles. Macromolecules 2017, 50, 7052–7061. DOI: 10.1021/acs.macromol.7b01318.
- Yassin, M. A.; Fuoco, T.; Mohamed-Ahmed, S.; Mustafa, K.; Finne-Wistrand, A. 3D and Porous RGDC-Functionalized Polyester Based Scaffolds as a Niche to Induce Osteogenic Differentiation of Human Bone Marrow Stem Cells. Macromol. Biosci. 2019, 1900049 (1–12).
- Fagerland, J.; Pappalardo, D.; Schmidt, B.; Syrén, P.-O.; Finne-Wistrand, A. Template-Assisted Enzymatic Synthesis of Oligopeptides from a Polylactide Chain. Biomacromolecules 2017, 18, 4271–4280. DOI: 10.1021/acs.biomac.7b01315.
- Fukushima, K. Poly(Trimethylene Carbonate)-Based Polymers Engineered for Biodegradable Functional Biomaterials. Biomater. Sci. 2016, 4, 9–24. DOI: 10.1039/C5BM00123D.
- Brannigan, R. P.; Dove, A. P. Synthesis, Properties and Biomedical Applications of Hydrolytically Degradable Materials Based on Aliphatic Polyesters and Polycarbonates. Biomater. Sci. 2017, 5, 9–21. DOI: 10.1039/C6BM00584E.
- Chen, W.; Zou, Y.; Jia, J.; Meng, F.; Cheng, R.; Deng, C.; Feijen, J.; Zhong, Z. Functional Poly(ε-Caprolactone) via Copolymerization of ε-Caprolactone and Pyridiyl Disulfide-Containing Ccyclic Carbonate: Controlled Synthesis and Facile Access to Reduction-Sensitive Biodegradable Graft Copolymer Micelles. Macromolecules 2013, 46, 699–707. DOI: 10.102/ma302499a.
- Chen, W.; Zou, Y.; Meng, F.; Cheng, R.; Deng, C.; Feijen, J.; Zhong, Z. Glyco-Nanoparticles with Sheddable Saccharide Shells: A Unique and Potent Platform for Hepatoma-Targeting Delivery of Anticancer Drugs. Biomacromolecules 2014, 15, 900–907. DOI: 10.102/bm401749t.
- Wang, R.; Chen, W.; Meng, F.; Cheng, R.; Deng, C.; Feijen, J.; Zhong, Z. Unprecedented Access to Functional Biodegradable Polymers and Coating. Macromolecules 2011, 44, 6009–6016. DOI: 10.1021/ma200824k.