Abstract
The outstanding performance of conventional thermosets arising from their covalently cross-linked networks directly results in a limited recyclability. The available commercial or close-to-commercial techniques facing this challenge can be divided into mechanical, thermal, and chemical processing. However, these methods typically require a high energy input and do not take the recycling of the thermoset matrix itself into account. Rather, they focus on retrieving the more valuable fibers, fillers, or substrates. To increase the circularity of thermoset products, many academic studies report potential solutions which require a reduced energy input by using degradable linkages or dynamic covalent bonds. However, the majority of these studies have limited potential for industrial implementation. This review aims to bridge the gap between the industrial and academic developments by focusing on those which are most relevant from a technological, sustainable and economic point of view. An overview is given of currently used approaches for the recycling of thermoset materials, the development of novel inherently recyclable thermosets and examples of possible applications that could reach the market in the near future.
1. Introduction
The increasing amount of fossil-based plastic waste ending up in our environment is one of the most pressing issues of this decade. The installment of a circular economy, as described by the Ellen MacArthur Foundation,Citation1 could contribute to a more sustainable future for generations to come. Two key aspects of this concept are that the vast majority of plastic materials should be recyclable and that those materials entering the chain should be bio-based. Although the recycling of plastic materials is currently achieved only for a very small percentage of all plastics produced (global average in 2016 was 10%Citation1), the recycling and reprocessing of thermoplastic materials can generally be performed relatively easily from a practical and technological point of view.Citation2–5 Thermoset materials, on the other hand, are classified amongst the most difficult materials to recycle, and for a long time this was even considered impossible. The defining characteristic of a thermoset is the presence of covalent intermolecular chemical cross-links that create increased strength and stiffness, and reduce the susceptibility to creep as compared to their thermoplastic counterparts. As a result, they become less susceptible to damage by thermal and chemical impulses from the nearby environment which makes them highly suitable for use in structural and protective (composite) applications (e.g. aerospace materials and wind turbines). However, a direct consequence of this high thermal and mechanical stability is that thermosets are very difficult to recycle. Major thermoset resin classes are isocyanates, unsaturated polyesters, formaldehydes, epoxies, and alkyds.Citation6,Citation7 These are usually applied as multicomponent reactive formulations to bind (functional) particles and reinforcing fibers to create, after curing, strong lightweight materials.
Currently, the majority of thermoset plastic waste is being managed by landfilling, which is, according to the Environmental Protection Agency’s (EPA) guidelines for solid waste management (1989), the least preferred waste management approach. Other industrial ways to handle thermoset waste are mainly limited to grinding and combustion.Citation8,Citation9 Ground thermosets are re-used, typically in the form of fillers, in lower quality applications without separation of the specific constituents and without changing its molecular structure. Combustion mainly targets to regain the more valuable reinforcing fibers and to recover energy by burning off the thermoset matrix, which is considered to be the lowest class of (partial) recycling.Citation10 However, in order to increase the composites’ contribution to the circular economy, it is critical that not only the fillers or fibers, but also the thermoset matrix itself is regained and re-used.
A more promising route to achieve recyclable thermosets is by the introduction of degradable or dynamic covalent bonds within their molecular structure. The dynamic response of these bonds is triggered by an external stimulus which can be of thermal, chemical or optical nature.Citation11,Citation12 In the past decades, polymers containing such linkages have been reported under a variety of nomenclatures, such as recyclable, reworkable, remendable, and self-healing materials.Citation12–17 The majority of the materials reported in these studies are industrially irrelevant due to complex expensive chemistry or a lack of relevant mechanical properties. Nevertheless, this new class of thermosets is slowly moving out of the academic spectrum, and the first examples of recyclable thermosets are approaching the market.Citation18–20 In the last decade, extensive reviews were published that either focus on the industrial approaches for thermoset recyclingCitation8,Citation9,Citation21 or on the wide range of available chemistries for dynamic covalently cross-linked polymers.Citation11–13 This review aims to connect these industrial and academic developments by focusing on those which are the most relevant from both an economic and sustainability point of view. In doing so, an overview is given of the currently applied solutions for the recycling of thermoset materials, the development of novel recyclable thermosets and examples of possible applications that could reach the market in the near future.
2. Recycling of conventional thermoset materials
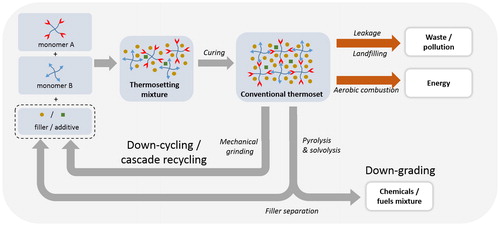
Due to an increasing number of energy-resourceful applications that use lightweight thermoset materials and restrictions on landfill solutions throughout the EU, the development of thermoset recycling strategies has accelerated in recent years.Citation8,Citation22,Citation23 Another important driver is the value of the regained products. Regarding thermosets, most attention is given to the recycling of fiber-reinforced composites, since the fibers are generally more valuable than the matrix material, especially when carbon fibers are used. As indicated by the European Union’s Waste Framework directive, the most favorable method of recycling is the direct re-use of components in similar or lower performance applications without any form of reprocessing.Citation24 Therefore, a first consideration in the recycling of high-quality demanding thermoset products should always be to verify the possibility of direct re-use in applications where a lower quality is acceptable. Examples of these applications can be found in housing and sports equipment.Citation25,Citation26 However, since the options for re-use are limited and waste material often has complex non-adaptable geometries, the direct re-use of thermoset products will most likely remain a specific niche market. Therefore direct re-use will not significantly contribute to solving the thermoset waste problem. Waste treatment technologies that are capable of processing thermosets via a more universal approach were therefore studied thoroughly in the past decades. The presently available techniques that are already commercial or are approaching a commercial status can be divided into mechanical, thermal and chemical processing.Citation9 A schematic overview of conventional thermoset composite waste processing and recycling routes is shown in .
Figure 1. Schematic overview of conventional thermoset composite waste processing and recycling routes.
Mechanical recycling most often involves a cutting and grinding step in order to transform scrap products of different shapes into uniformly sized flakes with a typical size of 50–100 mm using a speed cutting or crushing mill.Citation10 This form of mechanical recycling is applied to both thermoset and thermoplastic materials. The fundamental difference is that thermoplastics can be thermally reprocessed from this point onwards, while thermoset flakes need more advanced grinding procedures. These procedures use hammer mills or high-speed mills to grind the scrap flakes into finer products of several millimeters down to sizes within the micrometer range. For fiber-reinforced thermosets, a large disadvantage of this approach is that the matrix and the fibers are not treated separately and as a result, any orientational advantage of the fiber material is reduced or lost. This consequently decreases the superior mechanical properties and value of the product. Therefore, most investigations on mechanical recycling have targeted the processing of glass fiber-reinforced polymer (GFRP) products instead of their more expensive carbon fiber-reinforced polymer (CFRP) counterparts. Furthermore, the higher strength at break and stiffness of the carbon fibers also requires higher forces and more resistant machinery. The small-sized (<50 µm diameter) powder obtained after mechanical grinding can generally only be used as a filler in new thermoset and thermoplastic materials.Citation27 Unfortunately, these powders cannot compete economically with conventional filler material such as calcium carbonates and silicates.Citation28 To have any added value as filler, the ground products should therefore give an additional form of reinforcement as was intended in the original application. An example was given by Palmer et al., who developed a method to maintain the fibrous structure of the recyclates after grinding, which were successfully used as reinforcing filler in sheet molding compounds.Citation29
Thermal recycling can be divided into aerobic and anaerobic combustion where the latter is often defined as pyrolysis. The presence of oxygen is directly connected to the recycling output. The most straightforward aerobic thermal treatment is incineration, solely leading to energy recovery. However, as no material is actually recovered this is not classified as recycling and therefore not addressed in this review. The fluidized-bed combustion process developed by Pickering et al. is an example of an aerobic composite recycling process for GFRPs that is capable of fiber recovery while the thermoset matrix is broken down in CO2 and water vapor which is converted into energy.Citation30 A second promising example of aerobic thermal recycling is the use of scrap GFR thermosets as feedstock for cement processing in cement kilns. Again, the matrix material is converted into gas and energy, but the glass, mainly consisting of borosilicate and calcium carbonate, is used as resource material for the cement clinker which is later ground to cement. The European Composites Industry Association recommends GFR thermosets to be recycled via this approach as it is currently the only technically and economically feasible alternative to landfill.Citation28
Compared to aerobic combustion, pyrolysis will break down the thermoset matrix into lower molecular weight organic compounds of which the chemical nature is correlated to the original material.Citation31 The applied temperatures lie within the range of 300–800 °C and the resulting products are gaseous, liquid and solid carbon-based compounds that, contrary to those of aerobic combustion, can potentially be used as feedstock for further chemical processing.Citation8,Citation32 Another advantage is that, under anaerobic conditions, carbon fibers in CFRPs are not affected by oxidation which improves the recycling quality of these products. Compared to processing into cement kilns, pyrolysis gives rise to the option of re-using the thermoset product components more than once. With the assistance of specific catalysts, the thermoset pyrolysis recycling output can be enhanced. This is exemplified by the work of Ciprioti et al. who reported on improving the recycling yield of (thermoplastic) waste electrical and electronic equipment (WEEE) products by using fly ash (FA) as catalyst.Citation33,Citation34 As FA is a waste product originating from coal plants it actually adds to the circularity of the process and does not heavily impact the costs. They also showed that the thermoset recycling yield can be enhanced by modification of the catalyst prior to pyrolysis. Their concept is further illustrated in . These results add to the fact that, from a circular economy point of view, pyrolysis is considered to be the most optimal industrially available thermal recycling treatment at present. However, even though industrial pyrolysis processes are currently being developed, their economic feasibility is still questionable.Citation35 Continued research efforts on aspects such as reduction of pollutant emissionCitation36 and the required process energyCitation37 are crucial before pyrolysis can have a significant impact on the thermoset waste issue.
Figure 2. Overview of the FA-catalyzed pyrolysis process of WEEE plastics leading to exploitable fuels and feedstocks. The figure shows that the recycling output can be improved by enhancing the FA catalyst via acid (FAMA) and base (FAMB) treatments. Reprinted with permission from Benedetti et al.[Citation34] Copyright 2017 SpringerLink.
![Figure 2. Overview of the FA-catalyzed pyrolysis process of WEEE plastics leading to exploitable fuels and feedstocks. The figure shows that the recycling output can be improved by enhancing the FA catalyst via acid (FAMA) and base (FAMB) treatments. Reprinted with permission from Benedetti et al.[Citation34] Copyright 2017 SpringerLink.](/cms/asset/aa9bc91b-db7f-42af-b225-3ddd1437920a/lmsc_a_1673406_f0002_c.jpg)
Solvolysis (often referred to as chemical recycling) is a recycling strategy in which a liquid medium is used to degrade and dissolve the polymer matrix so that the reinforcing fibers or particles can be regained. Similar to thermal recycling, the main driver for development of solvolysis for thermosets is the potential to regain carbon fibers from thermoset composites. Additionally, as with pyrolysis, the degraded and dissolved organic compounds originating from the matrix can be reclaimed from the solvent and be re-used as molecular building blocks. Based on the applied dissolution medium, solvolysis can be divided into hydrolysis,Citation38,Citation39 glycolysis,Citation40–42 and acid digestion.Citation8,Citation43,Citation44 The selected solvent determines which type of molecular bonds can be broken. These solvolysis treatments were successfully reported for a wide range of polymer networks including those with amine-epoxy,Citation45,Citation46 anhydride-epoxy,Citation47 polyester,Citation40,Citation48,Citation49 and polyurethaneCitation50 linkages. A recent trend is the use of (near) supercritical fluids as recycling medium. These serve as good solvents for degradation chemistry due to their high mass transport coefficients, high diffusivities, and low viscosities. An additional advantage of using supercritical fluids over pure liquid solvents is that the solvent power, and thereby the reaction rates and selectivity, can be controlled by adjusting the applied pressure.Citation51–54 The main advantage of solvolysis over pyrolysis is that, at least conceptually, no thermal energy is required, which makes the process more energy-efficient. However, the majority of solvolysis methods investigated in the past decades are performed at temperatures above 200 °C and therefore they should be considered as thermo-chemical recycling.Citation9 Another issue is that the frequent use of hazardous solvents often results in poor life cycle analysis scores. Hence, the environmental impact of chemical recycling remains questionable.Citation55 Nevertheless, the concept of chemical recycling remains promising, provided that future research efforts focus on methods that are energy-efficient and produce organic components that can easily be reclaimed from the selected solvent. This topic and the, mainly academic, progress made so far is further discussed in the next chapter.
Citation3. Development of thermosets with inherent recyclability
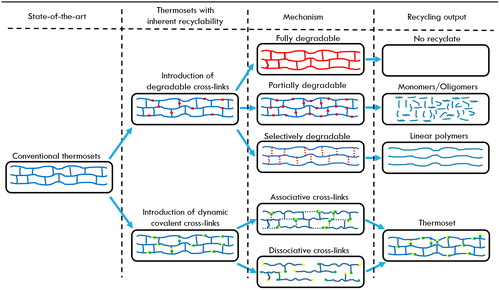
The aforementioned routes to recycle conventional thermoset polymers (e.g. epoxy-, polyurethane-, and polyester-based composites) most often require extensive amounts of energy and are generally not regenerating the polymer itself as is shown in . Therefore, there is a high demand for novel thermosets with polymer architectures that allow for low energy molecular debonding that enables easy matrix removal or recycling. This molecular debonding can be obtained by stimuli-triggered degradation or by introducing dynamic covalent bonds in the networks. These concepts can be subdivided into different mechanisms which are schematically depicted in . Stimuli-triggered degradation is achieved by modifying the conventional cross-linked resin via inclusion of labile bonds. It can result in different degradation products depending on the amount and location of the labile linkages within the polymer structure (): (a) total degradation of the polymer network into lower molecular weight organic substances (gases and char), b) degradation of the polymer backbone to yield monomers or oligomers, and (c) degradation of cross-linking bonds, resulting in a thermoplastic polymer.
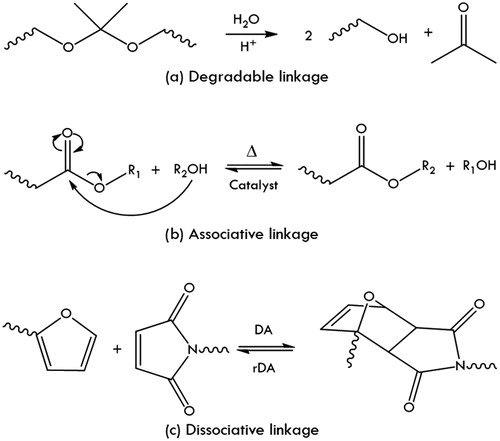
Even though stimuli-triggered degradation can lead to an improved thermoset recyclability, the original polymer architecture is irreversibly destroyed and as a consequence, the polymer recyclate needs to be resynthesized or be used in lower performing applications (i.e. down-cycling). This is illustrated in , which shows the molecular mechanism of a degradable acetal linkage indicating the irreversible nature of this type of bond. This drawback can be overcome by the use of dynamic covalent networks which function via either associative or dissociative mechanisms. The mechanism type affects the physical properties of the material during the recycling process, but both mechanisms yield a cross-linked structure with properties similar to the original thermoset. This is illustrated in which show the molecular mechanisms of a associative (transesterification) and dissociative (Diels-Alder [DA] reaction) linkage, respectively. The concepts depicted in and their practical implications and limitations are each further described in the upcoming sections.
Figure 4. Examples of molecular mechanisms describing the main concepts of recyclable thermosets; a degradable acetal linkage (a), an associative transesterification reaction, (b) and a dissociative Diels-Alder reaction (c).
3.1. Stimuli-triggered degradable thermosets
In essence, the vast majority of all industrially available routes that deal with the end of life of thermosets (e.g. grinding, pyrolysis, and solvolysis) are based on polymer degradation. However, these techniques focus on the degradation of polymers that were actually designed to remain stable under extreme conditions which leads to a very high energy consumption. Alternatively, thermoset polymers can be designed in such a way that low energy requiring external stimuli can be used to trigger degradation on demand, while maintaining their outstanding performance. This is achieved by the introduction of stimuli-triggered cleavable linkages in the polymer network. Examples of such linkages are esters, orthoesters, sulfur-, nitrogen-, and phosphorus-containing linkages, carbonate, acetal, hemiacetal/hemiketal ester, olefinic, tertiary ether and peroxide bonds, vicinal tricarbonyl, and (retro) DA addition bonds. The specific chemistry that is responsible for the degradative behavior of these bonds is not subject of this review but can be found in the extensive work by Ma and Webster.Citation17
Based on the type, amount, and location of the stimuli-responsive molecular moieties within the polymer (main or side) chain, the extent of degradation will differ. This leads to mechanisms that break down the polymer matrix into either (i) non-usable components (e.g. char and gases) or (ii) deliver directly reusable monomer/oligomer/polymer systems on the other side. This section reviews different classes of degradable thermosets based on the type of external stimulus that is required to trigger the degradation and intends to critically assess the current methods to develop degradable thermosets with respect to how efficient they are from the energy consumption and materials recovery point of view.
3.1.1. Triggered degradation without matrix recycling
The cumulative goal of stimuli-triggered degradable thermoset matrices is to decrease the energy required to degrade the polymers. This can be achieved by including more sensitive thermally cleavable bonds that lower the degradation temperature within the thermoset or by introducing chemical bonds that are cleaved upon exposure to other, alternative stimuli (pH, UV, and catalysts). However, the majority of the studies reported do not describe the attempt (or possibility) to recover the matrix. Nevertheless, these systems contribute to an enhanced thermoset (product) recyclability and are therefore covered in this review.
3.1.1.1. Thermally cleavable linkages
Thermosets with a lowered degradation onset temperature within the range of 200 °C up to 300 °C are often called thermally reworkable.Citation14,Citation56–58 Within this class of polymers, the main challenge is to have low degradation temperatures without affecting the material stability and lifetime during application.
An early example of degradation temperature reduction by introducing reworkable bonds in epoxy-based thermosets was given by Wang et al. who reported two series of reworkable cycloaliphatic diepoxides containing thermally cleavable carbamateCitation57 and carbonateCitation58 linkages. These materials undergo curing reactions with cyclic anhydride in a similar fashion as a commercial cycloaliphatic epoxide, except that the carbamate group within the diepoxides can act as the internal catalyst. Once cured, these thermosets decompose between 200–300 °C (as compared with 350 °C for the commercial cycloaliphatic epoxide). A second example is reported by Liu et al. who synthesized phosphate and sulfite-containing epoxides based on commercially available resins with tunable degradation temperatures within the reworking temperature range 180–300 °C by adjusting the ratio of the used monomers.Citation59–62 They demonstrated that the C–O bonds in O = P–O–C– and O = S–O–C are less stable than in the O = C–O–C– linkage, which suggests that the stability of C–O bond is strongly affected by the electron-withdrawing effect of the neighboring group. Another example of modification of commercially used thermoset monomers was reported by Zhang et al. who prepared, via a one-step reaction, a series of maleimides containing acetal ester linkages that can be used for electronic chip recycling. Their results showed that the degradation of the acetal ester linkage initiated from ∼225 °C which resulted in a significant decrease of adhesion strength.Citation63
Much lower degradation onset temperatures (Tdeg) were obtained by incorporating thermally cleavable secondary and tertiary ester linkages in epoxiesCitation14,Citation64–77 and (meth)acrylates.Citation78–82 While primary ester linkages have a typical Tdeg of 340 °C, Yang et al. managed to decrease this temperature to 120 °C by introducing the tertiary ester linkages into cycloaliphatic epoxies. The cured epoxies with tertiary esters retain the room temperature mechanical behavior of conventional epoxies comprising primary esters, while having lower mechanical properties at elevated temperatures, thereby offering the possibility of easier thermoset removal. Secondary ester linkages reduced the Tdeg with only 20 °C,Citation14 while hyperbranched aliphatic poly(ester-amides) showed a reduction of 100 °C.Citation75,Citation77 When air is used as the heating medium instead of nitrogen, Tdeg was further decreased by an additional 20 °C (tertiary ester linkages) and 85 °C (secondary esters), as is described by the work of Yang et al.Citation14 and Chen et al.,Citation65 respectively. The latter study also showed that it is possible to increase the content of non-cleavable linkages (from 36 mol.% to 50 mol.%) without impacting the degradation temperature. In order to prevent the uncontrolled hydrolysis of these ester linkages, Li et al. introduced benzoic rings into diepoxies without compromising Tdeg.Citation67 A bio-based approach to modify epoxy thermosets with polylactic acid was reported by Acebo et al. However, compared to earlier described concepts, the reported degradation temperature range (300–400 °C) is high, thereby potentially negating the initial gain in sustainability.Citation83
The energy required to achieve degradation of the cleavable linkages in degradable thermosets can be further decreased by using catalysts. In this respect, González et al. synthesized poly(ether esters) using a conventional epoxy resin (DGEBA) with 7,7‐dimethyl‐6,8‐dioxaspiro[3.5]nonane‐5,9‐dione catalyzed by waterproof environmentally friendly earth triflates. They found that an increase in Lewis acidity leads to a drop in degradation temperature down to a Tdeg of 214 °C.Citation84–86 Significantly lower degradation temperatures of tertiary esters are obtained when using photo-generated acids acting as catalysts as is shown by the work of Okamura et al.Citation82,Citation87,Citation88 They developed photo-cross-linkable reworkable methacrylate and thiol-ene resins with an onset Tdeg of 110 °C. The main drawback of these approaches is that the catalysts used in these syntheses are either not commercially available (9‐fluorenylideneimino-p‐toluenesulfonate)Citation87 or very expensive (di(tert-butylphenyl) iodonium trifluoromethanesulfonate).Citation88
3.1.1.2. Cleavability via alternative (non-thermal) triggers
Besides thermal activation, polymer degradation can be triggered by alternative stimuli such as acidic and basic environments. Many reworkable thermosets are shown to degrade via acidolysis, such as those containing acetal, ketal,Citation89,Citation90 and Schiff baseCitation91 linkages which are reported to degrade in aqueous HCl in combination with various solvents. An interesting comparison between thermal and alkaline degradation can be found in lactone-modified epoxies based on bisphenol-A diglycidyl ether (DGEBA) with tertiary ester groups. These thermosets are shown to degrade thermallyCitation92 or by alkaline hydrolysis.Citation93,Citation94 However, the alkaline hydrolysis proceeds relatively slow, measuring only around 5% weight loss after two months immersion.Citation94 It can be facilitated by hydrolyzing with reflux at elevated temperature (80 °C), where 25 wt.% becomes soluble after only 24 hr, as found by Giménez et al.Citation93
The low-temperature cleavage of C=C bonds via ozonolysis in anhydride containing copolymers was reported by Tsujii et al.Citation95,Citation96 These polymers are thermally stable up to ∼300 °C but can be degraded at temperatures ranging from −73 to 30 °C via ozonolysis in organic solvents. They showed that the degradation efficiency is inversely proportional to the temperature and that degradation occurs within minutes at low temperatures. Cross-linked poly(vinyl alcohol) and poly(vinyl acetate) have also shown ozone-triggered degradability, as reported by Lou et al. Advantageously, in these polymers, ozone degradation was performed in water, thus avoiding organic solvents.Citation97 When the use of organic solvents and cryogenic temperatures is minimized, ozonolysis is considered an environmentally friendly and up-scalable alternative to heavy metal oxidation reactions, as it gives high recyclate yields and does not require additional separation steps to remove unreacted reagents.Citation98
Gallagher et al. reported the synthesis of two new dimethacrylate polymers that contain rigid core structures derived from sugars, glucose and mannose.Citation99 Thermally initiated free radical polymerization of these substrates produced highly cross-linked bio-based thermosets with mechanical properties comparable to those reported for commercially available poly(dimethacrylates). These thermosets completely degrade in aqueous alkaline conditions after 17 days, while remaining stable in aqueous neutral and acidic conditions. Another bio-based stimuli-triggered degradable thermoset was reported by Ma et al., who cross-linked multifunctional carboxylic acids found in fruit juices with epoxidized sucrose soyate in aqueous environment without the use of catalysts. These thermosets, with Tg values in the range 9–96 °C and potential applications in coatings, could be degraded and completely dissolved in aqueous alkaline solutions within 13 min.Citation100 Bio-based thermosets that start degrading upon entering a specific biological environment (e.g. marine, freshwater, soil) could contribute to the thermoset waste problem of certain typical applications such as fishing gear and agricultural tools. However, it is questionable whether it is desirable and achievable to combine the typical high-performance behavior of thermosets with biodegradation. Nevertheless, examples of biodegradable thermosets are reported in soft materials, such as hydrogels and elastomers. Their degradation is often triggered by acidic, lysosomal, and endosomal conditions and these concepts could serve as a starting point for the development of biodegradable thermosets for structural applications.Citation101–106
3.2.1. Triggered degradation with matrix recycling
From a circular point of view, it is highly favorable to engineer a degradable thermoset in such a way that it can be recovered in the form of the original monomers, chemical building blocks or thermoplastic polymers. In this respect, a promising study on a family of recyclable poly(hexahydrotriazine) (PHT) thermosets which can be degraded by strong acid digestion to yield bisaniline monomers was reported by García et al.Citation107 More recently, a new PHT thermoset was prepared via the reaction of 2,2-bis[4-(4-aminophenoxy)phenyl]propane (BBAP) and formaldehyde by Yuan et al., which can be degraded via gentle depolymerization in special diluted acid solution (involving HCl and THF) within 18 hr. The breakdown process demonstrates two stages, dissolution and precipitation, which correspond to depolymerization into soluble oligomers and further generation of monomers,Citation108 as demonstrated in . The acid-catalyzed reversible cross-linking of bifunctional spiro orthoesters synthesized from terephthalic acid, succinic acid, and 1,4‐cyclohexanedicarboxylic acid were reported by Yoshida et al.Citation109 They found that the resulting thermoset could be depolymerized to regenerate the original monomers. Similar structures containing bicyclo orthoester moieties were explored by Hitomi et al.Citation110 By grafting the functional groups on the polymer side chains, they were able to de-cross-link the thermosets leading to regeneration of the starting linear polymers.
Figure 5. Illustrative example and kinetics of the triggered thermoset degradation in a 1 M HCL/THF solution with recovery of the BAPP-PHT matrix. (a) The resin’s degradation status at different times; (b) the resin’s first-stage depolymerization time (t1) and the contact angles of their corresponding H2O/THF solutions; (c) the depolymerization kinetics of the BAPP-PHT and a PHT-based CFRP, (d) 1H NMR spectra of the depolymerized products of the BAPP-PHT at different times; (e) the regeneration curve of BAPP. Reprinted with permission from Yuan et al.[Citation108] Copyright 2017 Nature Publishing Group.
![Figure 5. Illustrative example and kinetics of the triggered thermoset degradation in a 1 M HCL/THF solution with recovery of the BAPP-PHT matrix. (a) The resin’s degradation status at different times; (b) the resin’s first-stage depolymerization time (t1) and the contact angles of their corresponding H2O/THF solutions; (c) the depolymerization kinetics of the BAPP-PHT and a PHT-based CFRP, (d) 1H NMR spectra of the depolymerized products of the BAPP-PHT at different times; (e) the regeneration curve of BAPP. Reprinted with permission from Yuan et al.[Citation108] Copyright 2017 Nature Publishing Group.](/cms/asset/f9b70e5d-c709-46d9-aa47-2243cf86be89/lmsc_a_1673406_f0005_c.jpg)
A clear disadvantage of the aforementioned degradable thermosets with matrix recovery is that harmful solvents are used as degradation triggers. A more sustainable alternative is reported by Ogden and Guan who developed a vitrimer-like thermoset based on boroxine networks. In addition to unusual mechanical properties (strong and highly malleable), another important attribute was that, besides reprocessability, the polymer can be recycled completely back to its monomer upon disintegration and dissolution in boiling water. Subsequently, the monomer precipitated upon the cooling of the solution. A clear disadvantage of this polymer is that, due to its regeneration in aqueous environments, it shows a high sensitivity to moisture which largely affects the mechanical performance.Citation111
3.2. Recyclable thermosets based on dynamic covalent networks
Although the aforementioned routes to recycling via stimuli-triggered degradation can lead to increased thermoset circularity, the original polymer architecture is destroyed and as a consequence the polymer recyclate needs to be resynthesized or be used in lower performing applications (i.e. down-cycling). Alternatively, dynamic covalent chemistry enables the recycling of cross-linked polymers while maintaining the overall polymer structure. In addition, these polymers and their composites are often capable of damage healing or can be reprocessed prior to recycling. Comparable to the earlier described degradation mechanisms, dynamic covalent bonds are often (thermal, pH, light, moisture) stimuli-triggered. Reported covalent chemistries include carbon–carbon, carbon–nitrogen, carbon–oxygen, disulfide and boron–oxygen bonds.Citation11,Citation12,Citation112 Furthermore, the family of dynamic covalently bonded polymers can generally be divided into those with dissociative and those with associative exchange mechanisms, as schematically depicted in .Citation113,Citation114
The associative exchange mechanism involves the simultaneous exchange of one reaction partner for another, in which the original cross-link is only broken when a new covalent bond to another position is formed.Citation114 On the other hand, dissociative covalently bonded linkages are de facto broken before they are reconnected to another reactive site. This implies that resins containing dissociative networks are capable of a dramatic on-demand viscosity drop, which gives them a distinct benefit over associative networks: matrix and fiber/filler separation and recycling. Nevertheless, both mechanisms have been studied extensively in recent years and thus advanced the field of self-healing, reprocessable and recyclable thermoset materials.
3.2.1. Thermally reversible covalent bonds
As was observed for thermosets with inherent on-demand degradability, the most common trigger for dynamic covalent bonds is heat, and for that reason matrices containing thermally responsive linkages are described separately from the other available mechanisms.
Associative cross-link chemistries are frequently used to create healable and reprocessable thermosets. These materials are often called vitrimers, a hybrid class of polymers which show both thermoplastic and thermoset like behavior based on the pioneering work of Leibler et al.,Citation115 which is illustrated in . A clear mini review about different chemistries for vitrimers was already provided by Du Prez and coworkers in 2016.Citation114 In the present work, the aim is not to describe the mechanisms of different chemistries to obtain vitrimers, but to focus on the sustainability and technical feasibility of their usage in the industrial applications. The type of associative thermally reversible chemistries involve transesterification,Citation116,Citation117 transaminationCitation15,Citation114,Citation118, a selection of disulfide exchange mechanisms,Citation119–123 and alkene metathesis.Citation124,Citation125
Figure 6. Illustrative example of the vitrimer concept and capabilities focusing on the flow and insolubility properties of an epoxy network with 5 mol% Zn(Ac)2 catalyst. (A) Swelling during immersion in trichlorobenzene. (B) Normalized stress relaxation at different temperatures. (C) A cross-linked sample broken into pieces is reprocessed in an injection machine to recover its initial aspect and properties. (D) A fusilli-shaped elastomer made by local heating from a cross-linked ribbon of length 10 cm is reversibly deformed by a weight of 1.4 kg. Reprinted with permission from Montarnal et al.[Citation115] Copyright 2011 The American Association for the Advancement of Science.
![Figure 6. Illustrative example of the vitrimer concept and capabilities focusing on the flow and insolubility properties of an epoxy network with 5 mol% Zn(Ac)2 catalyst. (A) Swelling during immersion in trichlorobenzene. (B) Normalized stress relaxation at different temperatures. (C) A cross-linked sample broken into pieces is reprocessed in an injection machine to recover its initial aspect and properties. (D) A fusilli-shaped elastomer made by local heating from a cross-linked ribbon of length 10 cm is reversibly deformed by a weight of 1.4 kg. Reprinted with permission from Montarnal et al.[Citation115] Copyright 2011 The American Association for the Advancement of Science.](/cms/asset/3e002cc8-0d08-4d2d-b996-099f43405923/lmsc_a_1673406_f0006_c.jpg)
When vitrimers are heated above a certain temperature (100 °C < Tv < 200 °C), the reversible bonds cause network reorganization resulting in macroscopic flow without risking structural damage. Often catalysts are used to regulate the activation energy and Tv. For thermosets that work via transesterification a potential major drawback is that large amounts of a specific type of catalyst are used, which makes it difficult to tailor the desired properties.Citation114,Citation116,Citation118 Furthermore, these catalysts often deactivate over time which restricts the material lifetime. However, as long as the catalyst is present and active, the reversible nature enables welding, molding, reshaping, and recycling of fully cured materials. A new catalyst-free system based on the transamination in vinylogous urethanes was proposed recently. Interestingly, these vinylogous urethanes are stable towards hydrolysis, and can even be formed quantitatively in water as a solvent.Citation118 To increase the circularity of these thermosets bio-based monomers can be used. PartialCitation126 and fullCitation100,Citation127–131 bio-based vitrimers are already reported in recent literature.
Associative networks do not depolymerize upon heating as they retain cross-link density and hence insolubility at all temperatures. Even though the viscosity reduces following the Arrhenius relationship upon temperature increase, it remains at a rather high value of typically 1010 Pa·s at 200 °C.Citation115 This relatively high viscosity makes these chemistries very suitable for repairing the composite and reprocessing with methods that do not require a low matrix viscosity such as compression molding and (ultrasonic) welding. However, in the case of composite materials, a sole exposure to thermal stimuli is not sufficient for the separation of the fiber or filler material from the resin.
A seemingly more feasible alternative to obtain thermosets with an on-demand low viscosity is the use of dissociative reversible covalent thermosetting chemistries. Due to a net decrease in molecular linkages upon exposure to a thermal trigger, these materials can achieve very fast topology rearrangements (stress relaxation and flow). This temporary de-cross-linking typically results in a sudden viscosity drop, analogous to the melting of thermoplastics. Cross-links are re-formed upon cooling, which reestablishes the required thermosetting properties such as high moduli and insolubility. As a result, these dynamic cross-links could allow for thermal (re-)processing of polymeric networks as is typical for thermoplastic systems (e.g. via injection molding and extrusion).Citation114
One of the most extensively explored dissociative reversible exchange mechanisms is the [4 + 2] cycloaddition of a diene and a dienophile, also known as the DA reaction. The DA-reaction takes place, depending on the type of the diene and dienophile, usually at temperatures below 50 °C.Citation132 More interestingly, the DA-adduct is thermally unstable which results in its dissociation (i.e. retro-DA) back into diene and dienophile at elevated temperatures. This reversibility is very attractive for healable and/or recyclable thermosets.Citation133–137
The temperatures of the DA and retro-DA transitions largely depend on the choice of diene and dienophile. In literature, the furan-maleimide system is most often selected due to its suitable temperature profile for reversible bonding.Citation133,Citation134,Citation138 In these systems, the DA reaction takes place at temperatures below 40 °C while the retro DA occurs at temperatures above 140 °C. By adjusting the molecular weight and ratio of the individual constituents, the mechanical properties and the cross-linking density can be tuned, which in turn alters the viscosity of the retro-DA mixture. This is exemplified by the work of Billiet et al. who introduced DA-based thermoreversible 1,2,4-triazoline-3,5-dione groups into polyurethane and polymethacrylate materials, thereby creating a recyclable thermoset with a tunable dynamic behavior. The fast thermal recycling of their thermoset is illustrated in .Citation139
Figure 7. A polyurethane thermoset with DA-based 1,2,4-triazoline-3,5-dione groups that allows for the recycling of broken pieces by molding at elevated temperature and pressure. Reprinted with permission from Billiet et al.[Citation139] Copyright 2014 Springer Nature.
![Figure 7. A polyurethane thermoset with DA-based 1,2,4-triazoline-3,5-dione groups that allows for the recycling of broken pieces by molding at elevated temperature and pressure. Reprinted with permission from Billiet et al.[Citation139] Copyright 2014 Springer Nature.](/cms/asset/44cbf85c-fbb5-4bfa-9048-12dd13452fa1/lmsc_a_1673406_f0007_c.jpg)
Although DA-adducts are promising candidates for recyclable thermosets, two important characteristics must be accounted for; DA adduct formation is slow compared to conventional curing of thermosets and the reaction continues as long as there is some mobility in the polymer matrix. This results in an uncontrolled increase in cross-link density which changes the mechanical properties of the composite over time, usually manifested as polymer embrittlement.
3.2.2. Reversibility via alternative (non-thermal) triggers
Polyimine thermosets based on Schiff base reversible chemistry with heat or water-driven malleability were developed from a combination of diamine, dialdehyde, and triamine cross-linkers through imine condensation in the absence of any catalyst.Citation140,Citation141 It was demonstrated that the reversibility of imine bond formation and temperature‐dependent rate of the imine exchange render such malleable cross-linked polyimines.
The dynamic nature of sulfur–sulfur linkages and of disulfides, in particular, has been extensively studied because of the great interest for vulcanized rubbers in the chemical industry.Citation114 Recent reports show that the behavior of covalently exchanging disulfide bonds is a rather complex process and involves several mechanisms (both associative and dissociative) that also depend on the used conditions and substitution patterns of the disulfides. In the simplest form, disulfides can be reduced to two thiols and then oxidized again in rather mild conditions (involving low concentration of solvents such as HCl and iodine solution in diglyme).Citation142–144 Alternatively, dissociative disulfides can be reversible under UV,Citation122 shear, and/or heat triggers.Citation145,Citation146
Alternatively to the thermally induced [4 + 2] cycloaddition in DA reactions, photo-induced [2 + 2] and [4 + 4] cycloadditions are used for the synthesis of linear polymersCitation147 and reversible cross-linked networks.Citation148 The main advantage of photo-induced cycloaddition, compared to DA, can be found in the high rate of dimerization. For example, whereas DA adduct reformation typically spans over several hours, the cross-linking of cinnamoyl groups takes place within 10 min.Citation149 The photo-induced cycloadditions take place at other wavelengths than the reverse reaction, which enables tunable healing and recycling conditions.Citation149 Currently, the practical usage of photo-induced cycloaddition in composite applications remains unreported. Presumably, the main limitation for this application is the insufficient penetration depth of light into multicomponent applications.
4. Application perspective
Compared to thermoplastics, the market for thermoset resins is much more fragmented. The number of producers of thermoset resins is large and contains, besides a few multinational industries, many small- and medium-sized manufacturers. Hence, reliable data on market sizes of thermoset resins are difficult to find, and estimate vary considerably. Based on publicly accessible data, it is estimated that the global production volume of thermoset resins is currently somewhere between 40 and 50 million tonnes, which is about 10–15% of total ‘plastics’ production.Citation6 The global market size of thermoset products is expected to grow by 40% in the upcoming decade.Citation28 About 4–5 million tonnes (10%) are used to make FRP composites.Citation7 Glass fiber is used predominantly (>90%) due to cost-effectiveness. High-performance carbon fibers are also used, although the trend here is towards carbon-fiber/thermoplastic composites due to easier recycling and reclaiming of the carbon fiber.Citation7 In general, due to the intrinsic higher recyclability, the use of thermoplastic polymers in applications that were traditionally reserved for thermosets is increasing. Even though this could be a feasible approach for the recycling of a multitude of thermoset applications, this approach will not solve the thermoset recycling issue as a whole. As a result, due to continuously growing demand of high-performance light-weight materials, the overall number of thermoset applications is expected to grow regardless.
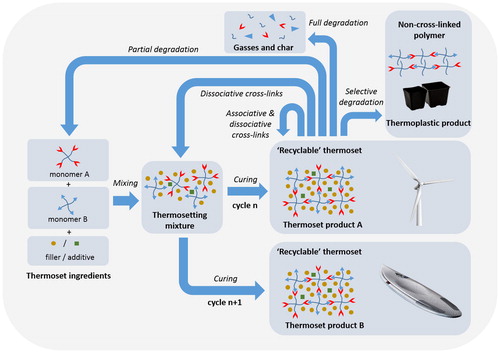
The increasing usage of thermoset materials will logically result in an increased amount of waste and higher demand for recycling options. As addressed in the preceding sections, the currently used waste treatment techniques require high amounts of energy and rarely take recycling of the polymer matrix itself into account (). In order to tackle this issue, many new academic concepts were developed in the past decades that focus on actual recycling of the thermoset. A closer look at these concepts reveals that there is no universal solution; every technology has its own unique selling points and disadvantages. In order to improve the recyclability of a specific product, it is crucial that individual application-based requirements are taken into account when selecting the most suitable recycling concept. This is further illustrated in which shows the circularity of the different concepts for thermosets with inherent recyclability (as shown in ) in combination with some application examples. Various concepts of both degradable and dynamic covalent systems are being translated to more application-oriented studies and some are already approaching the market. Examples of application-driven studies are found in typical thermoset fields such as structural composites, rubber, adhesive and electronic applications.Citation11,Citation13,Citation150,Citation151
In line with the current state of the art in thermoset recycling, the initial driver for application-oriented research often remains the ability to reclaim the more valuable fibers and therefore most developments are targeted towards structural composite applications. An example of stimuli-triggered degradation that is already approaching the market is based on the work performed by Pastine and coworkers. They developed a series of acetal-containing amine hardeners for cross-linking of commercially available epoxy resins which can be impregnated in glass and carbon fiber fabrics at conventional composite processing conditions. The resulting composites show mechanical performances comparable to commercially available composites. In addition, the acetal linkages degrade upon immersion into an acidic solution and the remaining linear polymer backbone can be regained and used as a thermoplastic polymer in different applications. Based on this technology Connora® was founded which focuses on the development of recyclable resins for sporting goods, automotive, and wind turbine applications.Citation18,Citation19,Citation152–154 A schematic overview of their technological concept is shown in .
Figure 9. Conceptual representations of two recently founded startup companies that focus on the development of recyclable thermoset for composite applications. Connora (a) uses degradable linkages to obtain thermoplastic recyclates. Mallinda (b) uses polyimine networks that can be separated by dissolution.[Citation18,Citation20]
![Figure 9. Conceptual representations of two recently founded startup companies that focus on the development of recyclable thermoset for composite applications. Connora (a) uses degradable linkages to obtain thermoplastic recyclates. Mallinda (b) uses polyimine networks that can be separated by dissolution.[Citation18,Citation20]](/cms/asset/5f488f0b-2fde-4324-b4c6-6eea0c44e5c3/lmsc_a_1673406_f0009_c.jpg)
Even though the described degradable thermoset concepts show a potential for thermoset composite recycling that was not yet realized till this date, the resulting matrix recyclates are still degraded versions of the original polymer. To obtain a higher quality of the recyclates, the infusion of dynamic covalent networks into fibrous composites was studied by many researchers. For instance, Chabert et al. transferred the vitrimer concept into GFRP composites by resin transfer molding of an epoxy-anhydride containing resin at 50 °C.Citation155 The resulting composites showed industrially relevant mechanical properties (i.e. Young’s modulus >20 GPa and strength at break >500 MPa) and good healing characteristics. However, due to the associative nature of vitrimer linkages the matrix-fiber separation and thereby the overall recycling of such composites will remain a challenge. A similar statement can be made for the recently developed epoxy-based FRPs with incorporated disulfide linkages. These composites (Young’s modulus >10 GPa) could be prepared by conventional vacuum infusion processing and are capable of low-temperature (70 °C) repair of cracks and delaminationCitation156 and reprocessing at temperatures above 200 °C.Citation157 However, fiber separation could only occur via solvolysis in thiol-containing solvent mixtures without any matrix recycling.Citation157 Taynton et al. reported another example of dynamic covalent linkages as a binder for CFRP composites by using a polyimine-based polymer. Via a wet lay-up approach, they prepared composites with near-commercial mechanical properties (Young’s moduli >10 GPa) that could be recycled by dissolution in an ethanol-based solution.Citation158 The development of this technology resulted in the founding of Mallinda, a recent startup company that focuses on the production of catalyst-free malleable CFRPs with short cycle times.Citation20 The circular philosophy of Mallinda is visualized in . Unfortunately, a clear disadvantage of these polyimine-based thermosets is that their mechanical performance is highly affected by moisture. Consequently, it is expected that their application field will most likely be limited to niche applications in low humidity conditions.
Thermosets with dissociative covalent linkages, where bonds are actually broken while being stimuli-triggered, can theoretically be designed in such a way that the drop in viscosity is sufficient for easy matrix removal. This technology has already been patented yet no recyclable composites that utilize dissociative bonds to separate matrix from fibers have reached the market up till this date.Citation159 The reason for the absence of such a polymer is most likely the complexity involved in designing a system that combines sufficient mechanical integrity with the required drop in viscosity. With this respect, DA reversible linkages seem to be the most promising mechanism for recyclable dissociative thermosets as they can be molecularly built into conventional composite resins (e.g. epoxy, polyurethane) with relative ease.Citation160 Furthermore, it is possible to graft DA groups (e.g. furan and maleimides) onto the reinforcing fibers in order to create repairable polymer–fiber interfaces.Citation161–164 However, processing of the DA functional resins into fiber fabrics tends to be very difficult since they are highly viscous or even in the solid state. Nevertheless, several researchers managed to produce FRPs-containing reversible DA linkages via injection molding,Citation165,Citation166 vacuum-assisted autoclave molding,Citation167 and compression molding.Citation133,Citation138 The produced composites showed good healing and easy repair capabilities, but full separation and recyclability of fibers and matrix were not yet reported.
Compared to FRP composites, the academic interest in the development of recycling methods via dynamic covalent linkages of other applications is rather limited. For example, recyclable cross-linked rubber products (e.g. car tires and sealing rings) are less investigated. This class of thermosets has intrinsically different mechanical properties compared to typical composite resins, but due to their permanent covalent cross-links the challenges for recycling are comparable. However, the fact that applications containing cross-linked rubbers are generally not hindered by continuous fiber networks makes that recycling is potentially easier to achieve.Citation168 As a result, the recycling of thermoset rubber tires has been developed to a greater extent, but similar to FRPs, high-energy-intensive mechanical and thermal recycling are also indicated as the most economically feasible options for this class of thermosets.Citation169,Citation170 The most straightforward approach to utilize dynamic covalent linkages in cross-linked rubbers seems to be via disulfide linkages which are already present in cross-linked (natural) rubbers due to sulfur vulcanization.Citation171,Citation172 By tuning of the molecular architecture and addition of CuCl2 catalyst, healing and easy repair of these rubbers are possible, but due to the associative nature of the disulfide linkages, the reprocessing and recycling is just as complex as it is for conventional rubbers.Citation151 In that respect, dissociative DA linkages show a higher recycling and reprocessing potential. Therefore, they account for the most investigated type of reversible bonds in rubbers, and it is expected that these concepts will approach the market in the upcoming decade.Citation173–175
The development of recyclable thermosets for applications in electronics is a relatively new field of interest. In this case, contrary to structural composites and rubbers, functional properties are more important than mechanical performance. Hence, recycling technology should focus on these aspects. For example, thermal interface materials were developed that show easy repair capacity of the thermal conductivity using associative disulfide linkages.Citation176,Citation177 Furthermore, flexible electronics that are capable of restoration of the electronic conductivity due to the presence of dynamic DACitation178,Citation179 and polyimineCitation180 linkages were reported. The repair in these concepts is often triggered by a thermal stimulus that is already applied due to intrinsic use and that the functional polymers are often shielded from environmental hazards such as moisture. As a result, less constraints need to be overcome and therefore the commercial development of these products is expected to arrive in the upcoming years.
5. Conclusions
Besides the issues and complications that exist for the recycling of thermoplastic materials, the recycling of thermosets suffers from additional technical complications due to the covalently cross-linked networks that are present in their molecular architecture. In other words, their intended high thermal stability and chemical resistance directly result in very limited recycling options. As a consequence, landfill is currently the most used end-of-life option for thermoset products. Nowadays, recycling methods such as reprocessing in cement kilns, pyrolysis, grinding, and solvolysis are growing towards an industrial level, but they typically require a high energy input and are de facto merely disposing instead of recycling the polymer. As recycling is crucial in realizing a circular economy, there is a high demand for reduced-energy solutions that allow for efficient thermoset recycling. One route to achieve this is by the introduction of degradable linkages or dynamic covalent bonds within the thermoset molecular structure. Unfortunately, regarding thermosets with degradable bonds, most research efforts end as soon as successful degradation is shown and no attention is given to the industrial exploitation of these concepts. Therefore, the development of low-energy methods for re-obtaining the polymer phase in the form of monomers, oligomers, or thermoplastic polymers should be a focal point in the upcoming years. Actual thermoset recycling can more easily be achieved when dynamic covalent bonds are implemented. From a recycling perspective, these systems should focus on the development of dissociative exchange mechanisms as they can allow a viscosity drop that is sufficient for (thermoplastic) reprocessing and separation of the matrix in polymer thermoset composite products. Degradable and dynamic covalent thermosets are gaining increasing attention in the application-oriented research and some concepts are slowly penetrating the market. In this respect, it has to be noted that there is no universal approach to energy-efficient thermoset recycling; for each application an individual assessment needs to be made as to which end-of-life option fits best with required product performance.
Additional information
Funding
References
- Ellen MacArthur Foundation. The New Plastics Economy: Rethinking the Future of Plastics and Catalysing Action; Ellen McArthur Foundation: Cowes, 2017.
- Soroudi, A.; Jakubowicz, I. Recycling of Bioplastics, Their Blends and Biocomposites: A Review. Eur. Polym. J. 2013, 49, 2839–2858. DOI: 10.1016/j.eurpolymj.2013.07.025.
- Ambrose, C. A.; Hooper, R.; Potter, A. K.; Singh, M. M. Diversion from Landfill: Quality Products from Valuable Plastics. Resour. Conserv. Recycl. 2002, 36, 309–318. DOI: 10.1016/S0921-3449(02)00030-7.
- Maris, J.; Bourdon, S.; Brossard, J.-M.; Cauret, L.; Fontaine, L.; Montembault, V. Mechanical Recycling: Compatibilization of Mixed Thermoplastic Wastes. Polym. Degrad. Stabil. 2018, 147, 245–266. DOI: 10.1016/j.polymdegradstab.2017.11.001.
- Brouwer, M. T.; Thoden van Velzen, E. U.; Augustinus, A.; Soethoudt, H.; De Meester, S.; Ragaert, K. Predictive Model for the Dutch Post-Consumer Plastic Packaging Recycling System and Implications for the Circular Economy. Waste Manage. 2018, 71, 62–85. DOI: 10.1016/j.wasman.2017.10.034.
- Biron, M.; Biron, M. 3 – Thermoplastics: Economic Overview. In Material Selection for Thermoplastic Parts; William Andrew Publishing: Oxford, 2016; pp 77–111.
- Biron, M.; Biron, M. 2 – The Plastics Industry: Economic Overview. In Thermosets and Composites, 2nd ed.; William Andrew Publishing: Oxford, 2014; pp 25–104.
- Yang, Y.; Boom, R.; Irion, B.; van Heerden, D.-J.; Kuiper, P.; de Wit, H. Recycling of Composite Materials. Chem. Eng. Process. Process Intens. 2012, 51, 53–68. DOI: 10.1016/j.cep.2011.09.007.
- Oliveux, G.; Dandy, L. O.; Leeke, G. A. Current Status of Recycling of Fibre Reinforced Polymers: Review of Technologies, Reuse and Resulting Properties. Prog. Mater. Sci. 2015, 72, 61–99. DOI: 10.1016/j.pmatsci.2015.01.004.
- Pickering, S. J. Recycling Technologies for Thermoset Composite Materials – Current Status. Compos. Part A: Appl. Sci. Manufact. 2006, 37, 1206–1215. DOI: 10.1016/j.compositesa.2005.05.030.
- Zhong, N.; Post, W. Self-Repair of Structural and Functional Composites with Intrinsically Self-Healing Polymer Matrices: A Review. Compos. Part A: Appl. Sci. Manufact. 2015, 69, 226–239. DOI: 10.1016/j.compositesa.2014.11.028.
- García, F.; Smulders, M. M. J. Dynamic Covalent Polymers. J. Polym. Sci. Part A: Polym. Chem. 2016, 54, 3551–3577. DOI: 10.1002/pola.28260.
- Fortman, D. J.; Brutman, J. P.; De Hoe, G. X.; Snyder, R. L.; Dichtel, W. R.; Hillmyer, M. A. Approaches to Sustainable and Continually Recyclable Cross-Linked Polymers. ACS Sustainable Chem. Eng. 2018, 6, 11145–11159. DOI: 10.1021/acssuschemeng.8b02355.
- Yang, S.; Chen, J.-S.; Körner, H.; Breiner, T.; Ober, C. K.; Poliks, M. D. Reworkable Epoxies: Thermosets with Thermally Cleavable Groups for Controlled Network Breakdown. Chem. Mater. 1998, 10, 1475–1482. DOI: 10.1021/cm970667t.
- Rivero, G.; Nguyen, L.-T. T.; Hillewaere, X. K. D.; Du Prez, F. E. One-Pot Thermo-Remendable Shape Memory Polyurethanes. Macromolecules 2014, 47, 2010–2018. DOI: 10.1021/ma402471c.
- Hayes, S. A.; Jones, F. R.; Marshiya, K.; Zhang, W. A Self-Healing Thermosetting Composite Material. Compos. Part A: Appl. Sci. Manufact. 2007, 38, 1116–1120. DOI: 10.1016/j.compositesa.2006.06.008.
- Ma, S.; Webster, D. C. Degradable Thermosets Based on Labile Bonds or Linkages: A Review. Prog. Polym. Sci. 2018, 76, 65–110. DOI: 10.1016/j.progpolymsci.2017.07.008.
- Pastine, S. J. Sustainable by Design: Introducing Recyclable Epoxytechnology. Presented at Society of Plastics Engineers – 13th Annual Automotive Composites Conference and Exhibition (ACCE), Novi, MI, USA, Sep 11–13, 2013.
- La Rosa, A.; Blanco, I.; Banatao, D.; Pastine, S.; Björklund, A.; Cicala, G. Innovative Chemical Process for Recycling Thermosets Cured with Recyclamines® by Converting Bio-Epoxy Composites in Reusable Thermoplastic – An LCA Study. Materials 2018, 11, 353. DOI: 10.3390/ma11030353.
- Kissounko, D. A.; Taynton, P.; Kaffer, C. New Material: Vitrimers Promise to Impact Composites. Reinforced Plast. 2018, 62, 162–166. DOI: 10.1016/j.repl.2017.06.084.
- Overcash, M.; Twomey, J.; Asmatulu, E.; Vozzola, E.; Griffing, E. Thermoset Composite Recycling – Driving Forces, Development, and Evolution of New Opportunities. J. Compos. Mater. 2018, 52, 1033–1043. DOI: 10.1177/0021998317720000.
- Ayre, D. Technology Advancing Polymers and Polymer Composites towards Sustainability: A Review. Curr. Opin. Green Sustain. Chem. 2018, 13, 108–112. DOI: 10.1016/j.cogsc.2018.06.018.
- Ribeiro, M.; Fiúza, A.; Ferreira, A.; Dinis, M.; Meira Castro, A.; Meixedo, J.; Alvim, M. Recycling Approach towards Sustainability Advance of Composite Materials’ Industry. Recycling 2016, 1, 178. DOI: 10.3390/recycling1010178.
- Asmatulu, E.; Twomey, J.; Overcash, M. Recycling of Fiber-Reinforced Composites and Direct Structural Composite Recycling Concept. J. Compos. Mater. 2014, 48, 593–608. DOI: 10.1177/0021998313476325.
- Jensen, J. P.; Skelton, K. Wind Turbine Blade Recycling: Experiences, Challenges and Possibilities in a Circular Economy. Renew. Sustain. Energy Rev. 2018, 97, 165–176. DOI: 10.1016/j.rser.2018.08.041.
- Bank, L.; Arias, F.; Yazdanbakhsh, A.; Gentry, T.; Al-Haddad, T.; Chen, J.-F.; Morrow, R. Concepts for Reusing Composite Materials from Decommissioned Wind Turbine Blades in Affordable Housing. Recycling 2018, 3, 3. DOI: 10.3390/recycling3010003.
- Bernardeau, F.; Perrin, D.; Caro-Bretelle, A.-S.; Benezet, J.-C.; Ienny, P. Development of a Recycling Solution for Waste Thermoset Material: Waste Source Study, Comminution Scheme and Filler Characterization. J. Mater. Cycles Waste Manag. 2018, 20, 1320–1336. DOI: 10.1007/s10163-017-0698-x.
- Job, S.; Mativenga, P.; Aizat Shuaib, N.; Oliveux, G. Composites Recycling: Where Are We Now? Composites UK Ltd.: Berkhamsted, 2016.
- Palmer, J.; Savage, L.; Ghita, O. R.; Evans, K. E. Sheet Moulding Compound (SMC) from Carbon Fibre Recyclate. Compos. Part A: Appl. Sci. Manufact. 2010, 41, 1232–1237. DOI: 10.1016/j.compositesa.2010.05.005.
- Pickering, S. J.; Kelly, R. M.; Kennerley, J. R.; Rudd, C. D.; Fenwick, N. J. A Fluidised-Bed Process for the Recovery of Glass Fibres from Scrap Thermoset Composites. Compos. Sci. Technol. 2000, 60, 509–523. DOI: 10.1016/S0266-3538(99)00154-2.
- Cunliffe, A. M.; Jones, N.; Williams, P. T. Pyrolysis of Composite Plastic Waste. Environ. Technol. 2003, 24, 653–663. DOI: 10.1080/09593330309385599.
- Al-Salem, S. M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A Review on Thermal and Catalytic Pyrolysis of Plastic Solid Waste (PSW). J. Environ. Manage. 2017, 197, 177–198. DOI: 10.1016/j.jenvman.2017.03.084.
- Santella, C.; Cafiero, L.; De Angelis, D.; La Marca, F.; Tuffi, R.; Vecchio Ciprioti, S. Thermal and Catalytic Pyrolysis of a Mixture of Plastics from Small Waste Electrical and Electronic Equipment (WEEE). Waste Manage. 2016, 54, 143–152. DOI: 10.1016/j.wasman.2016.05.005.
- Benedetti, M.; Cafiero, L.; De Angelis, D.; Dell’Era, A.; Pasquali, M.; Stendardo, S.; Tuffi, R.; Ciprioti, S. V. Pyrolysis of WEEE Plastics Using Catalysts Produced from Fly Ash of Coal Gasification. Front. Environ. Sci. Eng. 2017, 11, 11.
- Naqvi, S. R.; Prabhakara, H. M.; Bramer, E. A.; Dierkes, W.; Akkerman, R.; Brem, G. A Critical Review on Recycling of End-of-Life Carbon Fibre/Glass Fibre Reinforced Composites Waste Using Pyrolysis towards a Circular Economy. Resour. Conserv. Recycl. 2018, 136, 118–129. DOI: 10.1016/j.resconrec.2018.04.013.
- Alves, S. M. C.; da Silva, F. S.; Donadon, M. V.; Garcia, R. R.; Corat, E. J. Process and Characterization of Reclaimed Carbon Fiber Composites by Pyrolysis and Oxidation, Assisted by Thermal Plasma to Avoid Pollutants Emissions. J. Compos. Mater. 2018, 52, 1379–1398. DOI: 10.1177/0021998317724214.
- Kim, K. W.; Jeong, J.-S.; An, K.-H.; Kim, B.-J. A Low Energy Recycling Technique of Carbon Fibers-Reinforced Epoxy Matrix Composites. Ind. Eng. Chem. Res., 2018, 58, 618–624. DOI: 10.1021/acs.iecr.8b02554.
- Oliveux, G.; Bailleul, J. L.; Salle, E. L. G. L. Chemical Recycling of Glass Fibre Reinforced Composites Using Subcritical Water. Compos. Part A: Appl. Sci. Manufact. 2012, 43, 1809–1818. DOI: 10.1016/j.compositesa.2012.06.008.
- Oliveux, G.; Bailleul, J.-L.; Le Gal La Salle, E.; Lefèvre, N.; Biotteau, G. Recycling of Glass Fibre Reinforced Composites Using Subcritical Hydrolysis: Reaction Mechanisms and Kinetics, Influence of the Chemical Structure of the Resin. Polym. Degrad. Stabil. 2013, 98, 785–800. DOI: 10.1016/j.polymdegradstab.2012.12.010.
- Kuang, X.; Shi, Q.; Zhou, Y.; Zhao, Z.; Wang, T.; Qi, H. J. Dissolution of Epoxy Thermosets: Via Mild Alcoholysis: The Mechanism and Kinetics Study. RSC Adv. 2018, 8, 1493–1502. DOI: 10.1039/C7RA12787A.
- Li, J.; Xu, P.-L.; Zhu, Y.-K.; Ding, J.-P.; Xue, L.-X.; Wang, Y.-Z. A Promising Strategy for Chemical Recycling of Carbon Fiber/Thermoset Composites: Self-Accelerating Decomposition in a Mild Oxidative System. Green Chem. 2012, 14, 3260–3263. DOI: 10.1039/c2gc36294e.
- Sokoli, H. U.; Simonsen, M. E.; Søgaard, E. G. Investigation of Degradation Products Produced by Recycling the Solvent during Chemical Degradation of Fiber-Reinforced Composites. J. Reinforced Plast. Compos. 2017, 36, 1286–1296. DOI: 10.1177/0731684417707060.
- Buggy, M.; Farragher, L.; Madden, W. Recycling of Composite Materials. J. Mater. Process. Technol. 1995, 55, 448–456. DOI: 10.1016/0924-0136(95)02037-3.
- Dang, W.; Kubouchi, M.; Sembokuya, H.; Tsuda, K. Chemical Recycling of Glass Fiber Reinforced Epoxy Resin Cured with Amine Using Nitric Acid. Polymer 2005, 46, 1905–1912. DOI: 10.1016/j.polymer.2004.12.035.
- Ma, Y.; Kim, D.; Nutt, S. R. Chemical Treatment for Dissolution of Amine-Cured Epoxies at Atmospheric Pressure. Polym. Degrad. Stabil. 2017, 146, 240–249. DOI: 10.1016/j.polymdegradstab.2017.10.014.
- Ma, Y.; Nutt, S. Chemical Treatment for Recycling of Amine/Epoxy Composites at Atmospheric Pressure. Polym. Degrad. Stabil. 2018, 153, 307–317. DOI: 10.1016/j.polymdegradstab.2018.05.011.
- Zhang, L.; Liu, J.; Nie, W.; Wang, K.; Wang, Y.; Yang, X.; Tang, T. Degradation of Anhydride-Cured Epoxy Resin Using Simultaneously Recyclable Solvent and Organic Base Catalyst. J. Mater. Cycles Waste Manag. 2018, 20, 568–577. DOI: 10.1007/s10163-017-0623-3.
- Kuang, X.; Zhou, Y.; Shi, Q.; Wang, T.; Qi, H. J. Recycling of Epoxy Thermoset and Composites via Good Solvent Assisted and Small Molecules Participated Exchange Reactions. ACS Sustainable Chem. Eng. 2018, 6, 9189–9197. DOI: 10.1021/acssuschemeng.8b01538.
- Vallee, M.; Tersac, G.; Destais-Orvoen, N.; Durand, G. Chemical Recycling of Class a Surface Quality Sheet-Molding Composites. Ind. Eng. Chem. Res. 2004, 43, 6317–6324. DOI: 10.1021/ie049871y.
- Simón, D.; Rodríguez, J. F.; Carmona, M.; Serrano, A.; Borreguero, A. M. Glycolysis of Advanced Polyurethanes Composites Containing Thermoregulating Microcapsules. Chem. Eng. J. 2018, 350, 300–311. DOI: 10.1016/j.cej.2018.05.158.
- Henry, L.; Schneller, A.; Doerfler, J.; Mueller, W. M.; Aymonier, C.; Horn, S. Semi-Continuous Flow Recycling Method for Carbon Fibre Reinforced Thermoset Polymers by Near- and Supercritical Solvolysis. Polym. Degrad. Stabil. 2016, 133, 264–274. DOI: 10.1016/j.polymdegradstab.2016.09.002.
- Khalil, Y. F. Comparative Environmental and Human Health Evaluations of Thermolysis and Solvolysis Recycling Technologies of Carbon Fiber Reinforced Polymer Waste. Waste Manage. 2018, 76, 767–778. DOI: 10.1016/j.wasman.2018.03.026.
- Khalil, Y. F. Sustainability Assessment of Solvolysis Using Supercritical Fluids for Carbon Fiber Reinforced Polymers Waste Management. Sustainable Prod. Consump. 2019, 17, 74–84. DOI: 10.1016/j.spc.2018.09.009.
- Prinçaud, M.; Aymonier, C.; Loppinet-Serani, A.; Perry, N.; Sonnemann, G. Environmental Feasibility of the Recycling of Carbon Fibers from CFRPs by Solvolysis Using Supercritical Water. ACS Sustainable Chem. Eng. 2014, 2, 1498–1502. DOI: 10.1021/sc500174m.
- Shuaib, N. A.; Mativenga, P. T. Carbon Footprint Analysis of Fibre Reinforced Composite Recycling Processes. Proc. Manufact. 2017, 7, 183–190. DOI: 10.1016/j.promfg.2016.12.046.
- Wang, Z.; Xie, M.; Zhao, Y.; Yu, Y.; Fang, S. Synthesis and Properties of Novel Liquid Ester-Free Reworkable Cycloaliphatic Diepoxides for Electronic Packaging Application. Polymer 2003, 44, 923–929. DOI: 10.1016/S0032-3861(02)00873-X.
- Wang, L.; Wong, C. P. Syntheses and Characterizations of Thermally Reworkable Epoxy Resins. Part I. J. Polym. Sci. A Polym. Chem. 1999, 37, 2991–3001. DOI: 10.1002/(SICI)1099-0518(19990801)37:15<2991::AID-POLA32>3.0.CO;2-V.
- Wang, L.; Li, H.; Wong, C. P. Syntheses and Characterizations of Thermally Reworkable Epoxy Resins II. J. Polym. Sci. A Polym. Chem. 2000, 38, 3771–3782. DOI: 10.1002/1099-0518(20001015)38:20<3771::AID-POLA80>3.0.CO;2-4.
- Liu, W.; Wang, Z.; Xiong, L.; Zhao, L. Phosphorus-Containing Liquid Cycloaliphatic Epoxy Resins for Reworkable Environment-Friendly Electronic Packaging Materials. Polymer 2010, 51, 4776–4783. DOI: 10.1016/j.polymer.2010.08.039.
- Liu, W.; Wang, Z.; Chen, Z.; Zhao, L. Thermo-Initiated Cationic Polymerization of Phosphorus-Containing Cycloaliphatic Epoxides with Tunable Degradable Temperature. Polym. Degrad. Stabil. 2012, 97, 810–815. DOI: 10.1016/j.polymdegradstab.2012.01.028.
- Chen, Z.; Zhao, L.; Wang, Z. Synthesis of Phosphite-Type Trifunctional Cycloaliphatic Epoxide and the Decrosslinking Behavior of Its Cured Network. Polymer 2013, 54, 5182–5187. DOI: 10.1016/j.polymer.2013.07.048.
- Zhao, L.; Liu, Y.; Wang, Z.; Li, J.; Liu, W.; Chen, Z. Synthesis and Degradable Property of Novel Sulfite-Containing Cycloaliphatic Epoxy Resins. Polym. Degrad. Stabil. 2013, 98, 2125–2130. DOI: 10.1016/j.polymdegradstab.2013.09.007.
- Zhang, X.; Chen, G.-C.; Collins, A.; Jacobson, S.; Morganelli, P.; Dar, Y. L.; Musa, O. M. Thermally Degradable Maleimides for Reworkable Adhesives. J. Polym. Sci. A Polym. Chem. 2009, 47, 1073–1084. DOI: 10.1002/pola.23217.
- Chen, J.-S.; Ober, C. K.; Poliks, M. D. Characterization of Thermally Reworkable Thermosets: Materials for Environmentally Friendly Processing and Reuse. Polymer 2002, 43, 131–139. DOI: 10.1016/S0032-3861(01)00605-X.
- Chen, J.-S.; Ober, C. K.; Poliks, M. D.; Zhang, Y.; Wiesner, U.; Cohen, C. Controlled Degradation of Epoxy Networks: Analysis of Crosslink Density and Glass Transition Temperature Changes in Thermally Reworkable Thermosets. Polymer 2004, 45, 1939–1950. DOI: 10.1016/j.polymer.2004.01.011.
- Shirai, M. Reworkable UV Curing Materials. Prog. Organic Coat. 2007, 58, 158–165. DOI: 10.1016/j.porgcoat.2006.08.022.
- Li, H.; Wang, L.; Jacob, K.; Wong, C. P. Syntheses and Characterizations of Thermally Degradable Epoxy Resins. III. J. Polym. Sci. A Polym. Chem. 2002, 40, 1796–1807. DOI: 10.1002/pola.10258.
- Shirai, M.; Morishita, S.; Okamura, H.; Tsunooka, M. Photo-Cross-Linkable Polymers with Thermally Degradable Property. Chem. Mater. 2002, 14, 334–340. DOI: 10.1021/cm0103646.
- Okamura, H.; Yamauchi, E.; Shirai, M. Photo-Cross-Linking and de-Cross-Linking of Modified Polystyrenes Having Degradable Linkages. React. Funct. Polym. 2011, 71, 480–488. DOI: 10.1016/j.reactfunctpolym.2011.01.008.
- Okamura, H.; Toda, S.; Tsunooka, M.; Shirai, M. Photocrosslinking System Based on a Poly (Vinyl Phenol)/Thermally Degradable Diepoxy Crosslinker Blend. J. Polym. Sci. A Polym. Chem. 2002, 40, 3055–3062. DOI: 10.1002/pola.10394.
- Shirai, M.; Kawaue, A.; Okamura, H.; Tsunooka, M. Photo-Cross-Linkable Polymers Having Degradable Properties on Heating. Chem. Mater. 2003, 15, 4075–4081. DOI: 10.1021/cm0302734.
- Shirai, M.; Kawaue, A.; Okamura, H.; Tsunooka, M. Photocrosslinkable Polymers with Redissolution Property. Chem. Lett. 2002, 31, 940–942. DOI: 10.1246/cl.2002.940.
- Okamura, H.; Shirai, M. Novel Photo-Cross-Linkable Dendrimers Having Thermal de-Cross-Linking Properties. Polymer 2010, 51, 5087–5094. DOI: 10.1016/j.polymer.2010.09.010.
- Okamura, H.; Shin, K.; Shirai, M. Photocrosslinking System Using Highly-Functionalized Epoxy Crosslinkers Having Degradable Property. Polym. J. 2006, 38, 1237. DOI: 10.1295/polymj.PJ2006007.
- Morell, M.; Ramis, X.; Ferrando, F.; Yu, Y.; Serra, A. New Improved Thermosets Obtained from DGEBA and a Hyperbranched Poly(Ester-Amide). Polymer 2009, 50, 5374–5383. DOI: 10.1016/j.polymer.2009.09.024.
- Morell, M.; Erber, M.; Ramis, X.; Ferrando, F.; Voit, B.; Serra, A. New Epoxy Thermosets Modified with Hyperbranched Poly(Ester-Amide) of Different Molecular Weight. Eur. Polym. J. 2010, 46, 1498–1509. DOI: 10.1016/j.eurpolymj.2010.04.015.
- Morell, M.; Fernández-Francos, X.; Ramis, X.; Serra, A. Synthesis of a New Hyperbranched Polyaminoester and Its Use as a Reactive Modifier in Anionic Curing of DGEBA Thermosets. Macromol. Chem. Phys. 2010, 211, 1879–1889. DOI: 10.1002/macp.201000152.
- Ogino, K.; Chen, J.-S.; Ober, C. K. Synthesis and Characterization of Thermally Degradable Polymer Networks. Chem. Mater. 1998, 10, 3833–3838. DOI: 10.1021/cm9801183.
- Kilian, L.; Wang, Z.-H.; Long, T. E. Synthesis and Cleavage of Core-Labile Poly(Alkyl Methacrylate) Star Polymers. J. Polym. Sci. A Polym. Chem. 2003, 41, 3083–3093. DOI: 10.1002/pola.10885.
- Roper, T. M.; Kwee, T.; Lee, T. Y.; Guymon, C. A.; Hoyle, C. E. Photopolymerization of Pigmented Thiol-Ene Systems. Polymer 2004, 45, 2921–2929. DOI: 10.1016/j.polymer.2004.02.038.
- Montague, M. F.; Hawker, C. J. Secondary Patterning of UV Imprint Features by Photolithography. Chem. Mater. 2007, 19, 526–534. DOI: 10.1021/cm0622102.
- Adachi, M.; Okamura, H.; Shirai, M. A Reworkable Photothermal Dual-Curing System. Chem. Lett. 2013, 42, 1056–1058. DOI: 10.1246/cl.130415.
- Acebo, C.; Fernández-Francos, X.; Ferrando, F.; Serra, À.; Ramis, X. New Epoxy Thermosets Modified with Multiarm Star Poly(Lactide) with Poly (Ethyleneimine) as Core of Different Molecular Weight. Eur. Polym. J. 2013, 49, 2316–2326. DOI: 10.1016/j.eurpolymj.2013.05.015.
- González, L.; Ramis, X.; Salla, J. M.; Mantecón, A.; Serra, A. New Poly(Ether-Ester) Thermosets Obtained by Cationic Curing of DGEBA and 7,7-Dimethyl-6,8-Dioxaspiro[3.5] Nonane-5,9-Dione with Several Lewis Acids as Initiators. J. Polym. Sci. A Polym. Chem. 2008, 46, 1229–1239. DOI: 10.1002/pola.22464.
- González, L.; Ramis, X.; Salla, J. M.; Mantecón, A.; Serra, A. The Degradation of New Thermally Degradable Thermosets Obtained by Cationic Curing of Mixtures of DGEBA and 6, 6-Dimethyl (4,8-Dioxaspiro [2.5] Octane-5,7-Dione). Polym. Degrad. Stabil. 2007, 92, 596–604. DOI: 10.1016/j.polymdegradstab.2007.01.007.
- González, L.; Ramis, X.; Salla, J. M.; Serra, A.; Mantecón, A. New Thermosets Obtained from DGEBA and Meldrum Acid with Lanthanum and Ytterbium Triflates as Cationic Initiators. Eur. Polym. J. 2008, 44, 1535–1547. DOI: 10.1016/j.eurpolymj.2008.02.019.
- Okamura, H.; Shin, K.; Tsunooka, M.; Shirai, M. Photocrosslinking System Using Multifunctional Epoxy Crosslinkers Having Thermally Degradable Properties. J. Polym. Sci. A Polym. Chem. 2004, 42, 3685–3696. DOI: 10.1002/pola.20232.
- Okamura, H.; Shirai, M. Reworkable Resin Using Thiol-Ene System. J. Photopol. Sci. Technol. 2011, 24, 561–564. DOI: 10.2494/photopolymer.24.561.
- Buchwalter, S. L.; Kosbar, L. L. Cleavable Epoxy Resins: Design for Disassembly of a Thermoset. J. Polym. Sci. A Polym. Chem. 1996, 34, 249–260. DOI: 10.1002/(SICI)1099-0518(19960130)34:2<249::AID-POLA11>3.0.CO;2-Q.
- Hashimoto, T.; Meiji, H.; Urushisaki, M.; Sakaguchi, T.; Kawabe, K.; Tsuchida, C.; Kondo, K. Degradable and Chemically Recyclable Epoxy Resins Containing Acetal Linkages: Synthesis, Properties, and Application for Carbon Fiber-Reinforced Plastics. J. Polym. Sci. A Polym. Chem. 2012, 50, 3674–3681. DOI: 10.1002/pola.26160.
- Harada, M.; Ando, J.; Yamaki, M.; Ochi, M. Synthesis, Characterization, and Mechanical Properties of a Novel Terphenyl Liquid Crystalline Epoxy Resin. J. Appl. Polym. Sci. 2015, 132, 1–7. DOI: 10.1002/app.41296.
- González, S.; Fernández-Francos, X.; Salla, J. M.; Serra, A.; Mantecón, A.; Ramis, X. New Thermosets Obtained by the Cationic Copolymerization of Diglycidyl Ether of Bisphenol A with γ-Caprolactone with an Improvement in the Shrinkage. I. Study of the Chemical Processes and Physical Characteristics. J. Polym. Sci. A Polym. Chem. 2007, 45, 1968–1979. DOI: 10.1002/pola.21961.
- Giménez, R.; Fernández-Francos, X.; Salla, J. M.; Serra, A.; Mantecón, A.; Ramis, X. New Degradable Thermosets Obtained by Cationic Copolymerization of DGEBA with an s (γ-Butyrolactone). Polymer 2005, 46, 10637–10647. DOI: 10.1016/j.polymer.2005.09.026.
- Sangermano, M.; Tonin, M.; Yagci, Y. Degradable Epoxy Coatings by Photoinitiated Cationic Copolymerization of Bisepoxide with ε-Caprolactone. Eur. Polym. J. 2010, 46, 254–259. DOI: 10.1016/j.eurpolymj.2009.10.023.
- Tsujii, A.; Namba, M.; Okamura, H.; Matsumoto, A. Radical Alternating Copolymerization of Twisted 1,3-Butadienes with Maleic Anhydride as a New Approach for Degradable Thermosetting Resin. Macromolecules 2014, 47, 6619–6626. DOI: 10.1021/ma501555n.
- Nomura, K.; Tsujii, A.; Matsumoto, A. Synthesis and Ozone Degradation of Alternating Copolymers of N-Substituted Maleimides with Diene Monomers. Macromol. Chem. Phys. 2017, 218, 1700156. DOI: 10.1002/macp.201700156.
- Lou, L.; Okamura, H.; Matsumoto, A. Crosslinking of Poly(Vinyl Alcohol) and Poly(Vinyl Acetate) Using Poly(Maleic Anhydride-Alt-2,4-Dimethyl-1,3-Pentadiene) as Polyfunctional Crosslinker and Decrosslinking by Ozone Degradation. J. Appl. Polym. Sci. 2017, 134, 1–6. DOI: 10.1002/app.44229.
- Exner, M.; Pluim, H.; Dyer-Smith, P. Ozonolysis: The Green Oxidation. Chem. Today 2011, 29, 59–62.
- Gallagher, J. J.; Hillmyer, M. A.; Reineke, T. M. Degradable Thermosets from Sugar-Derived Dilactones. Macromolecules 2014, 47, 498–505. DOI: 10.1021/ma401904x.
- Ma, S.; Webster, D. C. Naturally Occurring Acids as Cross-Linkers to Yield VOC-Free, High-Performance, Fully Bio-Based, Degradable Thermosets. Macromolecules 2015, 48, 7127–7137. DOI: 10.1021/acs.macromol.5b01923.
- Zhang, Y.; Wang, R.; Hua, Y.; Baumgartner, R.; Cheng, J. Trigger-Responsive Poly(β-Amino Ester) Hydrogels. ACS Macro Lett. 2014, 3, 693–697. DOI: 10.1021/mz500277j.
- Guo, B.; Finne-Wistrand, A.; Albertsson, A.-C. Facile Synthesis of Degradable and Electrically Conductive Polysaccharide Hydrogels. Biomacromolecules 2011, 12, 2601–2609. DOI: 10.1021/bm200389t.
- Bulmus, V.; Chan, Y.; Nguyen, Q.; Tran, H. L. Synthesis and Characterization of Degradable p(HEMA) Microgels: Use of Acid-Labile Crosslinkers. Macromol. Biosci. 2007, 7, 446–455. DOI: 10.1002/mabi.200600258.
- Binauld, S.; Stenzel, M. H. Acid-Degradable Polymers for Drug Delivery: A Decade of Innovation. Chem. Commun. 2013, 49, 2082–2102. DOI: 10.1039/c2cc36589h.
- Halpern, J. M.; Urbanski, R.; Weinstock, A. K.; Iwig, D. F.; Mathers, R. T.; von Recum, H. A. A Biodegradable Thermoset Polymer Made by Esterification of Citric Acid and Glycerol. J. Biomed. Mater. Res. 2014, 102, 1467–1477. DOI: 10.1002/jbm.a.34821.
- Ulery, B. D.; Nair, L. S.; Laurencin, C. T. Biomedical Applications of Biodegradable Polymers. J. Polym. Sci. B Polym. Phys. 2011, 49, 832–864. DOI: 10.1002/polb.22259.
- García, J. M.; Jones, G. O.; Virwani, K.; McCloskey, B. D.; Boday, D. J.; ter Huurne, G. M.; Horn, H. W.; Coady, D. J.; Bintaleb, A. M.; Alabdulrahman, A. M. S.; et al. Recyclable, Strong Thermosets and Organogels via Paraformaldehyde Condensation with Diamines. Science 2014, 344, 732–735.
- Yuan, Y.; Sun, Y.; Yan, S.; Zhao, J.; Liu, S.; Zhang, M.; Zheng, X.; Jia, L. Multiply Fully Recyclable Carbon Fibre Reinforced Heat-Resistant Covalent Thermosetting Advanced Composites. Nat. Commun. 2017, 8, 14657. DOI: 10.1038/ncomms14657.
- Yoshida, K.; Sanda, F.; Endo, T. Synthesis and Cationic Ring-Opening Polymerization of Mono- and Bifunctional Spiro Orthoesters Containing Ester Groups and Depolymerization of the Obtained Polymers: An Approach to Chemical Recycling for Polyesters as a Model System. J. Polym. Sci. A Polym. Chem. 1999, 37, 2551–2558. DOI: 10.1002/(SICI)1099-0518(19990715)37:14<2551::AID-POLA28>3.3.CO;2-G.
- Hitomi, M.; Sanda, F.; Endo, T. Reversible Crosslinking-Decrosslinking of Polymers Having Bicyclo Orthoester Moieties in the Side Chains. Macromol. Chem. Phys. 1999, 200, 1268–1273. DOI: 10.1002/(SICI)1521-3935(19990601)200:6<1268::AID-MACP1268>3.0.CO;2-N.
- Ogden, W. A.; Guan, Z. Recyclable, Strong, and Highly Malleable Thermosets Based on Boroxine Networks. J. Am. Chem. Soc. 2018, 140, 6217–6220. DOI: 10.1021/jacs.8b03257.
- Kloxin, C. J.; Scott, T. F.; Adzima, B. J.; Bowman, C. N. Covalent Adaptable Networks (CANs): A Unique Paradigm in Crosslinked Polymers. Macromolecules 2010, 43, 2643–2653. DOI: 10.1021/ma902596s.
- Jin, Y.; Yu, C.; Denman, R. J.; Zhang, W. Recent Advances in Dynamic Covalent Chemistry. Chem. Soc. Rev. 2013, 42, 6634–6654. DOI: 10.1039/c3cs60044k.
- Denissen, W.; Winne, J. M.; Du Prez, F. E. Vitrimers: Permanent Organic Networks with Glass-like Fluidity. Chem. Sci. 2016, 7, 30–38. DOI: 10.1039/C5SC02223A.
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-Like Malleable Materials from Permanent Organic Networks. Science 2011, 334, 965–968. DOI: 10.1126/science.1212648.
- Capelot, M.; Montarnal, D.; Tournilhac, F.; Leibler, L. Metal-Catalyzed Transesterification for Healing and Assembling of Thermosets. J. Am. Chem. Soc. 2012, 134, 7664–7667. DOI: 10.1021/ja302894k.
- Lu, L.; Pan, J.; Li, G. Recyclable High-Performance Epoxy Based on Transesterification Reaction. J. Mater. Chem. A 2017, 5, 21505–21513. DOI: 10.1039/C7TA06397K.
- Denissen, W.; Rivero, G.; Nicolaÿ, R.; Leibler, L.; Winne, J. M.; Du Prez, F. E. Vinylogous Urethane Vitrimers. Adv. Funct. Mater. 2015, 25, 2451–2457. DOI: 10.1002/adfm.201404553.
- Azcune, I.; Odriozola, I. Aromatic Disulfide Crosslinks in Polymer Systems: Self-Healing, Reprocessability, Recyclability and More. Eur. Polym. J. 2016, 84, 147–160. DOI: 10.1016/j.eurpolymj.2016.09.023.
- Zhou, F.; Guo, Z.; Wang, W.; Lei, X.; Zhang, B.; Zhang, H.; Zhang, Q. Preparation of Self-Healing, Recyclable Epoxy Resins and Low-Electrical Resistance Composites Based on Double-Disulfide Bond Exchange. Compos. Sci. Technol. 2018, 167, 79–85. DOI: 10.1016/j.compscitech.2018.07.041.
- Canadell, J.; Goossens, H.; Klumperman, B. Self-Healing Materials Based on Disulfide Links. Macromolecules 2011, 44, 2536–2541. DOI: 10.1021/ma2001492.
- Michal, B. T.; Jaye, C. A.; Spencer, E. J.; Rowan, S. J. Inherently Photohealable and Thermal Shape-Memory Polydisulfide Networks. ACS Macro Lett. 2013, 2, 694–699. DOI: 10.1021/mz400318m.
- Pepels, M.; Filot, I.; Klumperman, B.; Goossens, H. Self-Healing Systems Based on Disulfide–Thiol Exchange Reactions. Polym. Chem. 2013, 4, 4955–4965. DOI: 10.1039/c3py00087g.
- Lu, Y.-X.; Tournilhac, F.; Leibler, L.; Guan, Z. Making Insoluble Polymer Networks Malleable via Olefin Metathesis. J. Am. Chem. Soc. 2012, 134, 8424–8427. DOI: 10.1021/ja303356z.
- Lu, Y.-X.; Guan, Z. Olefin Metathesis for Effective Polymer Healing via Dynamic Exchange of Strong Carbon–Carbon Double Bonds. J. Am. Chem. Soc. 2012, 134, 14226–14231. DOI: 10.1021/ja306287s.
- Ma, Z.; Wang, Y.; Zhu, J.; Yu, J.; Hu, Z. Bio-Based Epoxy Vitrimers: Reprocessibility, Controllable Shape Memory, and Degradability. J. Polym. Sci. Part A: Polym. Chem. 2017, 55, 1790–1799. DOI: 10.1002/pola.28544.
- Altuna, F. I.; Pettarin, V.; Williams, R. J. J. Self-Healable Polymer Networks Based on the Cross-Linking of Epoxidised Soybean Oil by an Aqueous Citric Acid Solution. Green Chem. 2013, 15, 3360–3366. DOI: 10.1039/c3gc41384e.
- Liu, T.; Hao, C.; Wang, L.; Li, Y.; Liu, W.; Xin, J.; Zhang, J. Eugenol-Derived Biobased Epoxy: Shape Memory, Repairing, and Recyclability. Macromolecules 2017, 50, 8588–8597. DOI: 10.1021/acs.macromol.7b01889.
- Brutman, J. P.; Delgado, P. A.; Hillmyer, M. A. Polylactide Vitrimers. ACS Macro Lett. 2014, 3, 607–610. DOI: 10.1021/mz500269w.
- Liu, T.; Hao, C.; Zhang, S.; Yang, X.; Wang, L.; Han, J.; Li, Y.; Xin, J.; Zhang, J. A Self-Healable High Glass Transition Temperature Bioepoxy Material Based on Vitrimer Chemistry. Macromolecules 2018, 51, 5577–5585. DOI: 10.1021/acs.macromol.8b01010.
- Henriksen, M. L.; Ravnsbaek, J. B.; Bjerring, M.; Vosegaard, T.; Daasbjerg, K.; Hinge, M. Epoxy Matrices Modified by Green Additives for Recyclable Materials. ChemSusChem 2017, 10, 2936–2944. DOI: 10.1002/cssc.201700712.
- Reutenauer, P.; Buhler, E.; Boul, P. J.; Candau, S. J.; Lehn, J.-M. Room Temperature Dynamic Polymers Based on Diels-Alder Chemistry. Chem. Eur. J. 2009, 15, 1893–1900. DOI: 10.1002/chem.200802145.
- Coope, T. S.; Turkenburg, D. H.; Fischer, H. R.; Luterbacher, R.; van Bracht, H.; Bond, I. P. Novel Diels-Alder Based Self-Healing Epoxies for Aerospace Composites. Smart Mater. Struct. 2016, 25, 084010. DOI: 10.1088/0964-1726/25/8/084010.
- Luo, K.; Li, J.; Duan, G.; Wang, Y.; Yu, J.; Zhu, J.; Hu, Z. Comb-Shaped Aromatic Polyamide Cross-Linked by Diels-Alder Chemistry: Towards Recyclable and High-Performance Thermosets. Polymer 2018, 142, 33–42. DOI: 10.1016/j.polymer.2018.03.026.
- Polgar, L. M.; Fortunato, G.; Araya-Hermosilla, R.; van Duin, M.; Pucci, A.; Picchioni, F. Cross-Linking of Rubber in the Presence of Multi-Functional Cross-Linking Aids via Thermoreversible Diels-Alder Chemistry. Eur. Polym. J. 2016, 82, 208–219. DOI: 10.1016/j.eurpolymj.2016.07.018.
- Zhang, Y.; Broekhuis, A. A.; Picchioni, F. Thermally Self-Healing Polymeric Materials: The Next Step to Recycling Thermoset Polymers? Macromolecules 2009, 42, 1906–1912. DOI: 10.1021/ma8027672.
- Chen, X.; Dam, M. A.; Ono, K.; Mal, A.; Shen, H.; Nutt, S. R.; Sheran, K.; Wudl, F. A Thermally Re-Mendable Cross-Linked Polymeric Material. Science 2002, 295, 1698–1702. DOI: 10.1126/science.1065879.
- Turkenburg, D. H.; Fischer, H. R. Diels-Alder Based, Thermo-Reversible Cross-Linked Epoxies for Use in Self-Healing Composites. Polymer 2015, 79, 187–194. DOI: 10.1016/j.polymer.2015.10.031.
- Billiet, S.; De Bruycker, K.; Driessen, F.; Goossens, H.; Van Speybroeck, V.; Winne, J. M.; Du Prez, F. E. Triazolinediones Enable Ultrafast and Reversible Click Chemistry for the Design of Dynamic Polymer Systems. Nat. Chem. 2014, 6, 815–821. DOI: 10.1038/nchem.2023.
- Taynton, P.; Yu, K.; Shoemaker, R. K.; Jin, Y.; Qi, H. J.; Zhang, W. Heat- or Water-Driven Malleability in a Highly Recyclable Covalent Network Polymer. Adv. Mater. Weinheim. 2014, 26, 3938–3942. DOI: 10.1002/adma.201400317.
- Whiteley, J. M.; Taynton, P.; Zhang, W.; Lee, S.-H. Ultra-Thin Solid-State Li-Ion Electrolyte Membrane Facilitated by a Self-Healing Polymer Matrix. Adv. Mater. 2015, 27, 6922–6927. DOI: 10.1002/adma.201502636.
- Tsarevsky, N. V.; Matyjaszewski, K. Reversible Redox Cleavage/Coupling of Polystyrene with Disulfide or Thiol Groups Prepared by Atom Transfer Radical Polymerization. Macromolecules 2002, 35, 9009–9014. DOI: 10.1021/ma021061f.
- Tesoro, G. C.; Sastri, V. Reversible Crosslinking in Epoxy Resins. I. Feasibility Studies. J. Appl. Polym. Sci. 1990, 39, 1425–1437. DOI: 10.1002/app.1990.070390702.
- Sastri, V. R.; Tesoro, G. C. Reversible Crosslinking in Epoxy Resins. II. New Approaches. J. Appl. Polym. Sci. 1990, 39, 1439–1457. DOI: 10.1002/app.1990.070390703.
- Takahashi, Y.; Tobolsky, A. V. Chemorheological Study on Natural Rubber Vulcanizates. Polym. J. 1971, 2, 457. DOI: 10.1295/polymj.2.457.
- Rajan, V. V.; Dierkes, W. K.; Joseph, R.; Noordermeer, J. W. M. Science and Technology of Rubber Reclamation with Special Attention to NR-Based Waste Latex Products. Prog. Polym. Sci. 2006, 31, 811–834. DOI: 10.1016/j.progpolymsci.2006.08.003.
- Wang, Z.; Miller, B.; Mabin, M.; Shahni, R.; Wang, Z. D.; Ugrinov, A.; Chu, Q. R. Cyclobutane-1,3-Diacid (CBDA): A Semi-Rigid Building Block Prepared by [2 + 2] Photocyclization for Polymeric Materials. Sci. Rep. 2017, 7, 13704. DOI: 10.1038/s41598-017-13983-z.
- Froimowicz, P.; Frey, H.; Landfester, K. Towards the Generation of self-healing Materials by Means of a Reversible Photo-Induced Approach. Macromol. Rapid Commun. 2011, 32, 468–473. DOI: 10.1002/marc.201000643.
- Chung, C.-M.; Roh, Y.-S.; Cho, S.-Y.; Kim, J.-G. Crack Healing in Polymeric Materials via Photochemical [2 + 2] Cycloaddition. Chem. Mater. 2004, 16, 3982–3984. DOI: 10.1021/cm049394+.
- Liu, Y.; Farnsworth, M.; Tiwari, A. A Review of Optimisation Techniques Used in the Composite Recycling Area: State-of-the-Art and Steps towards a Research Agenda. J. Clean. Prod. 2017, 140, 1775–1781. DOI: 10.1016/j.jclepro.2016.08.038.
- Hernández Santana, M.; den Brabander, M.; Garcia, S.; van der Zwaag, S. Routes to Make Natural Rubber Heal: A Review. Polym. Rev. 2018, 58, 585–609. DOI: 10.1080/15583724.2018.1454947.
- Liang, B, Qin, B, Pastine, S, Li, X. Methods for Recycling Reinforced Composites, 2014.
- La Rosa, A. D.; Banatao, D. R.; Pastine, S. J.; Latteri, A.; Cicala, G. Recycling Treatment of Carbon Fibre/Epoxy Composites: Materials Recovery and Characterization and Environmental Impacts through Life Cycle Assessment. Compos. Part B: Eng. 2016, 104, 17–25. DOI: 10.1016/j.compositesb.2016.08.015.
- Cicala, G.; La Rosa, A.D.;, Latteri, A.;, Banatao, R.;, Pastine, S. The use of recyclable epoxy and hybrid lay up for biocomposites: Technical and LCA evaluation (2016) CAMX 2016 - Composites and Advanced Materials Expo. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85010064568&partnerID=40&md5=43b1c027a0c4a0223483b6dac50c2c72
- Chabert, E.; Vial, J.; Cauchois, J.-P.; Mihaluta, M.; Tournilhac, F. Multiple Welding of Long Fiber Epoxy Vitrimer Composites. Soft Matter 2016, 12, 4838–4845. DOI: 10.1039/c6sm00257a.
- Post, W.; Cohades, A.; Michaud, V.; van der Zwaag, S.; Garcia, S. J. Healing of a Glass Fibre Reinforced Composite with a Disulphide Containing Organic-Inorganic Epoxy Matrix. Compos. Sci. Technol. 2017, 152, 85–93. DOI: 10.1016/j.compscitech.2017.09.017.
- Ruiz de Luzuriaga, A.; Martin, R.; Markaide, N.; Rekondo, A.; Cabañero, G.; Rodríguez, J.; Odriozola, I. Epoxy Resin with Exchangeable Disulfide Crosslinks to Obtain Reprocessable, Repairable and Recyclable Fiber-Reinforced Thermoset Composites. Mater. Horiz. 2016, 3, 241–247. DOI: 10.1039/C6MH00029K.
- Taynton, P.; Ni, H.; Zhu, C.; Yu, K.; Loob, S.; Jin, Y.; Qi, H. J.; Zhang, W. Repairable Woven Carbon Fiber Composites with Full Recyclability Enabled by Malleable Polyimine Networks. Adv. Mater. Weinheim. 2016, 28, 2904–2909. DOI: 10.1002/adma.201505245.
- Costanzo, P. J.; Beyer, F. L.; Jensen, R. E. Reversible Viscosity Reducing Polymer. U.S. Patent # 7,812,069, 2008.
- Dello Iacono, S.; Martone, A.; Pastore, A.; Filippone, G.; Acierno, D.; Zarrelli, M.; Giordano, M.; Amendola, E. Thermally Activated Multiple Self-Healing Diels-Alder Epoxy System. Polym. Eng. Sci. 2017, 57, 674–679. DOI: 10.1002/pen.24570.
- Khan, N. I.; Halder, S.; Gunjan, S.B.; Prasad, T. A Review on Diels-Alder Based Self-Healing Polymer Composites. IOP Conf. Ser: Mater. Sci. Eng. 2018, 377, 012007. DOI: 10.1088/1757-899X/377/1/012007.
- Zhang, W.; Duchet, J.; Gérard, J. F. Self-Healable Interfaces Based on Thermo-Reversible Diels–Alder Reactions in Carbon Fiber Reinforced Composites. J. Colloid Interface Sci. 2014, 430, 61–68. DOI: 10.1016/j.jcis.2014.05.007.
- Fei, J.; Duan, X.; Luo, L.; Zhang, C.; Qi, Y.; Li, H.; Feng, Y.; Huang, J. Grafting Methyl Acrylic onto Carbon Fiber via Diels-Alder Reaction for Excellent Mechanical and Tribological Properties of Phenolic Composites. Appl. Surf. Sci. 2018, 433, 349–357. DOI: 10.1016/j.apsusc.2017.09.211.
- Peterson, A. M.; Jensen, R. E.; Palmese, G. R. Thermoreversible and Remendable Glass-Polymer Interface for Fiber-Reinforced Composites. Compos. Sci. Technol. 2011, 71, 586–592. DOI: 10.1016/j.compscitech.2010.11.022.
- Ghezzo, F.; Smith, D. R.; Starr, T. N.; Perram, T.; Starr, A. F.; Darlington, T. K.; Baldwin, R. K.; Oldenburg, S. J. Development and Characterization of Healable Carbon Fiber Composites with a Reversibly Cross Linked Polymer. J. Compos. Mater. 2010, 44, 1587–1603. DOI: 10.1177/0021998310363165.
- Park, J. S.; Darlington, T.; Starr, A. F.; Takahashi, K.; Riendeau, J.; Thomas Hahn, H. Multiple Healing Effect of Thermally Activated Self-Healing Composites Based on Diels-Alder Reaction. Compos. Sci. Technol. 2010, 70, 2154–2159. DOI: 10.1016/j.compscitech.2010.08.017.
- Park, J. S.; Kim, H. S.; Hahn, H. T. Healing Behavior of a Carbon/Mendomer Composite. Presented at the American Society for Composites – 23rd Technical Conference of the American Society for Composites, Memphis, TN, Sep 9–11, 2008.
- Imbernon, L.; Norvez, S. From Landfilling to Vitrimer Chemistry in Rubber Life Cycle. Eur. Polym. J. 2016, 82, 347–376. DOI: 10.1016/j.eurpolymj.2016.03.016.
- Sienkiewicz, M.; Kucinska-Lipka, J.; Janik, H.; Balas, A. Progress in Used Tyres Management in the European Union: A Review. Waste Manag. 2012, 32, 1742–1751. DOI: 10.1016/j.wasman.2012.05.010.
- Diaz, R.; Colomines, G.; Peuvrel-Disdier, E.; Deterre, R. Thermo-Mechanical Recycling of Rubber: Relationship between Material Properties and Specific Mechanical Energy. J. Mater. Process. Technol. 2018, 252, 454–468. DOI: 10.1016/j.jmatprotec.2017.10.014.
- Hernández, M.; Grande, A. M.; Dierkes, W.; Bijleveld, J.; van der Zwaag, S.; García, S. J. Turning Vulcanized Natural Rubber into a Self-Healing Polymer: Effect of the Disulfide/Polysulfide Ratio. ACS Sustainable Chem. Eng. 2016, 4, 5776–5784. DOI: 10.1021/acssuschemeng.6b01760.
- Xiang, H. P.; Qian, H. J.; Lu, Z. Y.; Rong, M. Z.; Zhang, M. Q. Crack Healing and Reclaiming of Vulcanized Rubber by Triggering the Rearrangement of Inherent Sulfur Crosslinked Networks. Green Chem. 2015, 17, 4315–4325. DOI: 10.1039/C5GC00754B.
- Polgar, L. M.; van Duin, M.; Broekhuis, A. A.; Picchioni, F. Use of Diels–Alder Chemistry for Thermoreversible Cross-Linking of Rubbers: The Next Step toward Recycling of Rubber Products? Macromolecules 2015, 48, 7096–7105. DOI: 10.1021/acs.macromol.5b01422.
- Araya-Hermosilla, R.; Fortunato, G.; Pucci, A.; Raffa, P.; Polgar, L.; Broekhuis, A. A.; Pourhossein, P.; Lima, G. M. R.; Beljaars, M.; Picchioni, F.; et al. Thermally Reversible Rubber-Toughened Thermoset Networks via Diels-Alder Chemistry. Eur. Polym. J. 2016, 74, 229–240. DOI: 10.1016/j.eurpolymj.2015.11.020.
- Trovatti, E.; Lacerda, T. M.; Carvalho, A. J. F.; Gandini, A. Recycling Tires? Reversible Crosslinking of Poly(Butadiene). Adv. Mater. 2015, 27, 2242–2245. DOI: 10.1002/adma.201405801.
- Zhong, N.; Garcia, S. J.; Van Der Zwaag, S. The Effect of Filler Parameters on the Healing of Thermal Conductivity and Mechanical Properties of a Thermal Interface Material Based on a Self-Healable Organic-Inorganic Polymer Matrix. Smart Mater. Struct. 2016, 25, 084016. DOI: 10.1088/0964-1726/25/8/084016.
- Lafont, U.; Moreno-Belle, C.; van Zeijl, H.; van der Zwaag, S. Self-Healing Thermally Conductive Adhesives. J. Intell. Mater. Syst. Struct. 2014, 25, 67–74. DOI: 10.1177/1045389X13498314.
- Li, J.; Zhang, G.; Sun, R.; Wong, C.-P. A Covalently Cross-Linked Reduced Functionalized Graphene Oxide/Polyurethane Composite Based on Diels-Alder Chemistry and Its Potential Application in Healable Flexible Electronics. J. Mater. Chem. C 2017, 5, 220–228. DOI: 10.1039/C6TC04715G.
- Li, J.; Liang, J.; Li, L.; Ren, F.; Hu, W.; Li, J.; Qi, S.; Pei, Q. Healable Capacitive Touch Screen Sensors Based on Transparent Composite Electrodes Comprising Silver Nanowires and a Furan/Maleimide diels-Alder Cycloaddition Polymer. ACS Nano. 2014, 8, 12874–12882. DOI: 10.1021/nn506610p.
- Zou, Z.; Zhu, C.; Li, Y.; Lei, X.; Zhang, W.; Xiao, J. Rehealable, Fully Recyclable, and Malleable Electronic Skin Enabled by Dynamic Covalent Thermoset Nanocomposite. Sci. Adv. 2018, 4, eaaq0508. DOI: 10.1126/sciadv.aaq0508.