?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The superior biocompatibility and biodegradability of polyhydroxyalkanoates (PHAs) compared to man-made biopolymers such as polylactic acid promise huge potential in biomedical applications, especially tissue engineering (TE). Textile fiber-based TE scaffolds offer unique opportunities to imitate the anisotropic, hierarchical, or strain-stiffening properties of native tissues. A combination of PHAs’ enhanced biocompatibility and fiber-based TE scaffolds could improve the performance of TE scaffolds. However, the PHAs’ complex crystallization behavior and the resulting intricate spinning procedures remain a challenge. This review focuses on discussing the developments in PHA melt and wet spinning, their challenges, process parameters, and fiber characteristics while revealing the lack of an in-depth fiber characterization of wet-spun fibers compared to melt-spun filaments, leading to squandered potential in scaffold development. Additionally, the biomedical application of PHAs other than poly-4-hydroxybutyrate is hampered by a failure of polymer purity to meet the requirements for biomedical applications.
1. Introduction
The interdisciplinary field of tissue engineering (TE) has the goal of replacing or repairing damaged or diseased tissue or organs by artificially produced replacements. Traditionally such defects for instance in bones are treated with autografts (transplantation of tissue within one individual from one location to another[Citation1]) or allografts (transplantation of tissue from one individual to another individual[Citation2]). These methods have some constrains such a limited availability of donor supplies, immunogenicity risks and infections.[Citation3] To overcome the problem of limited donor supply and to reduce the risk of rejection by the host,[Citation4] TE tries to mimic the extracellular matrix’s structure seeded with the patients cells thus supporting new tissue growth as well as cell adherence and proliferation.[Citation3] TE has four interrelated prerequisites: suitable cells to colonize the scaffold and help the body to recover, a suitable environment/scaffold for cell adhesion and proliferation, appropriate biomolecules, and the right physical and mechanical forces for cell development.[Citation5,Citation6] It is challenging to mimic the versatile structures and mechanical properties of native tissues, but, among other technologies, textile technology has shown possibilities for conquering this challenge. Textile-based scaffolds produced by weaving, knitting, or braiding can imitate the anisotropic, hierarchical, or strain-stiffening properties of native tissues[Citation7] such as blood vessels, nerves, and muscles. An example of suitable textile structures are woven fabrics which can have similar anisotropic stress-strain curves to a heart valve or potentially other anisotropic tissues such as skin, skeletal muscles, and connective tissues.[Citation8,Citation9] Theoretically, yarns consisting of fibers or continuous filaments, which can also be combined with twinned ply yarns in a cord, could mimic (apart from the size) the hierarchical structure of bones (sub-nanostructure, nanostructure, as shown in ),[Citation10] and soft tissue’s strain-stiffening properties can be simulated by using knitted textiles.[Citation9]
Figure 1. Hierarchical structure of bones and the theoretical possibility of mimicking it with fibrous replacements. 1 represents mineralized collagen fibrils, 2 refers to a collagen fibril, and 3 shows a collagen molecule. To mimic the size of the collagen structure with the replacement is probably a major challenge. Figure adapted from Jiang et al.,[Citation9] Wang et al.[Citation10] and D’Elia.[Citation128]
![Figure 1. Hierarchical structure of bones and the theoretical possibility of mimicking it with fibrous replacements. 1 represents mineralized collagen fibrils, 2 refers to a collagen fibril, and 3 shows a collagen molecule. To mimic the size of the collagen structure with the replacement is probably a major challenge. Figure adapted from Jiang et al.,[Citation9] Wang et al.[Citation10] and D’Elia.[Citation128]](/cms/asset/242b9bb2-232c-4841-ac52-15de886ce99b/lmsc_a_2076693_f0001_c.jpg)
Choosing a suitable textile production method and pattern enables the controlling and tailoring of the porosity, architecture, physical, mechanical, and biological properties of the scaffolds.[Citation9]
Polyhydroxyalkanoates (PHAs) are a group of thermoplastic, bio-based, and biodegradable polyesters which have huge potential in medical applications and TE because of their superior biocompatibility compared to other biopolymers, such as polylactic acid (PLA) or polyglycolic acid (PGA).[Citation11,Citation12] However, due to their high crystallinity and narrow processing window, since the thermal degradation temperature is close to the thermal decomposition temperature, it is challenging to produce proper fibers from PHAs.
In TE, textiles can either be used as degradable substrate to culture cells in vitro which are then implanted into the body to recover the diseased area, or they can be used for an in vivo regeneration of the tissue by providing stimulation and the right preconditions for the organs and tissue to recover. Starting from a textile fiber or yarn, textile manufacturing techniques offer various possibilities for fabrication of a TE scaffold compared to other scaffold production processes. Versatile opportunities exist to adapt the physical and mechanical properties of the scaffolds in a way that closely mimics the properties of human tissues.[Citation9] The mechanical requirements of the scaffold depend on the replaced tissue types (connective tissue e.g., tendons, epithelial tissue such as skin, muscle tissue or nervous tissue). Human adult tendons like the flexor and extensor can endure a mean tensile strength of 31.25 and 26.23 MPa respectively[Citation13] while human skin has a mean ultimate tensile strength of 27.2 ± 9.3 MPa.[Citation14] Muscle tissue such as the human femora muscle shows a failure load of 26.7 ± 8.8 N [Citation15] whereas human sciatic nerves can withstand an average maximum stress of 3.88 MPa (peroneal) or 3.91 MPa (tibial).[Citation16] Classical textile production processes such as weaving, knitting, and braiding are, especially with very basic textile patterns, quite established within the tissue engineering community. Fibers for industrial use which are processed on common textile machinery should have a tensile strength of at least 500 MPa.[Citation17] Once the fibers meet the standard for industrial applications, they should also be applicable for biomedical textiles as fibers used in load-bearing applications such as tendon or bone replacements where they need to endure loads of approximately 150 MPa.[Citation18] Textile-based scaffolds are discussed by Tamayol et al.[Citation18]. Compared to other materials commonly used in medicine such as metals or ceramics, textile implants have several benefits such as an increased freedom/variety of patterns and structures. Further benefits of textile scaffolds include the relatively simple adjustability of their degradation time, and the possibility of controlling mechanical properties such as flexibility, elasticity, and porosity of the scaffold structure by varying the fiber type, diameter and length, surface characteristics, shape, and the fabric’s density or pattern.[Citation19]
However, it seems that even though being an important part of textile TE scaffolds by significantly affecting their final properties, textile fibers are often overlooked regarding their potential in TE. Fibers or filaments, respectively, are the building blocks of every textile structure. Without a well-elaborated spinning process, it is almost impossible to obtain a satisfying textile structure which meets end-use demands. This review therefore takes a textile engineering perspective when reviewing articles regarding fiber production. The paper briefly discusses the material properties of PHAs and follows with a detailed review of textile-fiber spinning processes for the preparation of biomedical textiles and tissue engineering scaffolds from PHAs using textile technologies. The focus is on melt- and wet-spinning techniques as these methods usually obtain fibers that are processable on common textile machinery into woven, knitted, or braided fabrics. Electrospinning is a well-known and established technique in the biomedical field because fibers within the nano-, micrometer range, having a large specific surface area can be produced.[Citation20] Often a three-dimensional interconnected pore network is produced in electrospinning which additionally helps to mimic the structure of the extracellular matrix and is therefore attractive for cell adhesion and proliferation.[Citation21] Electrospinning is extensively discussed in other reviews like Kanuik and Stachewicz [Citation22] or Zhao et al. [Citation23] for which reason it is excluded from this review. Additionally, spinning methods for staple fibers such as ring spinning are disregarded because they are designated for staple fibers (fibers with a specific length in contrast to endless filaments).
1.1. Polyhydroxyalkanoates
Polyhydroxyalkanoates (PHAs) is an umbrella term for many different types of related polymers, all of which are linear, nontoxic, biodegradable,[Citation24,Citation25] and thermoplastic to elastomeric natural polyesters produced intracellularly in different types of microorganisms and plants.[Citation26–28]
1.1.1. Origin of PHAs
More than 300 species of Archaea, gram-positive and gram-negative bacteria,[Citation29] photosynthetic bacteria, and mixtures of different microorganisms are capable of producing PHAs under aerobic as well as anaerobic conditions.[Citation26,Citation30] Usually, they are produced under unfavorable growing conditions with an oversupply of carbon sources and a limitation of other essential nutrients, for instance, oxygen, phosphorous, or nitrogen. The polymer is made intracellularly in an aqueous environment under ambient conditions and stored in the form of amorphous, water-insoluble granules (i.e., cytoplastic inclusions) [Citation31] inside the cell.[Citation32] Microorganisms use the PHA as a carbon and energy storage [Citation32] with bacteria commonly storing a PHA content of up to 80-90 wt% of the dry weight in their cells under regulated cultivation conditions in a fermenter.[Citation24,Citation33,Citation34] The production and extraction of PHAs is beyond the scope of this review; for further information see Steinbüchel and Hein,[Citation35] Kim and Lenz,[Citation36] Kurian and Das,[Citation37] and Laycock et al.[Citation24].
1.1.2. Properties of PHAs
Many factors influence PHA production, resulting in diverse polymers. The mechanical properties of one specific polymer within the group of PHAs are affected by the metabolic pathways and enzymes of the bacterial strain and substrate used.[Citation24] Reliant on that, the given carbon source is converted into 3-, 4- and 5-hydroxyalkanoyl-CoA precursors [Citation38] that are converted by an enzyme into high molecular weight (0.05-20 MDa [Citation39]) PHAs.[Citation32] Additionally, an increase of the carbon-nitrogen ratio favors PHA accumulation.[Citation40] Molecular weights in the range of 4.6 × 106 Da (at a polydispersity index (PI) around 2.0 [Citation41]) have been reported. More than 150 possible monomer constituents reflect the high structural variety and varying material properties of the corresponding PHAs. Polymer properties range therefore from thermoplastic to elastomeric and are mainly defined by the number of alkyl (R)-hydroxy fatty acid monomer units [Citation27] in the polymer backbone, the length and nature of the alkyl side groups (R in ), and the distance between the ester linkages (n in )[Citation38,Citation42,Citation43]. The pendant alkyl groups influence the polymer’s rigidity as PHAs with short pendant groups are usually hard and crystalline materials whereas PHAs with longer pendant groups become elastomeric.[Citation44] depicts the general structural formula of PHAs and gives examples of some specific polymers while gives an overview of selected copolymers. According to their monomer chain lengths (combination of n and R in ), PHAs are often divided into three different groups, i.e., short-chain length (scl) with less than five carbons, medium-chain length (mcl) with five to 14 carbons, and long-chain length (lcl) having more than 14 carbons in their chain.[Citation45] However, long-chain-length PHAs like poly(3-hydroxypentadecanoate) are very uncommon.[Citation30] Short-chain length PHAs, such as poly-3-hydroxybutyrate (P3HB) or poly(3-hydroxyvalerate) (PHV), show thermoplastic properties with elevated melting temperatures (Tm), are highly crystalline, stiff, and brittle [Citation46] whereas mcl-PHAs (e.g., poly(3-hydroxyoctanoate) (PHO)) have an elastomeric nature with low tensile strength and high elongation at break as well as a low crystallinity and lower glass transition temperature (Tg) compared to scl-PHAs.[Citation47,Citation48] These properties make mcl-PHAs suitable for many medical applications, such as cardio vascular grafts,[Citation44] artificial esophagus,[Citation49] and tendons or ligaments [Citation49] where elastic biomaterials are indispensable.
Table 1. General chemical structure of PHAs, examples of specific polymers and copolymers.
Table 2. Chemical structures of selected copolymers, m = repeat unit of the hydroxybutyrate block, p = repeat unit of copolymer block.
Since PHAs show similar properties to petrochemical polymers [Citation47] such as polypropylene (PP),[Citation44] compares selected examples of PHAs (mainly P3HB as it is one of the most studied) and polypropylene. Due to the manifoldness of the PHAs and their properties, this table does not claim to be all-inclusive, it rather gives an indication. For further information on how the mechanical properties are influenced by the polymer composition, see Laycock et al.[Citation24].
Table 3. Selected material properties of some PHAs compared with polypropylene.
As presented in , PHAs and PP are hydrophobic, linear, chiral polymers with a high degree of crystallinity (both >50%) and similar thermal transition stages (Tg and Tm). The main differences between PP and PHAs are the lower density of PP and its higher solvent resistance, which makes it harder to dissolve and not biodegradable in contrast to PHAs.
Besides the wide range of mechanical properties, PHAs are piezoelectric materials which enables many promising applications in the medical field.[Citation30] Piezoelectric properties are important for the stimulation of bone growth and support the healing of wounds so the medical application fields of PHAs can be seen in orthopedics, for instance, as screws, bone graft substitutes, or scaffolds for cartilage tissue engineering.
Because of their microbiological production, PHAs have a very high purity in cells [Citation24] and in the case of P3HBs, they are free of catalyst residues,[Citation50] making them promising for biomedical applications such as wound dressings, drug delivery systems,[Citation30] or substitutes for nerve guides.[Citation51] The high elongation to break of elastomeric PHAs could, for instance, facilitate use in cardiovascular tissue replacements as they are stronger under dynamic conditions than polyurethane-based elastomers.[Citation47] For an overview of P4HBs in commercial use in the medical field, see Manavitehrani et al.[Citation52].
Processing problems with PHAs arise from their being either too brittle (crystallinity >50%, often 60-80% [Citation41]) or too tacky (low viscosity and crystallinity).[Citation11] Thus, it is crucial to control crystallinity when processing PHAs. To meet the final product requirements, PHAs are often biologically or chemically modified, grafted, or blended.
PHA blending is a cost-effective and relatively straightforward method of adjusting the polymer’s end-use properties. However, this might also decrease the degradation time, making the blend unsuitable for some applications.[Citation24,Citation41,Citation53] Depending on the polymer blend and ratio, the blend’s properties change. Typically, thermal transitions like the glass transition, and melt temperature,[Citation11,Citation41,Citation46,Citation53,Citation54] the crystallinity, and the mechanical properties [Citation11,Citation41,Citation43,Citation54,Citation55] alter. Changes in crystallinity seem to affect the hygroscopicity, which might influence the cell adhesion when used as TE scaffold.[Citation55] Some blends, for instance, P3HB and poly(ethylene glycol) (PEG) are able to control blood clotting which makes them suitable for tissue engineering, surgery, and medical diagnostics applications.[Citation41] Some PHA constituents like 3-hydroxybutyric acid (a metabolite found in the blood) in PHBV occur naturally in the human body, which increases the biocompatibility.[Citation30,Citation56] Additionally, the degradation products of, for example, P4HB are 4HB, a natural mammalian and human metabolite present in various body parts such as the brain, heart, and muscles. This leads to a good in vivo tolerance and allows the human body to eliminate the 4HB within between 27 and 35 min by converting it via the Krebs cycle into carbon dioxide and then exhaling it.[Citation12,Citation57] Compared to PGA and PLA, 4HB has a lower pKa and fewer acidic degradation products, so inflammatory responses are less likely.[Citation11,Citation12]
Regardless of whether the PHAs degrade in the environment, water, or human bodies, the degradation process depends on physicochemical and microbial factors as well as on material properties and their processing history. Physicochemical factors are, for instance, temperature, humidity, and pH level.[Citation58] Microbiological factors include the influence of microorganisms such as microbial diversity and activity, or population density.[Citation58,Citation59] Moreover, material properties such as the molecular weight, crystallinity, hydrophobicity, porosity, and steric configuration as well as the processing (e.g., surface characteristics, material thickness, or additives) play an important role in PHA degradation.[Citation58] When conceptualizing a medical textile of PHA one should keep in mind that PHA degradation also varies within different human tissues and the size of the PHA artifact.[Citation51]
Thermal degradation of P3HB, for example, in the range of 170 to 200 °C (close to the melting temperature), is usually induced by a non-radical mechanism, involving ester bond decomposition via a random six-membered ring transition state.[Citation60] Besides the type of PHA, crystallinity and thermal properties such as Tg or Tm are influenced by the length of the polymer’s alkyl side chains.[Citation46,Citation61] Abe and coworkers found that the Tg of mcl-PHAs decreases with an increase of the side chain’s length up to seven carbons, whereas the Tm increases 24 °C (from 45 to 69 °C) with an increase of the side chain length from C4 to C7.[Citation62] They therefore suggested reconsidering the common categories in scl-, mcl-, and lcl-PHAs based on the number of carbon molecules and recommended a new classification in scl-, and mcl-PHAs based on crystallization behavior. According to their proposal, scl-P(3HA)s should be all compounds with less than C6 monomeric units and mcl-P(3HA)s compounds with more than C7 monomeric units.[Citation62] A polymer classification based on material properties seems, however, more reasonable than an apparently arbitrary division according to the chain length, so the common definition should be reassessed.
1.1.3. Sterilization
PHAs applied in medical products need to be sterilized. Rogers’ statement that heat treatment and gamma irradiation do not impact the strength of PHA films [Citation63] is controversial, according to Grigore and coworkers, who state that gamma irradiation reduced the mechanical properties and the molecular weight, at least for P3HB.[Citation30] They found that the same deterioration applied for steam sterilization, whereas sterilization with ethylene oxide or gaseous formaldehyde preserved the P3HB’s properties.[Citation30] Martin and Williams also reported the sterilization of P4HB in a cold cycle of ethylene oxide (commonly used for surface sterilization [Citation64]) without significantly affecting the polymer. Martin and Williams furthermore found that P4HB lost molecular weight and showed a higher polydispersity when sterilized with gamma-irradiation at 25-50 kGy. The material deterioration became more prominent with increasing radiation.[Citation12] Alternative sterilization methods like UV radiation at 260 nm for 24 hr [Citation34] and submerging the samples for 2 hr in 70% aqueous ethanol solution [Citation56] were also applied by other researchers. However, it seems that the effect of a sterilization method on the material properties was not studied widely, especially not for fibrous materials.
1.1.4. Drawback of PHAs
Reported endotoxin residues, a decomposition product of gram-negative bacteria that can lead to several physiological reactions in humans, are a drawback of PHAs. It is thus indispensable to eliminate endotoxins with an appropriate method before applying the material in medical products. Several researchers solved this problem by using solvents that add cost and environmental impact to the PHA production process.[Citation41] For the purification of PHAs other than P4HB, further research is necessary, since to the best of our knowledge, no other PHA has clearance for biomedical applications.
PHAs have high production costs – between five to 15 times greater [Citation26,Citation40,Citation65] than petroleum-derived polymers. The main costs are due to the substrates,[Citation66] which account for 30-40% [Citation67] of the total production costs. Microbial growing, extraction, and purification processes add further expense and hinder the widespread replacement of petroleum-based plastics. Researchers are trying to use cheaper substrates such as waste material; however, impurities and variances in material composition may reduce the polymer yield. Additionally, waste feedstock needs further purification processes for the production of medical applications with the risk of bacterial, viral, plasmid, or genetic contamination.[Citation40]
1.2. Fibers and fiber spinning
Generally, fibers include filaments (fibers of theoretically indefinite length), typically man-made from natural or synthetic polymers, such as viscose or polyethylene terephthalate, and staple fibers with a definite length. Traditionally, staple fibers were of natural origin, for instance, cotton or wool fibers. However, for some applications filaments are cut into staple fibers for further processing. Additionally, staple fibers should have a length of at least 100 times their diameter. The molecular structure of each fiber significantly contributes to its properties.[Citation68] One distinctive property of fibers and yarns is their co-possession of strength and flexibility.[Citation69] Hardly any other material type shows stiff and strong properties against tensile stress along one axis (in this case, the fiber axis) while simultaneously being flexible and soft for bending, twisting, and deformation. These characteristics originate from the fiber’s long and thin shape and is further enhanced by alignment of the polymer’s chains. Alignment (the orientation of molecular chains along a certain direction in the material, while assuming uniaxial symmetry) increases the mechanical properties because the covalent bonds of the straightened polymer chains take the load. Without alignment, the weight has to be taken by a random and probably weaker part of the polymer chain.[Citation69] Thus, the main objective of fiber spinning is to process polymers with a specific directionality as the polymer orientation (maximization of the polymer chain orientation [Citation69]) and control of the crystallization during cooling greatly influences the mechanical properties of the filament and therefore the functionality of the end product.[Citation70] Further, especially cellulose based man-made fibers, polyaramide, and polyamides form intramolecular bonds between the aligned fiber polymer molecules supporting the fiber structure.
Fundamentally, the process of fiber spinning starts with transferring the polymer into a liquid state; in melt spinning this occurs by melting whereas a solvent is used in the case of wet spinning. Afterwards, the liquid polymer passes through a die, followed by a drawing unit to align the macromolecules in the direction of the material flow while in a rubbery state. Before the fibers are wound up and collected on a bobbin, they are either cooled or the solvent is removed, depending on the process.[Citation70] To put it simply, the polymer viscosity and rheology, both in liquid and solid state, control the workability of the fiber, while the spinning process forms the shape of the fiber and the structure of the fiber is mainly adjusted by the drawing process.[Citation69]
1.2.1 Melt spinning
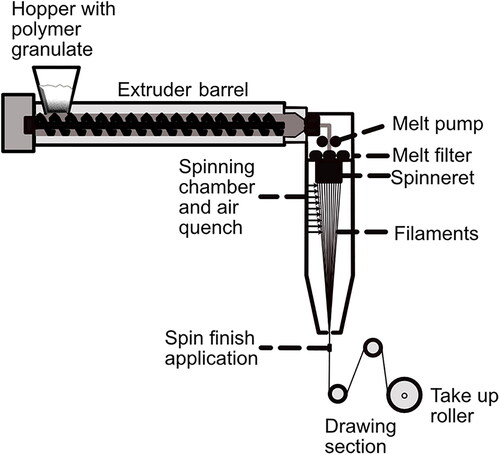
Melt spinning is a favored spinning method for thermoplastic polymers as it is usually considered a comparatively economical,[Citation71] simple, and environmentally friendly process (because of the absence of solvents).[Citation69] Melt spinning can be done at very high filament-forming speed, with take-up speeds of up to 10 km/min [Citation72] whereas 0.5 km/min was reported for PHBHs.[Citation73]
During melt spinning, polymer granules are fed by a hopper into the machine at the top of the spinning tower/facility. The granules are usually melted in an extruder and pumped toward the spinneret. In between, it passes a metering melt pump [Citation74] that builds up and maintains the spin pressure at a constant value and controlled melt throughput, and then the melt passes through steel-screen or sand-melt filters to remove unwanted impurities and prevent damage to the machinery.[Citation75] The melt filters build up additional melt pressure and shear forces because of their narrow meshes. This improves the melt quality and homogeneity which is indispensable to achieve good processability and reproducibility. Additionally, this may have a positive effect on the mechanical properties of the spun fibers. When the polymer exits the die, extrudate or die swell (the process of the polymer expanding to a size bigger than the spinneret holes) often occurs. This is due to the polymer releasing elastic energy and molecular orientation after being exposed to shear flow when being forced through the small holes of the spin pack. After exiting the die, the spun filaments are wound up on rotating and heatable godet rolls at a higher speed than the throughput rate to achieve a uniaxial drawing. The filaments are cooled by an air stream and in this section of the spinning process, the draw-down ratio (DDR) is set, which is defined as shown in EquationEquation (1)(1)
(1) with v0 being the average extrusion speed and vf the take-up velocity.[Citation76]
(1)
(1)
Together with quench rate and spin-line stress, the DDR defines the orientation of the filament. Decreasing melt temperatures and increasing molecular weight builds up spin-line stress. The filaments needs to be solidified either through glass transition or crystallization before being wound up by the take-up roll to avoid it sticking to the godet, winder, or to each other. Afterwards, the filaments are led through a set of godets run at different revolving speeds to obtain the main molecular orientation in the solidified filaments. The relationship between the velocities of the take-up roll and winder is called draw ratio (DR). To perfect the microstructure, the filaments can be stretched and annealed (heat set) under strain. This helps to a certain extent to relax frozen stresses and stabilizes the filament’s microstructure. The main molecular orientation is obtained by post drawing in the solidified state.[Citation75]
Melt spinning enables the production of mono- and multifilaments and of complex fiber cross sections such as fractal-like [Citation77] and hollow fibers [Citation78] which can support cell alignment and topographical control of cell orientation or bi-component fibers.[Citation79] A schematic drawing of a melt-spinning facility is shown in .
The main challenges in the melt spinning of virgin PHAs, especially P3HB, are the rapid thermal degradation, low melt elasticity, and control of the crystalline structure because it has a low crystallization rate due to its low nucleation density and brittleness,[Citation80] and variations in the molecular weight and in polymer quality.[Citation75] The crystal orientation is influenced by environmental conditions i.e., spinning parameters such as applied temperatures, stresses, or solvents.[Citation81]
1.2.2. Wet spinning
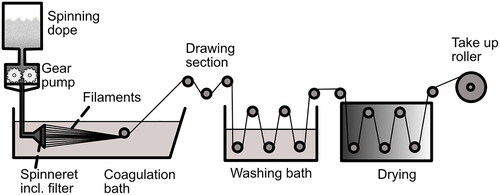
Wet spinning is the oldest method of producing man-made fibers and a well-known process for producing cellulose-derived fibers such as rayon or viscose.[Citation82] In wet spinning, the polymer is first dissolved in a suitable solvent without being degraded and then coagulated in a non-solvent bath (for the polymer) to obtain the fibers.[Citation83] Before coagulation, the polymer solution is forced by a gear pump through the fine orifices of a spinneret that is equipped with a filter to avoid clogging of the spinneret holes by contaminants. The spinneret is submerged in the non-solvent so that there is immediate contact between the exiting polymer solution and coagulation bath which leads to the removal of the solvent from the polymer solution, and the filaments solidify.[Citation83] The contact of these two solvents causes chemical and physical changes to the dissolved polymer, which leads to a coagulation of the polymer in the spinning bath and forms the final filament. depicts the wet-spinning process. The fiber properties are mainly influenced by the reaction between the bath and the fiber, wherefore additives might be used to control the coagulation and regeneration rate. Usually, a rather fast coagulation is preferred for wet spinning which almost immediately forms the fiber skin when the polymer solution is extruded in the coagulation bath to obtain a fiber with some structural integrity before the core is coagulated and is able to contribute to the fiber’s stability.[Citation83] Hydraulic drag causes the fibers to stretch in the bath. The filaments are drawn off the coagulation bath by rollers. In an additional step, the fibers are further drawn by a set of paired rollers operating at different rotational speeds after the filament leaves the coagulation bath. Fiber drawing increases the orientation of the polymer molecules in the fiber parallel to the fiber axis and thus enhances the mechanical properties. In general, fiber properties such as breaking strength or elongation modulus are influenced by the spinning conditions, composition of the spinning bath, and potential additives. Wet-spun fibers often consist of a core and skin structure since, due to contact with the coagulation bath, they start to solidify from the outside in.[Citation82–84]
Because of its low processing temperatures compared to melt spinning, wet spinning is a preferable method for the processing of thermally sensitive or non-thermoplastic polymers to avoid thermal degradation.[Citation82] Compared to melt-spinning, the spinning speeds in wet spinning are lower (around 30-300 m/min) because the procedure is conducted in fluids with higher viscosity to expose the filament to higher tensions. Lower processing speeds help to reduce the stress on the fibers.
The mass transfer rate determines the resulting properties and microstructures of the as-spun fibers during the coagulation of the dope outer layer. Wet spinning tolerates up to 30% of solid particles [Citation84] which could open up opportunities for the incorporation of temperature-sensitive additives such as pharmaceuticals.
2. Spinning methods and process parameters for PHA fiber
The reported spinning methods for fabricating PHA fibers, including process parameters, are compared and discussed in detail below, according to type of spinning method.
2.1. Melt spinning
Several varying melt spinning processes to produce PHA fibers are found in the literature. For good process control and sufficient fiber properties, Hufenus and coworkers suggested five crucial properties a polymer should have.[Citation75]
The polymer should be thermally stable enough to withstand the extrusion temperature and the shear strain during the processing, with minimal degradation and without crosslinking.
As the melt is subject to forces of gravity, air or water friction, and inertia, the polymer needs an intermediate molecular weight to provide sufficient melt strength and avoid filament break during processing, while at the same time not being so viscous as to impair processability.
For a constant melt flow, the polymer should have a rather low polydispersity index to guarantee a consistent melt flow rheology. In a rough estimation, the polydispersity index should not exceed a value of three to achieve a stable melt-spinning process.
Linear polymers are the most suitable for melt spinning because their molecular chains are mobile enough to be unfolded and aligned along strain direction.
The polymer should be uniform and pure to prevent clogging of the machine and fluctuations in the processing.
With respect to the requirements mentioned above, PHAs should be melt spinnable since they are linear polymers (despite having different side-chain length),[Citation24] their polydispersity index has an average of two below three [Citation41] and their MW is usually above 500,000 g/mol which can be considered sufficient.[Citation85] Challenges are the rapid thermal degradation of PHAs and a lack of polymer purity for some PHA qualities. The thermal degradation can be prevented, for instance, by the use of plasticizers or copolymers that lower the melting temperature and thus increase the processing window.[Citation46,Citation86] Even though Hufenus et al. reported a lack of PHA purity originating from the biotechnological process, the polymer purification processes should be sufficient to enable the spinning procedure. Most PHAs do not meet purity standards for medical applications, for instance, due to bioactive contaminants [Citation87] – which is why the FDA have not approved PHAs (except for P4HB).
In industrial textile applications, fibers need to withstand stresses of between 400 and 700 MPa, so it is desirable to develop PHA fibers within this range to enable them to be more widely applied in the field.[Citation25]
The main challenge when melt spinning PHAs is their crystallization behavior for which reason researchers have tried several approaches to controlling crystallization during melt spinning, from typical parameters such as the change/control of temperature and drawing, to using several additives or electron beams.
The crystalline structure of the most commonly investigated PHA (P3HB) shows two molecular conformations, namely the α-form with a 21 helix conformation and the β-form which has a planar-zigzag shape. The β-form is only found in drawn films or fibers and is assumed to be one of the main reasons for high tensile strength, whereas the α-form is universally observed in P3HB materials, including films, injection-molded items, or fibers.[Citation25] Additionally, the crystalline structure, especially the planar-zigzag conformation, improves the mechanical properties of the fibers.[Citation88] Iwata and Tanaka found that the orientation of isothermally crystallized and one-step drawn fibers of the α-form as well as the intensity of the β-form rose with increasing draw ratio. The isothermal crystallization influenced the orientation and crystallinity of the fibers, as both increased compared to non-isothermally crystallized fibers.[Citation88]
2.1.1. Crystallization control by temperature and drawing
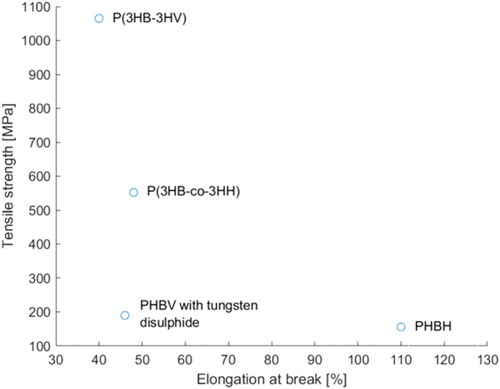
Gordeyev and Nekrasov[Citation89] as well as Schmack et al.[Citation90] started to study the influence of P3HB’s crystallization behavior with temperature control and drawing. Gordeyev and Nekrasov used an extruder to produce fiber precursors with a DR of 2 followed by hot drawing and annealing. The results showed that each additional process step further increased the tensile strength and modulus of the fiber while the strain at break steadily decreased with increasing strength. By using this method, they managed to obtain fibers with a maximum tensile strength of 190 MPa.[Citation89] Using similar temperature conditions but a fiber melt spinning facility instead of an extruder led to improved strength of pure fibril-like P3HB fibers (physical break stress of the fibers: 330 MPa) with two different crystalline structures.[Citation90] The researchers found 21 helical α-modifications as well as planar-zigzag β-conformations. The magnitude of α- and β-crystals reflects the degree of fiber orientation. The higher the degree of orientation, the higher the values of the β- and α-structures, where the α-configuration is influenced to a greater extent by the tensile stress.[Citation90] So far there has been a numerous number of studies which report the mechanical properties for melt spun PHA fibers. These have been compiled in . For a better comparison, summarizes the tensile strength and elongation at break of melt spun P3HB filaments at different draw ratios, showing that the draw ratio influences the tensile strength. Similarly, the mechanical properties for some PHA fibers with different composition are illustrated in .
Figure 4. Tensile strength and elongation at break of melt spun P3HB filaments at different draw ratios.
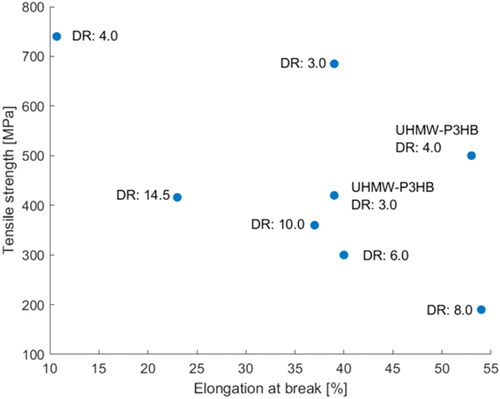
Table 4. Maximum mechanical properties achieved by melt spinning.
Both production methods – fiber extrusion from an extruder and a melt spinning facility – provided fibers; however, both technologies exposed the biopolymer to high temperatures that led to considerable thermal degradation.[Citation90] One way to reduce the thermal degradation is to supply the polymer in the form of a gel instead of pellets or powder. Gordeyev and Nekrasov [Citation91] produced a polymer gel by dissolving 20 wt.% P3HB powder in 1,2 dichlorethane (under stirring at 80 °C) and letting the solvent evaporate for several hours until a solid gel with a P3HB content of 30-32 wt% was obtained. The gel was treated in a three-step process consisting of extrusion in a capillary rheometer and pre-stretching, followed by hot drawing and annealing. The melting temperature during the extrusion in the capillary rheometer was 170 °C instead of 180 °C in an extruder, which led to improved fiber properties (tensile strength of 360 MPa) because of the reduced thermal degradation during the spinning process, with otherwise comparable processing/treatment conditions to fibers produced in the extruder. The hot-drawn and annealed fibers spun from gel were stable for six months whereas aging occurred for the only-hot-drawn fibers. Their stiffness increased while strength decreased,[Citation91] which means that the crystallization process was not fully completed at the end of the spinning process as result of the slow crystallization rate of P3HB, and secondary crystallization occurred.
To avoid aging accompanied by spherulitic crystallization and brittle fibers which cannot be drawn, Furuhashi and coworkers [Citation92] quenched the P3HB fibers immediately after exiting the die in a − 40 °C dry ice/methanol bath. This way, the filaments were kept in an amorphous state below their Tg to induce molecular orientation while drawing them just above the Tg in the rubbery state. A second-step drawing and annealing followed the spinning procedure to reach the final fiber properties. The produced fibers had a maximum tensile strength of 416 MPa, [Citation91,Citation92] which is higher compared to the fibers prepared from gel, even though the applied extrusion temperatures were 15 °C higher than in the gel-extrusion process. Additionally, the researchers investigated the crystallization behavior of P3HB and found α- and β-form reflections in the wide-angle X-ray diffraction (WAXD) patterns of fibers drawn once in the amorphous state. The second-stage drawing increased the β-formation because the amorphous tie molecules that are located between the α-form crystals are stretched again and turned into β-form crystals. This led to improved mechanical properties after the second drawing. However, in the final annealing process, molecular relaxation occurred, and the number of α-form crystals increased while the content of β-form crystals decreased to values lower than the unannealed fibers. Thus, the researchers concluded that the mechanical properties of the fiber did not only depend on the relative amounts of α- and β-form crystals, but also on the molecular orientation, total crystalline contents, and the presence of voids.[Citation92] These results indicate that the crystalline orientation/structure has a higher impact on the mechanical properties of the resulting PHA fibers than the thermal degradation arising from the processing. Thus, focusing rather on an increased chain orientation than on a refined process for the reduction of thermal degradation is recommended.
Qin and coworkers tried to obtain ideal fiber structures by high-speed spinning of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBH).[Citation93] They tried to induce crystallization by means of high tensile stress and molecular orientation in the spin line. Higher molecular orientation was obtained by increased throughput rates because of the higher viscosity that derived from the suppression of thermal decomposition. Indeed, an increase in the take-up velocity led to an improved crystalline orientation of the as-spun fibers. Additionally, WAXD patterns showed β-form crystals that are usually formed during a drawing procedure. In this case, the β-form crystals are evidence of the stress-induced crystallization, which took place in the spin line when the PHBH was placed under high tensile stress. Further, it was observed that the molecular orientation and therefore the tensile strength and tensile modulus were improved with increasing throughput rate. An increase in the take-up velocity and a decreasing extrusion temperature had a similar effect on the mechanical properties of the PHBH fibers (increase in tensile modulus and tensile strength and decrease of elongation at break). The highest achieved tensile strength and tensile modulus were 156 MPa and 2.43 GPa, respectively.[Citation93] These are comparatively low values; however, Qin et al. did not use any additives or drawing or annealing procedures.[Citation93] Follow-up research from this group showed an improvement in the fiber’s tensile strength (215 MPa) when immersing the as-spun fibers in a 20 cm deep, 40 °C, water-soluble polypropylene glycol isothermal bath and applying a take-up velocity of 1.0 km/min.[Citation94] Regarding tensile strengths obtained by other researchers when applying a different drawing and annealing procedure, it seems that these results might be further improved by choosing different process parameters. However, other researchers such as Tanaka et al.[Citation17] mostly used P3HB and not PHBH, which likely influences the results.
It appeared that many researchers successfully focused on the improvement of drawing and annealing procedures, leading to several patents for high-strength fibers [Citation95,Citation96] and medical textiles.[Citation97] Sophisticated drawing and annealing result in an increase in the mechanical properties of PHA fibers without the addition of nucleation aids or the like that (depending on the additives used) may cause problems during degradation or impair biocompatibility.
Hufenus et al.[Citation60] modified the drawing setup for the fiber-spinning process by adding an intermediate godet in the draw-off division to induce an oriented crystallization to the as-spun fibers with a low melt strength before the fibers were solidified. This led to improved melt strength so the fibers could withstand the subsequent drawing procedure. The crystalline structure obtained by this procedure differed from previously known structures which describe a crystalline formation in β-form, as the researchers discovered a highly ordered amorphous phase that is “kinetically trapped between the aligned lamellae of the crystalline α-phase”.[Citation60] This theory was supported by the findings of longitudinally oriented lamellae in the produced fibers instead of the commonly seen spherulites. The fibers did not show secondary crystallization and had a long-term stability with tensile strength of maximum 215 MPa at 20% tensile strain.[Citation60] However, it should be noted that these results cannot be attributed solely to the drawing procedure since the authors also used nucleating aids.
2.1.2. Crystallization control with nucleation and processing aids
Even though Iwata et al.[Citation98] have successfully produced high-strength P3HB fibers (1 GPa) by means of their elaborated quenching, drawing, and annealing techniques,[Citation98] the procedure is probably too complex for an industrial implementation. Thus, other researchers investigated the use of nucleation agents to introduce more inhomogeneities into the melt that serve as a nucleus for the crystallization.[Citation99,Citation100] Thereby, the number of nuclei and the crystallization rate are increased while the spherulite’s dimension is decreased. The size of the nucleation agents is usually around 3 µm, and boron nitride (BN) was reported to be one of the most effective nucleation agents for P3HB.[Citation101,Citation102] The drawback of BN is that it is not biocompatible which would inhibit the use of PHAs in the medical field and hence alternatives are necessary.[Citation60]
Yamane and coworkers [Citation103] used non-purified P3HB homopolymers (which still contained protein and lipid from the culture medium) to serve as nucleating agents for their spinning trial, thus increasing the crystallization rate. They found that the contaminations seemingly acted as plasticizers, facilitating a cold-drawing process at 110 °C of the as-spun fibers directly after the spinning procedure. In contrast, aged as-spun fibers showed spherulite formation, resulting in very brittle fibers that were impossible to draw. Fibers with a diameter of around 300 µm were obtained by the melt-spinning process with a maximum DR of six. As expected, the fiber’s mechanical properties increased with increasing DR. Annealing under load improved the mechanical properties of the fibers. A maximum tensile strength of around 300 MPa (tensile modulus ≈ 3.75 GPa, elongation at break ≈ 60%) was achieved at an annealing temperature of 125 °C and a load of 100 MPa. With these mechanical properties, the fibers could be applied in load-bearing applications in bone or tendon TE (tensile strength > 150 MPa), but they still do not fulfill the requirements for industrial applications (tensile strength > 500 MPa). The WAXD pattern of the drawn fibers showed a higher crystalline orientation with increasing DR and crystallites in the orthorhombic lattice α-form as well as a rather imperfect twisted planar-zigzag conformation (β-form).[Citation103]
Many experimental trials to melt-process P3HB without the addition of nucleation agents faced the problem of and not completely crystallized fibers that did not show sufficient textile properties wherefore research was focused on nucleation agents to inhibit secondary crystallization.[Citation90,Citation104] Because of the huge interests in PHAs for medical applications, traditional nucleation aids such as boron nitride or talc [Citation105] cannot be used due to their blood incompatibility.[Citation85] Thus, Vogel et al. used α-cyclodextrin (CD) or, rather, supramolecular crystalline inclusion complexes (Ics) thereof as these seem to be more effective.[Citation85] To obtain Ics, CD molecules were threaded onto the P3HB’s polymer chain. The study showed that P3HB with CD Ics supported crystalline nucleation; however, stress-induced crystallization during spinning was necessary to obtain fast and complete crystallization in the spin line. The fibers produced were not sticky and had sufficient mechanical properties for further textile processing.[Citation85] The researchers state that the fibers show sufficient mechanical properties for textile processing [Citation50] but it seems as they did not produce a textile out of their fibers. An indication of the processability of the fibers into textiles and the textile’s properties would have been interesting.
As the preparation of CD/P3HB complexes is laborious, Vogel et al.[Citation106] used electron-beam-irradiated P3HB as a nucleation agent. The exposure to electro-beam irradiation causes a drastic drop in the number-average and weight-average molecular weight of the P3HB which would have a negative impact on the mechanical properties of the fiber. Thus, not all the material was exposed to the radiation, but virgin P3HB was blended with 2 wt% of P3HB which was exposed to 750 kGy. According to the researchers, this composition supported the formation of a stable nucleus for a dramatic improvement of the P3HB’s crystallization behavior.[Citation106]
Vogel and coworkers [Citation50] tried to use reactive extrusion with dicumyl peroxide (DCP) to improve the crystallization behavior of P3HB. The crystallization behavior of pure P3HB limited the DR to 4.0, whereas DCP at concentrations of 0.2, 0.3 and 0.5 wt.% improved the crystallization behavior so that a ratio of up to 7.5 was feasible. The neat P3HB with a DR of 4.0 achieved a tensile modulus of 4.44 GPa, a tensile stress at break of 164.38 N/mm2, and an elongation at break of 52.82%. All samples modified with DCP showed decreased tensile moduli and elongation at break compared to the pure reference sample, even though the influence of the DR was still visible (the higher the DR, the higher the tensile modulus). However, the tensile stress at break and the molecular weight increased for some samples compared to the neat P3HB. P3HB with 0.2 wt.-% DCP shows the best results with a tensile stress at break of 203.20 and 242.70 N/mm2 at a DR of 7.0 and 7.5, respectively. According to the authors, this clearly improved the textile properties of the produced fibers. The conducted WAXD measurements showed that the fibers were predominantly oriented in the orthorhombic α-modification with a 21 helical chain conformation and no β-forms were found.[Citation50] However, when placing the mechanical properties in relation to the results from Furuhashi et al.[Citation92] and Yamane et al.,[Citation103] the fibers produced by Vogel et al.[Citation50] had inferior mechanical properties. Even though some information is missing, which precludes a thorough comparison of the different processes, it appears that higher DRs and annealing improve the mechanical properties more than reactive extrusion with DCP. While the concept of reactive extrusion has some potential, DCP is the wrong component since the molecular weight reduction due to its application is probably too high.
Hufenus and coworkers [Citation60] studied the influence of commercial P3HB (from Biomer) blended with boron nitride (BN) and tri-n-butyl citrate (TBC) as a nontoxic and biodegradable plasticizer on the fiber spinnability. Moreover, they tested the effect of three different types of P3HB: neat P3HB without additives, P209 (a P3HB with nucleating agent and plasticizers), and P309 (a P3HB with nucleation agent but no plasticizers). The addition of TBC led to a melting point reduction of approximately 15 °C for the sample containing neat P3HB with added TBC and P209, which had a positive effect on the melt processing of the materials. Further, it turned out that materials with both BN and TBC could effectively stabilize the melt spinning process by suppressing viscosity fluctuations so that the processing temperatures could be decreased, leading to reduced thermal degradation.[Citation60]
2.1.3. Crystallization control by copolymers and polymer blending
Polymer blends and copolymers have been shown to improve the mechanical properties of PHAs, wherefore copolymers are also used for fiber production, for example, by Tanaka and coworkers,[Citation107] who produced high-strength fibers with a poly[(R)-3-hydroxybutyrate-co-3-hydroxyvalerate] (P(3HB-co-3HV)) copolymer without the use of nucleation agents or other additives.[Citation107] With increasing HV content, the crystallinity of the polymer decreased while the flexibility of the copolymer was enhanced. In this case, an HV content of 8% was used and led to high-strength fibers with a tensile strength of 1065 MPa (Young’s modulus: 8.0 GPa, elongation to break: 40%). By producing fibers with a tensile strength of 1065 MPa, they accomplished a fiber strength comparable to well-established textile fibers such as polyamide 6.6 (1000 MPa),[Citation108] polyester (polyethylene terephthalate: 800 MPa) [Citation108] or Nomex® (poly(m-phenylenediamine isophthalamide): 640 MPa).[Citation108] Compared to the mechanical properties of other fibers based on P(3HB-co-3HV) copolymers with tensile strength of 183 ± 2 MPa (Young’s modulus of 9.0 ± 0.7 GPa, P(3HB-co-3HV) with 14% 3HV) [Citation109] or 210 MPa (Young’s modulus: 1.8 GPa),[Citation104] Tanaka and coworkers achieved extraordinary mechanical properties, especially considering that both of the comparative values were achieved using nucleation agents whereas their study did not. The success is probably a result of the special spinning conditions they applied - directly quenched the fibers in ice water to obtain amorphous fibers. The fibers were kept for 24 h in ice water to obtain small crystal nuclei during isothermal crystallization before they were subjected to drawing at room temperature and a subsequent annealing procedure. Additionally, it was found that the one-step drawing led to a uniform crystalline structure with highly oriented 21 helix conformations (α-form) and planar-zigzag conformation (β-form), which is depicted in . The β-form arose from the stretching of molecular chains in the amorphous region between the small crystal nuclei, which were obtained during isothermal crystallization. The small crystal nuclei in turn acted as cross-linking points for the generation of the α-form during annealing of the fibers. This generation of crystalline structure is different from the previously described mechanisms for cold-drawn and two-step drawn fibers and films.[Citation107]
Figure 6. Scheme of the crystalline changes during quenching, drawing, and annealing procedure. The red boxes in the last step represent the α-structures formed while the zigzag lines represent the β-crystals formed. Figure adapted from Tanaka et al.[Citation107]
![Figure 6. Scheme of the crystalline changes during quenching, drawing, and annealing procedure. The red boxes in the last step represent the α-structures formed while the zigzag lines represent the β-crystals formed. Figure adapted from Tanaka et al.[Citation107]](/cms/asset/9ce22366-d34b-4733-9a3d-0577167fb487/lmsc_a_2076693_f0006_c.jpg)
One drawback of this outstanding process is the time- and probably also labor-intensive procedure, which will likely hinder its widespread industrial use.
Chen and coworkers investigated the spinnability and fiber properties of poly(3-hydroxybutyrate-co-4-hydroxybutyrate)/poly (lactic acid) blends (P3HB4HB/PLA).[Citation110] As the 4HB phase in the comonomer cannot crystallize in the 3HB phase, it led to a disruption of the stereoregularity in the 3HB phase and therefore reduced the overall crystallinity. Depending on the 4HB content, the copolymer properties ranged from semicrystalline (<10% 4HB) to elastomeric (>40% 4HB). A blend ratio of 35/65 P3HB4HB/PLA (based on the weight), led to fibers with the highest mechanical properties (tensile strength: 904 MPa, tensile modulus: 28.42 GPa, strain at break: 81%).[Citation110] In addition to the favorable mechanical properties, the 35/65 blend also showed good spinnability with no obvious adhesion or fiber breakage during spinning. According to the researchers, an increase in the P3HB4HB component reduced the spinnability (fibers became more adhesive) and increased the fiber breakage due to the poor compatibility of the P3HB4HB and PLA which was confirmed by clear-phase separation morphologies in SEM.[Citation110] Even though the tensile strength of these fibers is slightly lower than that produced by Tanaka et al.[Citation107] it is considerably higher than the desired 500 MPa for industrial application of the filament. At the same time, Chen and coworkers [Citation110] seemingly used a more convenient and less time-intensive process, probably enabling easier transfer to industrial applications.
Li and coworkers [Citation111] aimed to improve PLA fibers by blending PLA with PHBV. In general, the PHBV/PLA blends showed inferior melt spinnability compared to pure PLA, and at PHBV contents of more than 40% the fibers could not be wound which further impairs the spinnability. While mechanical properties such as tenacity, modulus, and elongation at break of the blend fibers constantly decreased with increasing PHBV content, they were still acceptable for basic textile applications. Despite these drawbacks, the polymer blend led to superior softness owing to the rubbery and predominantly amorphous domains of the PHBV. Surprisingly, the heat resistance of the polymer blend was also enhanced compared to pure PLA, which could improve the performance of traditional PLA fibers which turn rigid and fragile after heat treatment.[Citation111]
In general, copolymers might be more advisable because polymer incompatibilities are avoided, and mechanical strength and recyclability/degradability (which is usually more complicated with an increasing number of different materials within one product) is not impaired. Chen et al.'s copolymer-blend fibers showed a clear phase separation in the SEM pictures, which likely reduced the mechanical properties so that similar results to Tanaka et al.[Citation17] could not be achieved. However, because of its simplicity and good tensile strength results, Chen et al.'s [Citation110] copolymer blend is presumably more interesting for industrial applications.
2.1.4. Ultra-high molecular weight P3HB (UHMW-P3HB)
Iwata and coworkers [Citation112] conducted extensive research in the field of ultra-high molecular weight P3HB (UHMW-P3HB) which they obtained from an engineered Escherichia coli strain, XL1-Blue. They developed a two-step spinning procedure in which the fibers were quenched immediately after spinning in an ice bath to obtain amorphous fibers. These fibers were cold drawn in the ice bath up to 600% of their initial length. Afterwards, a second drawing step at room temperature was used against the oriented fibers, followed by an annealing procedure in an autoclave at 50 °C with weak tension. The annealing was done to increase the crystallinity of the high-strength fibers (tensile strength: 1.3 GPa, elongation to break: 35%, Young’s modulus: 18.1 GPa at a fiber diameter of 40 µm). Investigations by microbeam X-ray diffraction, WAXD, and different microscopes revealed the core-sheath structure of the obtained fiber. The sheath showed only α-form crystals while the core consisted of α- and β-form crystals. Because of the quenching procedure, the fiber sheath cools faster, i.e., at a higher crystallization rate than the core, so that a higher crystallinity exists in the core region. Although a sheath-core structure is not unusual in fibers with two polymer components, it is rather special for a fiber consisting of one single polymer. The researchers assumed that this structure was a result of the drawing procedure. They proposed that the β-form crystals were generated from the amorphous molecular chains between the α-form lamellar crystals because of the two-step drawing. The crystal rotation angles (θ) obtained by microbeam X-ray diffraction showed that the lamellar crystals aligned perpendicular to the fiber axis whereas the molecular chains were aligned along the drawing direction. The lamellar crystals in the sheath region rotated more than they did in the core region and were slightly elongated by the rotation of the lamellar crystals during the two-step drawing. The molecular chains between the lamellar crystals, however, were not elongated which led to the core-sheath structure of the fiber.[Citation112]
Even though the molecular weight usually influences the tensile strength of a material, Tanaka et al.[Citation17] found that in their experimental setup, the molecular weight difference between the commercial P3HB and their UHMW-P3HB did not improve the tensile strength of the produced fibers. Thus, this experiment showed that the molecular orientation obtained by drawing has a greater influence on the mechanical properties than the molecular weight.
2.1.5. Bicomponent fibers
Because of the superior biocompatibility of PHBV compared to PLA, Hufenus and coworkers [Citation79] tried to produce bicomponent fibers with a PLA core and a PHBV sheath. The processing of these two biopolymers turned out to be problematic due to their different thermal properties. The PHBV in the outer sheath thermally degraded and exhibited a sticky nature (coagulation of the fibers on the godets and bobbins) as well as immediately starting secondary crystallization. The researchers therefore decided to have a PHBV core and a PLA sheath. With this setup, it was possible to achieve fibers with a maximum tensile modulus of 7.1 GPa for a core of 36 wt% PHBV and a sheath of 54% PLA. Further tensile tests and WAXD analysis revealed that the PLA fiber took the load alone because the PHBV macromolecular structure was not oriented. It would be interesting to investigate whether a bicomponent fiber of two PHAs is feasible, since two polymers with closer thermal properties should be easier to process even though the mechanical properties might be challenging. In contrast to all the other reviewed articles about melt spinning, this research group also investigated the in vitro biocompatibility of human dermal fibroblast with the produced fibers. The results showed the anticipated production of lactic acid resulting from the degradation of the PLA shares but the in vitro test with human dermal fibroblast did not show a toxicity of the bicomponent fibers.[Citation79] Fibroblast grew along single filaments from cell re-aggregates, covering the fibers after one week of cultivation. Well expressed cytoskeletons showed cell adherence on the fibers and extract tests according to ISO 10993-5:2009 showed no adverse effect on 3T3 cells so that the fibers are promising candidates for medical applications.[Citation79]
2.1.6. Hollow fibers
Hinüber and coworkers [Citation78,Citation113] investigated different possibilities for melt spinning hollow P3HB fibers. A future potential application for these fibers is found in nerve repair with artificial tubes to bridge the gap between two nerve stumps.[Citation114] They investigated different nucleation agents to produce the hollow P3HB fibers. Boron nitride (BN), a common nucleation agent, was used as a reference nucleating agent in the study but it is not suitable for biomedical applications. The two biocompatible nucleation agents, hydroxyapatite (HAP) and thymine (THY) were therefore studied. Boron nitride showed the best crystallization with no secondary crystallization being observed. THY showed secondary crystallization only to a small extent and had crystallization properties comparable to BN, whereas HAP showed a poor ability to improve the crystallization and had a remarkable secondary crystallization. However, when it came to melt spinning in a conventional extrusion spin line, the nucleation effect did not appear, and the fibers were sticky and not processable. Fibers produced by a piston spinning machine had a smooth and regular surface and an inner diameter of between 50 and 500 µm, depending on the variations in the take-up speed or contaminations. As the nucleating effect did not occur as expected when using the nucleating agents, the authors concluded that nucleating agents might be used for further optimization but are not necessary for achieving a stable hollow-fiber structure.[Citation78]
To improve the mechanical properties, especially the flexibility of the hollow fibers, a 70/30 wt% polymer blend of P3HB and poly-ε-caprolactone (PCL) obtained from dissolving and precipitation was used to melt-spin hollow fibers. Even though the tensile modulus and yield stress was slightly reduced due to the blending, the polymer blend was less brittle compared to the pure P3HB, which can be regarded as improvement of the fiber properties for the planned application. An adequate porosity for the permeation of nutrients and metabolites remained a challenge.[Citation113]
The previous reports about bicomponent and hollow fibers demonstrated the possibility of producing more complex PHA fibers which could open opportunities for more advanced textile technology applications in the future.
2.1.7. Future work in melt spinning
The reviewed articles showed an enormous development in the melt spinning of PHAs to fibers that can compete with conventional man-made polymers in terms of mechanical properties. With regard to medical textiles – for instance, woven bone scaffolds for large segmental bone defects – the physiological flow transportation of nutrients and wastes remains a challenge. Research showed that the permeability of the fabric and the wicking behavior by capillary action of the fiber could be beneficial for nutrient and waste transport. Gilmore and coworkers [Citation115] produced grooved wicking fibers of poly-l-lactide and poly-l-lactide-co-ε-caprolactone and processed them into simple woven fabrics (plain weave and crowfoot weave). Their results showed that the weaving configuration, material combination, and the fiber geometry were crucial factors, influencing the scaffold’s permeability potentially improving the nutrient and waste transportation.[Citation115] The reviewed articles mainly focused on the production of round monofilaments, whereas the production of specially shaped/grooved wicking fibers for PHAs seems to be rather unexplored. Because of PHAs’ biocompatibility and their promising applications in medical textiles and for TE scaffolds, further research on PHA wicking fibers and fabrics is advisable. Additionally, the influence of further fiber-processing methods such as crimping or twisting on permeability, cell adhesion, and cell growth could be interesting, although twisting and crimping are a challenge because of the potential brittleness of the PHA fibers.
2.1.8. Wet spinning of PHAs
Due to the narrow thermal processing window of PHAs, wet spinning could be an alternative to melt spinning to avoid thermal degradation. Wet spinning can be described in very simple terms as the continuous precipitation of polymer from a solution in a coagulation bath. As the basic principle of wet spinning appears to be simple, it soon became a common practice in PHA research involving wet spinning to use an improvised and simplified spinning setup instead of a laboratory-size wet-spinning machine. Often, the gear pump is replaced with a syringe pump, while a syringe with a needle is used as a spinneret and the filament is extruded as a huddle into a coagulation bath without further drawing. Commonly, the feeding speed of the syringe pump, the polymer concentration/composition, and sometimes the coagulation bath temperature are controlled.[Citation116] Wet spinning with a needle setup produces fibers with diameters from approximately 30 to 600 µm, depending on the needle diameter.[Citation18] It also appears that wet spinning with a syringe produces more porous fibers than electrospinning and could be useful for the preparation of porous membrane materials.[Citation18,Citation116] From a textile engineering perspective, it is imprecise to use the term wet spinning for such a syringe setup since it conveys the assumption that an entire wet-spinning line, as is common in the textile industry, would have been used. However, the syringe setup is a very simplified procedure compared to the industrial wet-spinning process with significantly fewer parameters being controllable. As this fundamentally influences the fiber properties, we suggest the term “syringe wet spinning” for simplified syringe setups to clarify whether one is dealing with a simplified setup or an actual wet-spinning process.
2.1.9. Bone tissue engineering using wet spinning of PHAs
In bone tissue engineering, especially for large bone grafts, the transportation of nutrients and waste materials to and from cells to ensure their survival remains a challenge. To tackle this problem, Alagoz and coworkers [Citation56] spun a 3D bone-tissue engineering scaffold of PHBV, coated with elastin-like recombinamer (ELR) peptide with an endothelial cell-attracting REDV sequence (REDV: arginine- glutamic- aspartic acid- valine tetrapeptide. The peptide is supposed to improve cell adhesion by supporting the cell binding of fibronectin [Citation117] which is regulating cell attachment and motility and thus is crucial for tissue repair [Citation118]) to promote early vascularization.[Citation56] The fibers produced by syringe wet spinning had an average fiber diameter of 90.0 ± 1.5 µm at a porosity of 75% and 250.0 ± 5.3 µm pore size which is within the range of 200-350 µm as the ideal pore size for bone regeneration and mineralization. The obtained fibers were placed in a cylindrical mold to obtain 3D scaffolds for further investigation. The 3D scaffolds underwent a compression test where they showed a Young’s modulus of 4.65 ± 0.69 MPa in the dry state. This is not sufficient for replacing trabecular or cortical bones with Young’s moduli of 0.1-2 GPa and 15-20 GPa, respectively.[Citation119] Although the scaffold’s mechanical properties are not sufficient, the ELR-modified scaffolds attracted a higher number of HUVECs (human umbilical vein endothelial cells) cells because the REDV sequence is specific for endothelial cells. Thus, PHBV scaffolds functionalized with ELR_REDV could serve as carries for bone tissue engineering even though the in vitro experiments with rat bone marrow-derived mesenchymal stem cells did not show a significant improvement regarding cell attachment, proliferation and differentiation due to a non-recognition of the sequences by the modified scaffold.[Citation56] An overview of the (syringe) wet spinning procedures used, and their processing conditions reviewed in this article is provided in .
Table 5. Overview (syringe) wet spinning.
Degeratu and coworkers [Citation34] prepared wet-spun 2D and 3D PHBV scaffolds which were intended for use in bone grafts. Like Alagoz et al.,[Citation56] the challenge was that the structures did not meet the mechanical properties required for bone graft use, which was reflected in an unfavorable bone apposition in vivo even though the scaffolds showed good colonization, spread, and surface development of SaOs2 osteoblast-like cells.[Citation34] The inferior mechanical properties in these two cases supposedly arose from the unsuitable scaffold production method for this purpose. From a textile engineering perspective, the mechanical properties of the PHA scaffold might be improved by carrying out an adjusted fiber-production method with more parameters being controlled to obtain the strongest possible fibers. If this fiber were to be combined with another scaffold production method, for example, 3D weaving or warp-knitted spacer fabrics and maybe even impregnated with a suitable resin to produce a biocomposite, the requisite mechanical properties could probably be created. In a recent study, Singhi and coworkers [Citation120] investigated the influence of spinning parameters (dissolution and coagulation of P4HB, polymer concentration in the spinning dope, coagulation bath temperature, and draw ratio) on the mechanical properties and fiber structure of syringe wet-spun P4HB fibers.[Citation120] They concluded that a chloroform and acetone mixture with a polymer concentration of 15% was ideal for dissolving P4HB while achieving a faster coagulation in the alcohol-based, room temperature coagulation bath compared to methylene dichloride and pure chloroform. The fiber’s modulus (tensile modulus: 101.7 ± 23.6 gf/denier ≈ 1.15 cN/tex, tenacity: 1.88 ± 0.24 gf/denier ≈ 0.021 cN/tex), and crystallinity increased with a DR of 18 (compared to 12) while the fiber diameter (approximately between 2.79 and 5.39 µm with an average linear density of 0.097 tex) and elongation at break (55.9 ± 10.9%) were reduced.[Citation120] Compared to the tenacity of other natural (e.g., silk 25-50 cN/tex) or man-made (Alginate (Ca-Alginate) 11-18 cN/tex) filaments,[Citation121] the wet-spun P4HB’s tenacity is rather low.
These results give a first indication of the mechanical properties of wet-spun P4HB fibers and identify further research needs for an improvement of the fiber’s mechanical properties so that appropriate application fields for wet-spun PHA fibers can be identified in the future. Additionally, it shows that the (syringe) wet-spun fibers in general should be characterized in more detail. This additional knowledge could help to identify the scaffold’s failure, which might be the PHA filament’s weakness, or an improper structural or material choice.
2.1.10. Porous fibers and scaffolds
Kundrat and coworkers [Citation116] developed porous wet-spun P3HB fibers for cosmetics and wound-healing applications with a simplified syringe setup. The obtained fibers were between 20-120 µm in diameter and had a maximum surface area of 55 m2/g. According to the researchers, the ideal process conditions were a feed rate of 0.5 mL/h and 5 wt% of P3HB. Compared to a lab-size wet-spinning procedure with spinning speeds of 22-76 m/min for cellulosic fibers [Citation122]), a feed rate of 0.5 mL/h seems to be very low. Additionally, the researchers coated the fibers with plant extracts and found that clove extracts show significant antibacterial and antimycotic effects.[Citation116]
In a recent study, Polyák and coworkers [Citation123] prepared porous scaffolds for drug release from P3HB by syringe wet spinning and incorporated quercetin, a model drug. The obtained fibers showed a regular, cylindrical shape with narrow diameter distribution and a linear dependency of the fiber diameter from the spinning rate. They showed that the spinning rate can be used to control the fiber thickness which in turn controls the kinetics of drug release. The thinner the fiber diameter, the faster the drug diffusion into the surrounding medium; thick fibers are thus necessary when a slow release is desired.[Citation123] Apart from the fiber’s diameter, how the diameter was influenced by spinning rate, and how the diameter influenced drug release, the fibers were not further characterized. An investigation of the mechanical properties and processability into a textile would be beneficial.
2.1.11. Additive manufacturing by wet spinning
The research group around Puppi [Citation53,Citation124–126] investigated the possibilities of additive manufacturing for PHAs, one of them being computer-aided wet spinning (CAWS). CAWS is a syringe wet-spinning process where either the syringe pump or needle are movable in the z- and x-direction (up/down and forward/backwards) and the plate with the coagulation bath in the y-direction (left/right) to achieve a layer-by-layer deposition process of the fibers in the coagulation bath. In principle, the technique is comparable with a fused filament fabrication 3 D printer except that the polymer is not melted and deposited on a plate but coagulated and deposited in a bath.[Citation125,Citation126] According to the researchers, it was possible to produce layered 3 D scaffolds from porous and aligned poly[(R)-3-hydroxybutyrate-co-(R)-3-hydroxyhexanoate] (PHBHHx) fibers with good control of the shapes and pore sizes of the scaffold while showing good cytocompatibility because they could sustain murine pre-osteoblast proliferation and differentiation toward an osteoblastic phenotype.[Citation124] In a follow-up study in 2017, the researchers developed a CAWS PHBHHx/PCL blend scaffold shown in to cost-effectively combine the advantages of both polymers.[Citation53] The results showed that it was possible to produce scaffolds with tunable thermal and mechanical properties as well as morphological features. After 28 days of culture, the scaffolds were fully colonized by MC3T3-E1 pre-osteoblast cells that showed good cell adhesion and proliferation as shown in .[Citation53]
Figure 7. Scanning electron microscope images of the top (left column) and cross-sectional view (right column) of a PHBHHx (A) and PHBHHx/poly(ε-caprolactone) (PCL) scaffold at different ratios. (B) PHBHHx/PCL 3:1 (C) PHBHHx/PCL 2:1, (D) PHBHHx/PCL 1:1. The high magnification insets show the porosity (left) as well as cross-section (right) of single fibers. Images were taken from Puppi et al.[Citation53] without any modification and licensed under CC BY 4.0.
![Figure 7. Scanning electron microscope images of the top (left column) and cross-sectional view (right column) of a PHBHHx (A) and PHBHHx/poly(ε-caprolactone) (PCL) scaffold at different ratios. (B) PHBHHx/PCL 3:1 (C) PHBHHx/PCL 2:1, (D) PHBHHx/PCL 1:1. The high magnification insets show the porosity (left) as well as cross-section (right) of single fibers. Images were taken from Puppi et al.[Citation53] without any modification and licensed under CC BY 4.0.](/cms/asset/e19afccf-7720-4ab9-90e3-94423c86c8bb/lmsc_a_2076693_f0007_b.jpg)
Figure 8. Confocal laser scanning microscopy microphotographs of PHBHHx and PHBHHx/PCL scaffolds, showing MC3T3-E1 cells cultured on the surface after 7, 14, 21 and 28 days of cell culture. Images were taken from Puppi et al.[Citation53] without any modification and licensed under CC BY 4.0.
![Figure 8. Confocal laser scanning microscopy microphotographs of PHBHHx and PHBHHx/PCL scaffolds, showing MC3T3-E1 cells cultured on the surface after 7, 14, 21 and 28 days of cell culture. Images were taken from Puppi et al.[Citation53] without any modification and licensed under CC BY 4.0.](/cms/asset/5b09d0c1-48a0-4b66-8735-842e523b6177/lmsc_a_2076693_f0008_c.jpg)
This approach seems to be promising for tailored scaffold production, yet rather in the field of soft tissue engineering applications than bone replacements. For one specific process condition, the researchers reported a fusion of the fiber-fiber contact points in the z-direction.[Citation124] However, for all other process conditions it appears that the individual layers were only stacked on top of each other so the layers had only a very weak or no connection in the z-direction. As the coagulation probably did not provide sufficient adhesion of the fibers in this direction, this could limit the application fields of the scaffold to certain uses where high stability is not necessary in all directions. This assumption was supported by the mechanical characterization of the PHBHHx scaffolds which revealed that the mechanical properties significantly failed to meet the requirements for the desired application as a replacement for load-bearing tissues. This shows that the chosen production method might not be ideal for load-bearing applications. A clear benefit of the CAWS, however, is the freedom of design so the scaffold can easily be adapted to the patient’s needs. Nevertheless, the produced filament was not exposed to any drawing in the chosen setup which would orient the molecular chains within the fiber and therefore possibly increase the fiber’s mechanical strength. Combined with the rather weak connection/bonding within the scaffold and the fiber’s porosity, this seems not to be an ideal starting point to obtain high-strength scaffolds.
2.1.12. Dry-jet wet spinning
Dry-jet wet spinning is related to the wet-spinning process since the polymer is also dissolved in a solvent. However, instead of being directly extruded into the coagulation bath, the extruded polymer solution passes an air gap so that it is possible to separately control the temperature of the dope extrusion and the coagulation temperature to develop specific structures during coagulation. After coagulation in the bath, the fiber is usually washed, drawn, and wound up on a bobbin.[Citation82]
P4HB, for example, can be processed by dry-jet wet spinning because it is soluble in several polar solvents that evaporate easily and leave very low amounts of residual solvent.[Citation57] Chiono and coworkers [Citation54] investigated the dry-jet wet spinnability of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/poly(ε-caprolactone) (PHBHV/PCL) blend in hollow fibers that can be used in tissue-engineering applications.[Citation54] The PCL is not miscible with PHBHV but the two polymers are compatible with each other so PCL acted as a plasticizer and significantly lowered the elastic modulus of the PHBHV. Blends with more than 50 wt% PCL were ductile and showed similar tensile properties to pure PCL. The degree of crystallinity and crystalline morphology as well as the degradation behavior depended on the blend ratio of the polymers. The higher the PCL content, the more the blend properties shift toward the properties of pure PCL. These blends showed improved cell adhesion and proliferation, which depended on the fiber composition, wall porosity, and thickness, as well as the inner diameter and mechanical characteristics.[Citation54] Additionally, the PHBHV/PCL blends showed biocompatibility and it was identified that more ductile blends lead to a higher fibroblast response.[Citation66]
2.1.13. Future work in wet spinning
The reviewed articles about wet spinning discussed mainly fibrous PHAs from an application point of view, e.g., in bone tissue engineering or regarding the production of scaffolds, leading to a less-detailed study of the filament’s properties. In our opinion, this is a missed opportunity to gain more knowledge about the fiber’s mechanical properties and thus to identify a suitable application field. Additionally, the filament production process itself could often be improved by adding a drawing zone, leading to increased molecular orientation. Moreover, a change in the scaffold production process to established textile production processes should improve the mechanical properties of fiber-based scaffolds.
2.2. Outlook on spinning procedures
To reflect on the spinning procedures, advantages and drawbacks of wet and melt spinning for PHAs are discussed. Besides the absence of solvents in the melt spinning process compared to the wet spinning and the resulting environmental and processing benefits (no need to remove or recover solvents),[Citation127] melt spinning offers a considerably higher production speed of PHA filaments than wet spinning (0.5 km/h for melt spinning[Citation73] versus 0.5 mL/h wet spinning[Citation116]) which is beneficial for a commercialization of fiber based PHA products. Some of the PHAs are temperature sensitive because their melting temperature is close to their thermal degradation temperature[Citation80] which is a weakness when considering melt spinning. This problem can be overcome by several earlier stated methods like the usage of additives. Additives can cause problems for biomedical applications of PHAs as they also need to be biocompatible and biodegradable while adding costs to the final product. Additional challenges in PHA filament extrusion is the cooling/solidification process which usually is very sophisticated to avoid the filaments to stick on the godets/winders or each other and which is often accompanied with a post-treatment process like annealing to increase the fiber’s mechanical properties.
Filaments produced via wet spinning are hardly characterized for which reason it is harder to draw specific conclusions on the drawbacks and benefits of wet spinning PHAs for instance whether an annealing procedure like in melt spinning would be beneficial. Even though solvents are used and need to be recovered for wet spinning, it is an advantageous process for temperature sensitive PHAs because they are not exposed to as high temperatures as in melt spinning. Lower processing temperatures also enable the incorporation of temperature sensitive active substances supporting wound healing or cell adhesion and proliferation if the additives are compatible with the used solvents. Apart from the essential filament purification processes to pass biomedical regulations without exceeding solvent residues, the formation of precise fiber cross sections is harder to control by wet spinning because of inward and outward mass transfer processes.
3. Conclusions and outlook
This review showed the huge research effort that has been undertaken to produce and investigate the properties of spun PHA fibers. Sophisticated drawing and annealing processes to orient the polymer chain seem to have a greater influence on the mechanical properties of melt-spun fibers than the reduction of thermal degradation by lower processing temperatures, even though degradation prevention is always beneficial. Additionally, copolymers often achieve more promising results than blends. The polymer’s molecular weight appears to have a secondary influence on the mechanical properties of the fibers since UHMW-P3HB is not superior to conventional P3HB.
A conspicuous difference between the spinning methods is observed. While the processing conditions of the melt-spinning process is well studied and the produced fibers are characterized in detail, the fiber properties obtained by (syringe) wet spinning are rather unexplored and need further investigation to improve the processing conditions and identify the most suitable application fields for these fibers. To the best of the authors knowledge no research group investigated the formation of a skin-core structure for wet spun PHAs. Depending on the spinning conditions different skin-core ratios can be obtained where the skin fraction usually show higher mechanical properties than the core fraction.[Citation127] This is one way besides the alteration of the molecular weight and degree of orientation to develop distinct fibers from a given raw material and make it suitable for different applications.[Citation127] Thus, studying the influence of the skin-core ratio on the in vivo and in vitro degradation and maybe cell adhesion of PHA filaments could be interesting and a beneficial feature compared to melt spun fibers.
First trials on special filaments such as bi-component or hollow PHA fibers for specified applications have been undertaken that seem to be rather a proof of concept without tapping the full potential. To the best of our knowledge, no other filament cross sections but round was tried to produce from PHAs although different fiber cross sections might have a beneficial effect on cell adhesion and proliferation. The scaffold’s overall performance may be improved through further research on special filaments and fiber cross sections since enhanced filament wicking properties could lead to improved cell adhesion, nutrition, and waste transportation.
For a potential biomedical application of PHAs, the polymer purity needs to be improved so that polymers other than P4HB are approved for biomedical applications. Additionally, a detailed study of PHA filament sterilization, processability on textile machines, and fabric characterization thereof should be conducted to obtain the full picture of PHAs’ potential in textile-fiber TE scaffolds. Depending on the processability on textile machinery, knitted fabrics could be produced for scaffolds used in skin regeneration after burns, braids or 3 D weaves for bone tissue engineering scaffolds.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Crawford, M. E. Chapter 20 - Autografts, Allografts and Xenografts in Cutaneous Surgery; In: Lower Extremity Soft Tissue & Cutaneous Plastic Surgery. Dockery, G. D. and Crawford, M. E., Eds.; W.B. Saunders: Oxford, 2012; pp. 225–230
- Amini, A. R.; Laurencin, C. T.; Nukavarapu, S. P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. DOI: 10.1615/critrevbiomedeng.v40.i5.10.
- Mani, M. P.; Jaganathan, S. K.; Khudzari, A. Z. M. Evaluation of Electrospun Polyurethane Scaffolds Loaded with Cerium Oxide for Bone Tissue Engineering. J. Ind. Text. 2021, 152808372110066. DOI: 10.1177/15280837211006668.
- Madiwale, P.; Singh, G. P.; Biranje, S.; Adivarekar, R. Advances of Textiles in Tissue Engineering Scaffolds; In: Advances in Functional Finishing of Textiles. Shahid, MAdivarekar, R., Eds.; Springer Singapore: Singapore, 2020; pp. 169–194
- Abbah, S. A.; Delgado, L. M.; Azeem, A.; Fuller, K.; Shologu, N.; Keeney, M.; Biggs, M. J.; Pandit, A.; Zeugolis, D. I. Harnessing Hierarchical Nano- and Micro-Fabrication Technologies for Musculoskeletal Tissue Engineering. Adv. Healthc. Mater. 2015, 4, 2488–2499. DOI: 10.1002/adhm.201500004.
- Miao, S.; Zhu, W.; Castro, N. J.; Leng, J.; Zhang, L. G. Four-Dimensional Printing Hierarchy Scaffolds with Highly Biocompatible Smart Polymers for Tissue Engineering Applications. Tissue Eng. Part C Methods 2016, 22, 952–963. DOI: 10.1089/ten.tec.2015.0542.
- Fallahi, A.; Khademhosseini, A.; Tamayol, A. Textile Processes for Engineering Tissues with Biomimetic Architectures and Properties. Trends Biotechnol. 2016, 34, 683–685. DOI: 10.1016/j.tibtech.2016.07.001.
- Chanda, A.; Callaway, C. Tissue Anisotropy Modeling Using Soft Composite Materials. Appl. Bionics Biomech. 2018, 2018, 4838157. DOI: 10.1155/2018/4838157.
- Jiang, C.; Wang, K.; Liu, Y.; Zhang, C.; Wang, B. Application of Textile Technology in Tissue Engineering: A Review. Acta Biomater. 2021, 128, 60–76 DOI: 10.1016/j.actbio.2021.04.047.
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y. M. Topological Design and Additive Manufacturing of Porous Metals for Bone Scaffolds and Orthopaedic Implants: A Review. Biomaterials 2016, 83, 127–141. DOI: 10.1016/j.biomaterials.2016.01.012.
- Basnett, P.; Ching, K. Y.; Stolz, M.; Knowles, J. C.; Boccaccini, A. R.; Smith, C.; Locke, I. C.; Keshavarz, T.; Roy, I. Novel Poly(3-Hydroxyoctanoate)/Poly(3-Hydroxybutyrate) Blends for Medical Applications. React. Funct. Polym 2013, 73, 1340–1348. DOI: 10.1016/j.reactfunctpolym.2013.03.019.
- Martin, D. P.; Williams, S. F. Medical Applications of Poly-4-Hydroxybutyrate: A Strong Flexible Absorbable Biomaterial. Biochem. Eng. J. 2003, 16, 97–105. DOI: 10.1016/S1369-703X(03)00040-8.
- Blanton, P. L.; Biggs, N. L. Ultimate Tensile Strength of Fetal and Adult Human Tendons. J. Biomech. 1970, 3, 181–189. DOI: 10.1016/0021-9290(70)90005-9.
- Gallagher, A. J.; Ní Anniadh, A.; Bruyere, K.; Otténio, M.; Xie, H.; Gilchrist, M. D. Dynamic Tensile Properties of Human Skin. 2012 IRCOBI Conference Proceedings 2012, 12, 494–502.
- Schleifenbaum, S.; Schmidt, M.; Möbius, R.; Wolfskämpf, T.; Schröder, C.; Grunert, R.; Hammer, N.; Prietzel, T. Load and Failure Behavior of Human Muscle Samples in the Context of Proximal Femur Replacement. BMC Musculoskelet. Disord. 2016, 17, 149–149. DOI. 10.1186/s12891-016-0998-7
- Kerns, J.; Piponov, H.; Helder, C.; Amirouche, F.; Solitro, G.; Gonzalez, M. Mechanical Properties of the Human Tibial and Peroneal Nerves following Stretch with Histological Correlations. Anat. Rec. (Hoboken) 2019, 302, 2030–2039. DOI: 10.1002/ar.24250.
- Tanaka, T.; Yabe, T.; Teramachi, S.; Iwata, T. Mechanical Properties and Enzymatic Degradation of Poly[(R)-3-Hydroxybutyrate] Fibers Stretched after Isothermal Crystallization near Tg. Polym. Degrad. Stab. 2007, 92, 1016–1024. DOI: 10.1016/j.polymdegradstab.2007.02.017.
- Tamayol, A.; Akbari, M.; Annabi, N.; Paul, A.; Khademhosseini, A.; Juncker, D. Fiber-Based Tissue Engineering: Progress, Challenges, and Opportunities. Biotechnol. Adv. 2013, 31, 669–687. DOI: 10.1016/j.biotechadv.2012.11.007.
- Qin, Y. 6 - Superabsorbent Polymers and Their Medical Applications; In: Medical Textile Materials. Qin, Y., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 71–88
- Brunetti, L.; Degli Esposti, M.; Morselli, D.; Boccaccini, A. R.; Fabbri, P.; Liverani, L. Poly(Hydroxyalkanoate)s Meet Benign Solvents for Electrospinning. Mater. Lett. 2020, 278, 128389. DOI: 10.1016/j.matlet.2020.128389.
- Gupta, B. S.; Moghe, A. K. 2 - Nanofiber Structures for Medical Biotextiles; In: Biotextiles as Medical Implants. King, M. W., Gupta, B. S. and Guidoin, R., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 48–90
- Kaniuk, Ł.; Stachewicz, U. Development and Advantages of Biodegradable PHA Polymers Based on Electrospun PHBV Fibers for Tissue Engineering and Other Biomedical Applications. ACS Biomater. Sci. Eng. 2021, 7, 5339–5362. DOI: 10.1021/acsbiomaterials.1c00757.
- Zhao, X.-H.; Niu, Y.-N.; Mi, C.-H.; Gong, H.-L.; Yang, X.-Y.; Cheng, J.-S.-Y.; Zhou, Z.-Q.; Liu, J.-X.; Peng, X.-L.; Wei, D.-X. Electrospinning Nanofibers of Microbial Polyhydroxyalkanoates for Applications in Medical Tissue Engineering. J. Polym. Sci. 2021, 59, 1994–2013. DOI: 10.1002/pol.20210418.
- Laycock, B.; Halley, P.; Pratt, S.; Werker, A.; Lant, P. The Chemomechanical Properties of Microbial Polyhydroxyalkanoates. Prog. Polym. Sci. 2013, 38, 536–583. DOI: 10.1016/j.progpolymsci.2012.06.003.
- Kabe, T.; Hongo, C.; Tanaka, T.; Hikima, T.; Takata, M.; Iwata, T. High Tensile Strength Fiber of Poly[(R)-3-Hydroxybutyrate-co-(R)-3-Hydroxyhexanoate] Processed by Two-Step Drawing with Intermediate Annealing. J. Appl. Polym. Sci. 2015, 132, 41258. DOI: 10.1002/app.41258.
- Sharma, V.; Sehgal, R.; Gupta, R. Polyhydroxyalkanoate (PHA): Properties and Modifications. Polymer 2021, 212, 123161. DOI: 10.1016/j.polymer.2020.123161.
- Mitra, R.; Xu, T.; Xiang, H.; Han, J. Current Developments on Polyhydroxyalkanoates Synthesis by Using Halophiles as a Promising Cell Factory. Microb. Cell Fact. 2020, 19, 86. DOI: 10.1186/s12934-020-01342-z.
- Karthik, T.; Rathinamoorthy, R. 8 - Sustainable Synthetic Fibre Production; In: Sustainable Fibres and Textiles. Muthu, S. S., Eds.;Woodhead Publishing: Duxford, United Kingdom, 2017; pp. 191–240
- Rehm, B. H. A. Polyester Synthases: Natural Catalysts for Plastics. Biochem. J. 2003, 376, 15–33. DOI: 10.1042/BJ20031254.
- Grigore, M. E.; Grigorescu, R. M.; Iancu, L.; Ion, R.-M.; Zaharia, C.; Andrei, E. R. Methods of Synthesis, Properties and Biomedical Applications of Polyhydroxyalkanoates: A Review. J. Biomater. Sci. Polym. Ed. 2019, 30, 695–712. DOI: 10.1080/09205063.2019.1605866.
- Anderson, A. J.; Dawes, E. A. Occurrence, Metabolism, Metabolic Role, and Industrial Uses of Bacterial Polyhydroxyalkanoates. Microbiol. Rev. 1990, 54, 450–472. DOI: 10.1128/mr.54.4.450-472.1990.
- Sudesh, K.; Abe, H. Background; In Practical Guide to Microbial Polyhydroxyalkanoates. Eds.; Smithers Rapa: Shawbury, 2010; pp. 1–2
- Sudesh, K.; Abe, H. Polyhydroxyalkanoates (PHA) Types; In: Practical Guide to Microbial Polyhydroxyalkanoates. Eds.; Smithers Rapa: Shawbury 2010; pp. 5–12
- Degeratu, C. N.; Mabilleau, G.; Aguado, E.; Mallet, R.; Chappard, D.; Cincu, C.; Stancu, I. C. Polyhydroxyalkanoate (PHBV) Fibers Obtained by a Wet Spinning Method: Good in Vitro Cytocompatibility but Absence of in Vivo Biocompatibility When Used as a Bone Graft. Morphologie 2019, 103, 94–102. DOI: 10.1016/j.morpho.2019.02.003.
- Steinbüchel, A.; Hein, S. Biochemical and Molecular Basis of Microbial Synthesis of Polyhydroxyalkanoates in Microorganisms; In: Biopolyesters. Babel, W. and Steinbüchel, A., Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2001; pp. 81–123
- Kim, Y. B.; Lenz, R. W. Polyesters from Microorganisms; In: Biopolyesters. Babel, W. and Steinbüchel, A., Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2001; pp. 51–79
- Kurian, N. S.; Das, B. Comparative Analysis of Various Extraction Processes Based on Economy, Eco-Friendly, Purity and Recovery of Polyhydroxyalkanoate: A Review. Int. J. Biol. Macromol. 2021, 183, 1881–1890. DOI: 10.1016/j.ijbiomac.2021.06.007.
- Steinbüchel, A.; Valentin, H. E. Diversity of Bacterial Polyhydroxyalkanoic Acids. FEMS Microbiol. Lett. 1995, 128, 219–228. DOI: 10.1016/0378-1097(95)00125-O.
- Lee, S. Y. Bacterial Polyhydroxyalkanoates. Biotechnol. Bioeng. 1996, 49, 1–14. DOI: 10.1002/(SICI)1097-0290(19960105)49:1 < 1::AID-BIT1 > 3.0.CO;2-P.
- Raza, Z. A.; Abid, S.; Banat, I. M. Polyhydroxyalkanoates: Characteristics, Production, Recent Developments and Applications. Int. Biodeterior. Biodegrad. 2018, 126, 45–56. DOI: 10.1016/j.ibiod.2017.10.001.
- Raza, Z. A.; Khalil, S.; Abid, S. Recent Progress in Development and Chemical Modification of Poly(Hydroxybutyrate)-Based Blends for Potential Medical Applications. Int. J. Biol. Macromol. 2020, 160, 77–100. DOI: 10.1016/j.ijbiomac.2020.05.114.
- Alcântara, J. M. G.; Distante, F.; Storti, G.; Moscatelli, D.; Morbidelli, M.; Sponchioni, M. Current Trends in the Production of Biodegradable Bioplastics: The Case of Polyhydroxyalkanoates. Biotechnol. Adv. 2020, 42, 107582. DOI: 10.1016/j.biotechadv.2020.107582.
- Larrañaga, A.; Fernández, J.; Vega, A.; Etxeberria, A.; Ronchel, C.; Adrio, J. L.; Sarasua, J. R. Crystallization and Its Effect on the Mechanical Properties of a Medium Chain Length Polyhydroxyalkanoate. J. Mech. Behav. Biomed. Mater. 2014, 39, 87–94. DOI: 10.1016/j.jmbbm.2014.07.020.
- Williams, S. F.; Martin, D. P.; Horowitz, D. M.; Peoples, O. P. PHA Applications: Addressing the Price Performance Issue: I. Tissue Engineering. Int. J. Biol. Macromol. 1999, 25, 111–121. DOI: 10.1016/S0141-8130(99)00022-7.
- Kunasundari, B.; Sudesh, K. Isolation and Recovery of Microbial Polyhydroxyalkanoates. Express Polym. Lett. 2011, 5, 620–634. DOI: 10.3144/expresspolymlett.2011.60.
- Puppi, D.; Pecorini, G.; Chiellini, F. Biomedical Processing of Polyhydroxyalkanoates. Bioengineering 2019, 6, 108. DOI: 10.3390/bioengineering6040108.
- Rodriguez-Contreras Recent Advances in the Use of Polyhydroyalkanoates in Biomedicine. Bioengineering 2019, 6, 82. DOI: 10.3390/bioengineering6030082.
- Rai, R.; Keshavarz, T.; Roether, J. A.; Boccaccini, A. R.; Roy, I. Medium Chain Length Polyhydroxyalkanoates, Promising New Biomedical Materials for the Future. Mater. Sci. Eng. R Rep. 2011, 72, 29–47. DOI: 10.1016/j.mser.2010.11.002.
- Chen, G.-Q.; Wu, Q. The Application of Polyhydroxyalkanoates as Tissue Engineering Materials. Biomaterials 2005, 26, 6565–6578. DOI: 10.1016/j.biomaterials.2005.04.036.
- Vogel, R.; Tändler, B.; Voigt, D.; Jehnichen, D.; Häussler, L.; Peitzsch, L.; Brünig, H. Melt Spinning of Bacterial Aliphatic Polyester Using Reactive Extrusion for Improvement of Crystallization. Macromol. Biosci. 2007, 7, 820–828. DOI: 10.1002/mabi.200700041.
- Iftikhar, A.; Nazia, J. Polyhydroxyalkanoates: Current Applications in the Medical Field. Front. Biol. 2016, 11, 19–27. DOI: 10.1007/s11515-016-1389-z.
- Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Negahi Shirazi, A.; Dehghani, F. Biomedical Applications of Biodegradable Polyesters. Polymers 2016, 8, 20. DOI: 10.3390/polym8010020.
- Puppi, D.; Morelli, A.; Chiellini, F. Additive Manufacturing of Poly(3-Hydroxybutyrate-co-3-Hydroxyhexanoate)/Poly(ε-Caprolactone) Blend Scaffolds for Tissue Engineering. Bioengineering 2017, 4, 49. DOI: 10.3390/bioengineering4020049.
- Chiono, V.; Ciardelli, G.; Vozzi, G.; Sotgiu, M. G.; Vinci, B.; Domenici, C.; Giusti, P. Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate)/Poly(Epsilon-Caprolactone) Blends for Tissue Engineering Applications in the Form of Hollow Fibers. J. Biomed. Mater. Res. A 2008, 85, 938–953. DOI: 10.1002/jbm.a.3151310.1002/jbm.a.31513.
- Mármol, G.; Gauss, C.; Fangueiro, R. Potential of Cellulose Microfibers for PHA and PLA Biopolymers Reinforcement. Molecules 2020, 25, 4653–4670. DOI: 10.3390/molecules25204653.
- Alagoz, A.; Rodríguez-Cabello, J.; Hasirci, V. PHBV Wet Spun Scaffold Coated with ELR-REDV Improves Vascularization for Bone Tissue Engineering. Biomed. Mater. 2018, 13, 055010. DOI: 10.1088/1748-605X/aad139.
- Williams, S. F.; Rizk, S.; Martin, D. P. Poly-4-Hydroxybutyrate (P4HB): A New Generation of Resorbable Medical Devices for Tissue Repair and Regeneration. Biomed. Tech. (Berl) 2013, 58, 439–452. DOI: 10.1515/bmt-2013-0009.
- Brandi, H.; Bachofen, R.; Mayer, J.; Wintermantel, E. Degradation and Applications of Polyhydroxyalkanoates. Can. J. Microbiol. 1995, 41, 143–153. DOI: 10.1139/m95-181.
- Jendrossek, D.; Handrick, R. Microbial Degradation of Polyhydroxyalkanoates. Annu. Rev. Microbiol. 2002, 56, 403–432. DOI: 10.1146/annurev.micro.56.012302.160838.
- Hufenus, R.; Reifler, F. A.; Fernández-Ronco, M. P.; Heuberger, M. Molecular Orientation in Melt-Spun Poly(3-Hydroxybutyrate) Fibers: Effect of Additives, Drawing and Stress-Annealing. Eur. Polym. J. 2015, 71, 12–26. DOI: 10.1016/j.eurpolymj.2015.07.039.
- Gopi, S.; Kontopoulou, M.; Ramsay, B. A.; Ramsay, J. A. Manipulating the Structure of Medium-Chain-Length Polyhydroxyalkanoate (MCL-PHA) to Enhance Thermal Properties and Crystallization Kinetics. Int. J. Biol. Macromol. 2018, 119, 1248–1255. DOI: 10.1016/j.ijbiomac.2018.08.016.
- Abe, H.; Ishii, N.; Sato, S.; Tsuge, T. Thermal Properties and Crystallization Behaviors of Medium-Chain-Length Poly(3-Hydroxyalkanoate)s. Polymer 2012, 53, 3026–3034. DOI: 10.1016/j.polymer.2012.04.043.
- Rogers, W. J. 7 - Sterilisation Techniques for Polymers; In: Sterilisation of Biomaterials and Medical Devices. Lerouge, S. and Simmons, A., Eds.; Woodhead Publishing: Cambridge, UK, 2012; pp. 151–211
- Shalaby, S. W.; Nagatomi, S. D.; Powell, E. F. 6 - Sterilization Techniques for Biotextiles for Medical Applications; In: Biotextiles as Medical Implants. King, M. W., Gupta, B. S. and Guidoin, R., Eds.; Cambridge, UK: Woodhead Publishing, 2013; pp. 157–168
- Shahid, S.; Razzaq, S.; Farooq, R.; Nazli, Z-i-H. Polyhydroxyalkanoates: Next Generation Natural Biomolecules and a Solution for the World's Future Economy. Int. J. Biol. Macromol. 2021, 166, 297–321. DOI: 10.1016/j.ijbiomac.2020.10.187.
- Lee, G. N.; Na, J. Future of Microbial Polyesters. Microb. Cell Fact. 2013, 12, 54–54. DOI: 10.1186/1475-2859-12-54.
- Andler, R.; Vivod, R.; Steinbüchel, A. Synthesis of Polyhydroxyalkanoates through the Biodegradation of Poly(Cis-1,4-Isoprene) Rubber. J. Biosci. Bioeng. 2019, 127, 360–365. DOI: 10.1016/j.jbiosc.2018.08.015.
- Sinclair, R. Chapter 1 - Understanding Textile Fibres and Their Properties: What is a Textile Fibre?; In: Textiles and Fashion. Sinclair, R., Eds.; Woodhead Publishing: Cambridge,UK, 2015; pp. 3–27
- Kikutani, T. 5 - Structure Development in Synthetic Fiber Production; In: Handbook of Textile Fibre Structure. Eichhorn, S. J., Hearle, J. W. S., Jaffe, M. and Kikutani, T., Eds.; Woodhead Publishing: Cambridge, UK, 2009; 12, pp. 157–180
- Devaux, E. 2 - Understanding the Behaviour of Synthetic Polymer Fibres during Spinning; In: Advances in Filament Yarn Spinning of Textiles and Polymers. Zhang, D., Eds.; Woodhead Publishing: Cambridge, UK, 2014; pp. 31–47
- Rawal, A.; Mukhopadhyay, S. 4 - Melt Spinning of Synthetic Polymeric Filaments; In: Advances in Filament Yarn Spinning of Textiles and Polymers. Zhang, D., Eds.; Woodhead Publishing: Cambridge, UK, 2014; pp. 75–99
- Wu, G.; Li, Q.; Cuculo, J. A. Fiber Structure and Properties of Poly(Ethylene-2,6-Naphthalate) Obtained by High-Speed Melt Spinning. Polymer 2000, 41, 8139–8150. DOI: 10.1016/S0032-3861(00)00122-1.
- Qin, Q.; Takarada, W.; Kikutani, T. Fiber Structure Formation in Melt Spinning of Bio-Based Aliphatic co-Polyesters. Proceedings of PPS-30: The 30th International Conference of the Polymer Processing Society - Conference Papers 2014, 1664 . DOI: 10.1063/1.4918460.
- Qin, Y. 14 - Biocompatibility Testing for Medical Textile Products; In: Medical Textile Materials. Qin, Y., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 191–201
- Hufenus, R.; Yan, Y.; Dauner, M.; Kikutani, T. Melt-Spun Fibers for Textile Applications. Materials 2020, 13, 4298. DOI: 10.3390/ma13194298.
- Rawal, A., Mukhopadhyay, S. 4 - Melt spinning of synthetic polymeric filaments; In: Advances in Filament Yarn Spinning of Textiles and Poymers. Deopura, B. L., Alagirusamy, R., Joshi, M. and Gupta, B., Eds.; Woodhead Publishing: Cambridge, UK, 2014; pp. 77.
- Sinclair, K. D.; Webb, K.; Brown, P. J. The Effect of Various Denier Capillary Channel Polymer Fibers on the Alignment of NHDF Cells and Type I Collagen. J. Biomed. Mater. Res. A 2010, 95, 1194–1202. DOI: 10.1002/jbm.a.32941.
- Hinüber, C.; Häussler, L.; Vogel, R.; Brünig, H.; Werner, C. Hollow Poly(3-Hydroxybutyrate) Fibers Produced by Melt Spinning. Macromol. Mater. Eng. 2010, 295, 585–594. DOI: 10.1002/mame.201000023.
- Hufenus, R.; Reifler, F. A.; Maniura-Weber, K.; Spierings, A.; Zinn, M. Biodegradable Bicomponent Fibers from Renewable Sources: Melt-Spinning of Poly(Lactic Acid) and Poly[(3-Hydroxybutyrate)-co-(3-Hydroxyvalerate)]. Macromol. Mater. Eng. 2012, 297, 75–84. DOI: 10.1002/mame.201100063.
- Barham, P. J.; Keller, A. The Relationship between Microstructure and Mode of Fracture in Polyhydroxybutyrate. J. Polym. Sci. B Polym. Phys 1986, 24, 69–77. DOI: 10.1002/polb.1986.180240108.
- Xiang, H.; Chen, Z.; Zheng, N.; Zhang, X.; Zhu, L.; Zhou, Z.; Zhu, M. Melt-Spun Microbial Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) Fibers with Enhanced Toughness: Synergistic Effect of Heterogeneous Nucleation, Long-Chain Branching and Drawing Process. Int. J. Biol. Macromol. 2019, 122, 1136–1143. DOI: 10.1016/j.ijbiomac.2018.09.063.
- Gupta, B. S. Manufactured Textile Fibers; In: Handbook of Industrial Chemistry and Biotechnology. Kent, J. A., Bommaraju, T. V. and Barnicki, S. D., Eds.; Springer International Publishing: Cham, 2017; pp. 1325–1396
- Ozipek, B.; Karakas, H. 9 - Wet Spinning of Synthetic Polymer Fibers; In: Advances in Filament Yarn Spinning of Textiles and Polymers. Zhang, D., Eds.; Woodhead Publishing: Cambridge, UK, 2014; pp. 174–186
- Gupta, V. B. Solution-Spinning Processes; In: Manufactured Fibre Technology. Gupta, V. B. and Kothari, V. K., Eds.; Springer Netherlands: Dordrecht, 1997; pp. 124–138
- Vogel, R.; Tändler, B.; Häussler, L.; Jehnichen, D.; Brünig, H. Melt Spinning of poly(3-hydroxybutyrate) fibers for tissue engineering using alpha-cyclodextrin/polymer inclusion complexes as the nucleation agent . Macromol. Biosci. 2006, 6, 730–736. DOI: 10.1002/mabi.200600116.
- Shah, D. T.; Tran, M.; Berger, P. A.; Aggarwal, P.; Asrar, J.; Madden, L. A.; Anderson, A. J. Synthesis and Properties of Hydroxy-Terminated Poly(Hydroxyalkanoate)s. Macromolecules 2000, 33, 2875–2880. DOI: 10.1021/ma991773e.
- Singh, A. K.; Srivastava, J. K.; Chandel, A. K.; Sharma, L.; Mallick, N.; Singh, S. P. Biomedical Applications of Microbially Engineered Polyhydroxyalkanoates: An Insight into Recent Advances, Bottlenecks, and Solutions. Appl. Microbiol. Biotechnol. 2019, 103, 2007–2032. DOI: 10.1007/s00253-018-09604-y.
- Iwata, T.; Tanaka, T. Manufacturing of PHA as Fibers; In: Plastics from Bacteria. Chen, G. G.-Q., Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2010; pp. 257–282
- Gordeyev, S. A.; Nekrasov, Y. P. Processing and Mechanical Properties of Oriented Poly(β-Hydroxybutyrate) Fibers. J. Mater. Sci. Lett. 1999, 18, 1691–1692. DOI: 10.1023/A:1006629800491.
- Schmack, G.; Jehnichen, D.; Vogel, R.; Tändler, B. Biodegradable Fibers of Poly(3-Hydroxybutyrate) Produced by High-Speed Melt Spinning and Spin Drawing. J. Polym. Sci. B Polym. Phys. 2000, 38, 2841–2850. # DOI: 10.1002/1099-0488(20001101)38:21 < 2841::AID-POLB130 > 3.0.CO;2-.
- Gordeyev, S. A.; Nekrasov, Y. P.; Shilton, S. J. Processing of Gel-Spun Poly(β-Hydroxybutyrate) Fibers. J. Appl. Polym. Sci. 2001, 81, 2260–2264. DOI: 10.1002/app.1665.
- Furuhashi, Y.; Imamura, Y.; Jikihara, Y.; Yamane, H. Higher Order Structures and Mechanical Properties of Bacterial Homo Poly(3-Hydroxybutyrate) Fibers Prepared by Cold-Drawing and Annealing Processes. Polymer 2004, 45, 5703–5712. DOI: 10.1016/j.polymer.2004.05.069.
- Qin, Q.; Takarada, W.; Kikutani, T. Fiber Structure Development of PHBH through Stress-Induced Crystallization in High-Speed Melt Spinning Process. JFST 2017, 73, 49–60. DOI: 10.2115/fiberst.2017-0007.
- Miyao, Y.; Takarada, W.; Kikutani, T. Improvement of Mechanical Properties of Biodegradable PHBH Fibers through High-Speed Melt Spinning Process Equipped with a Liquid Isothermal Bath. Europe-Africa Regional Conference of the Polymer Processing Society 2019, 2289, 020038. DOI: 10.1063/5.0028686.
- Iwata, T.; Tanaka, T.; Yoshiharu, D. High-Strength Fiber of Biodegradable Aliphatic Polyester and Process for Producing the Same. Internationalb 13 April 2006, Patent No.: US 7,938,999B2.
- Hong, C.; Iwata, T.; Tamura, M. Biodegradable polyester fiber having exvellent thermal stability and strength, and method for producing same. Internationalb US 2014/0088288 A1, Mar. 27, 2014.
- Martin, D. P.; Rizk, S.; Ahuja, A.; Williams, S. F. Process of Making Polyhydroxyalkanoate Medical Textiles. Internationalb 24 June 2014, Patent No.: US 8,758,657B2.
- Iwata, T.; Aoyagi, Y.; Fujita, M.; Yamane, H.; Doi, Y.; Suzuki, Y.; Takeuchi, A.; Uesugi, K. Processing of a Strong Biodegradable Poly[(R)-3-Hydroxybutyrate] Fiber and a New Fiber Structure Revealed by Micro-Beam X-Ray Diffraction with Synchrotron Radiation. Macromol. Rapid. Commun. 2004, 25, 1100–1104. DOI: 10.1002/marc.200400110.
- Puente, J. A. S.; Esposito, A.; Chivrac, F.; Dargent, E. Effect of Boron Nitride as a Nucleating Agent on the Crystallization of Bacterial Poly(3-Hydroxybutyrate). J. Appl. Polym. Sci. 2013, 128, 2586–2594. DOI: 10.1002/app.38182.
- Zhang, J.; Wang, L.; Sun, J.; Jiang, S.; Li, H.; Zhang, S.; Yang, W.; Gu, X.; Qiao, H. A Novel Hollow Microsphere Acting on Crystallization, Mechanical, and Thermal Performance of Poly(3-Hydroxybutyrate-co-4-Hydroxybutyrate). Polym. Cryst. 2021, 4, e10204. DOI: 10.1002/pcr2.10204.
- Qian, J.; Zhu, L.; Zhang, J.; Whitehouse, R. S. Comparison of Different Nucleating Agents on Crystallization of Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerates). J. Polym. Sci. B Polym. Phys. 2007, 45, 1564–1577. DOI: 10.1002/polb.21157.
- Kai, W.; He, Y.; Inoue, Y. Fast Crystallization of Poly(3-Hydroxybutyrate) and Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) with Talc and Boron Nitride as Nucleating Agents. Polym. Int. 2005, 54, 780–789. DOI: 10.1002/pi.1758.
- Yamane, H.; Terao, K.; Hiki, S.; Kimura, Y. Mechanical Properties and Higher Order Structure of Bacterial Homo Poly(3-Hydroxybutyrate) Melt Spun Fibers. Polymer 2001, 42, 3241–3248. DOI: 10.1016/S0032-3861(00)00598-X.
- Yamamoto, T.; Kimizu, M.; Kikutani, T.; Furuhashi, Y.; Cakmak, M. The Effect of Drawing and Annealing Conditions on the Structure and Properties of Bacterial Poly(3-Hydroxybutyrate-co-3 Hydroxyvalerate) Fibers. Int. Polym. Process 1997, 12, 29–37. DOI: 10.3139/217.970029.
- Liu, W. J.; Yang, H. L.; Wang, Z.; Dong, L. S.; Liu, J. J. Effect of Nucleating Agents on the Crystallization of Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate). J. Appl. Polym. Sci. 2002, 86, 2145–2152. DOI: 10.1002/app.11023.
- Vogel, R.; Voigt, D.; Tändler, B.; Gohs, U.; Häussler, L.; Brünig, H. Melt Spinning of Poly(3-Hydroxybutyrate) for Tissue Engineering Using Electron-Beam-Irradiated Poly(3-Hydroxybutyrate) as Nucleation Agent. Macromol. Biosci. 2008, 8, 426–431. DOI: 10.1002/mabi.200700225.
- Tanaka, T.; Fujita, M.; Takeuchi, A.; Suzuki, Y.; Uesugi, K.; Ito, K.; Fujisawa, T.; Doi, Y.; Iwata, T. Formation of Highly Ordered Structure in Poly[(R)-3-Hydroxybutyrate-co-(R)-3-Hydroxyvalerate] High-Strength Fibers. Macromolecules 2006, 39, 2940–2946. DOI: 10.1021/ma0527505.
- Bunsell, A. R. 1 - Introduction to the Science of Fibers; In: Handbook of Properties of Textile and Technical Fibres. Bunsell, A. R., Eds.; Woodhead Publishing: Duxford, United Kingdom, 2018; pp. 1–20
- Ohura, T.; Aoyagi, Y.; Takagi, K-i.; Yoshida, Y.; Kasuya, K-i.; Doi, Y. Biodegradation of Poly(3-Hydroxyalkanoic Acids) Fibers and Isolation of Poly(3-Hydroxybutyric Acid)-Degrading Microorganisms under Aquatic Environments. Polym. Degrad. Stab. 1999, 63, 23–29. DOI: 10.1016/S0141-3910(98)00057-3.
- Chen, Z.; Zhao, Z.; Hong, J.; Pan, Z. Novel Bioresource-Based Poly(3-Hydroxybutyrate-co-4-Hydroxybutyrate)/Poly(LacticAcid) Blend Fibers with High Strength and Toughness via Melt-Spinning. J Appl. Polym. Sci. 2020, 137, 48956. DOI: 10.1002/app.48956.
- Li, L.; Huang, W.; Wang, B.; Wei, W.; Gu, Q.; Chen, P. Properties and Structure of Polylactide/Poly (3-Hydroxybutyrate-co-3-Hydroxyvalerate) (PLA/PHBV) Blend Fibers. Polymer 2015, 68, 183–194. DOI: 10.1016/j.polymer.2015.05.024.
- Iwata, T.; Aoyagi, Y.; Tanaka, T.; Fujita, M.; Takeuchi, A.; Suzuki, Y.; Uesugi, K. Microbeam X-Ray Diffraction and Enzymatic Degradation of Poly[(R)-3-Hydroxybutyrate] Fibers with Two Kinds of Molecular Conformations. Macromolecules 2006, 39, 5789–5795. DOI: 10.1021/ma060908v.
- Hinüber, C.; Häussler, L.; Vogel, R.; Brünig, H.; Heinrich, G.; Werner, C. Hollow Fibers Made from a Poly(3-Hydroxybutyrate)/Poly-ε-Caprolactone Blend. Express Polym. Lett. 2011, 5, 643–652. DOI: 10.3144/expresspolymlett.2011.62.
- Battiston, B.; Geuna, S.; Ferrero, M.; Tos, P. Nerve Repair by means of Tubulization: Literature Review and Personal Clinical Experience Comparing Biological and Synthetic Conduits for Sensory Nerve Repair. Microsurgery 2005, 25, 258–267. DOI: 10.1002/micr.20127.
- Gilmore, J.; Yin, F.; Burg, K. J. L. Evaluation of Permeability and Fluid Wicking in Woven Fiber Bone Scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 306–313. DOI: 10.1002/jbm.b.34122.
- Kundrat, V.; Matouskova, P.; Marova, I. Facile Preparation of Porous Microfiber from Poly-3-(R)-Hydroxybutyrate and Its Application. Materials 2019, 13, 86. DOI: 10.3390/ma13010086.
- Li, Z.; Zhou, P.; Zhou, F.; Zhao, Y.; Ren, L.; Yuan, X. Antimicrobial Eugenol-Loaded Electrospun Membranes of Poly(ε-Caprolactone)/Gelatin Incorporated with REDV for Vascular Graft Applications. Colloids Surf. B Biointerfaces 2018, 162, 335–344. DOI: 10.1016/j.colsurfb.2017.12.004.
- Bierbaum, S.; Hintze, V.; Scharnweber, D. 2.8 Artificial Extracellular Matrices to Functionalize Biomaterial Surfaces; In: Comprehensive Biomaterials II. Ducheyne, P., Eds.; Elsevier: Oxford, 2017; pp. 147–178
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent Advances in Bone Tissue Engineering Scaffolds. Trends Biotechnol. 2012, 30, 546–554. DOI: 10.1016/j.tibtech.2012.07.005.
- Singhi, B.; Ford, E. N.; King, M. W. The Effect of Wet Spinning Conditions on the Structure and Properties of Poly-4-Hydroxybutyrate Fibers. J. Biomed. Mater. Res. - B Appl. Biomater. 2020, 109, e34763–e34763. DOI: 10.1002/jbm.b.34763.
- Schultze-Gebhardt, F.; Herlinger, K.-H. Fibers, 1. Survey. In: Ullmann's Fibers; Wiley-VCH: Weinheim, 2008 pp. 16
- Vehviläinen, M.; Kamppuri, T.; Rom, M.; Janicki, J.; Ciechańska, D.; Grönqvist, S.; Siika-Aho, M.; Elg Christoffersson, K.; Nousiainen, P. Effect of Wet Spinning Parameters on the Properties of Novel Cellulosic Fibres. Cellulose 2008, 15, 671–680. DOI: 10.1007/s10570-008-9219-3.
- Polyák, P.; Bartha, K.; Pukánszky, B. Quantitative Determination of Release Kinetics from Fibrous Poly(3-Hydroxybutyrate) Scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 114, 111026. DOI: 10.1016/j.msec.2020.111026.
- Mota, C.; Wang, S.-Y.; Puppi, D.; Gazzarri, M.; Migone, C.; Chiellini, F.; Chen, G.-Q.; Chiellini, E. Additive Manufacturing of Poly[(R)-3-Hydroxybutyrate-co-(R)-3-Hydroxyhexanoate] Scaffolds for Engineered Bone Development. J. Tissue Eng. Regen. Med. 2017, 11, 175–186. DOI: 10.1002/term.1897.
- Puppi, D.; Chiellini, F. Wet-Spinning of Biomedical Polymers: From Single-Fibre Production to Additive Manufacturing of Three-Dimensional Scaffolds. Polym. Int 2017, 66, 1690–1696. DOI: 10.1002/pi.5332.
- Puppi, D.; Mota, C.; Gazzarri, M.; Dinucci, D.; Gloria, A.; Myrzabekova, M.; Ambrosio, L.; Chiellini, F. Additive Manufacturing of Wet-Spun Polymeric Scaffolds for Bone Tissue Engineering. Biomed. Microdevices 2012, 14, 1115–1127. DOI: 10.1007/s10544-012-9677-0.
- Gupta, B. S. 1 - Manufacture, Types and Properties of Biotextiles for Medical Applications; In: Biotextiles as Medical Implants. King, M. W., Gupta, B. S. and Guidoin, R., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 3–47
- D'Elia, E. Self-Healing organic/inorganic composites. Doctor of Philosophy, Department of Materials, Imperial College London, U.K., 2015.
- Crangle, A. 1 - Types of Polyolefin Fibres; In: Polyolefin Fibres . Ugbolue, S. C. O., Eds.; Woodhead Publishing: Duxford, United Kingdom, 2009; pp. 3–34
- Cowie, J. M. G.; Arrighi, V. Introduction; In: Polymers: Chemistry and Physics of Modern Materals, third edition. Eds.; Taylor and Francis Group: Boca Raton, 2007; pp. 14, 20
- Barham, P. J.; Keller, A.; Otun, E. L.; Holmes, P. A. Crystallization and Morphology of a Bacterial Thermoplastic: Poly-3-Hydroxybutyrate. J. Mater. Sci. 1984, 19, 2781–2794. DOI: 10.1007/BF01026954.
- Ugbolue, S. 3 - The Structural Mechanics of Polyolefin Fibrous Materials and Nanocomposites; In: Polyolefin Fibres. Ugbolue, S. C. O., Eds.; Woodhead Publishing: Duxford, United Kingdom, 2009; pp. 57–80
- Mehrpouya, M.; Vahabi, H.; Barletta, M.; Laheurte, P.; Langlois, V. Additive Manufacturing of Polyhydroxyalkanoates (PHAs) Biopolymers: Materials, Printing Techniques, and Applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 127, 112216. DOI: 10.1016/j.msec.2021.112216.
- Cowie, J. M. G.; Arrighi, V. The Glassy State and Glass Transition; In: Polymers: Chemistry and Physics of Modern Materials, third edition Eds.; Taylor and Francis Group: Boca Raton, 2007; pp. 329–340.
- Chen, Y.; Chou, I.-N.; Tsai, Y.-H.; Wu, H.-S. Thermal Degradation of Poly(3-Hydroxybutyrate) and Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) in Drying Treatment. J. Appl. Polym. Sci. 2013, 130, 3659–3667. DOI: 10.1002/app.39616.
- Esmizadeh, E.; Tzoganakis, C.; Mekonnen, T. H. Degradation Behavior of Polypropylene during Reprocessing and Its Biocomposites: Thermal and Oxidative Degradation Kinetics. Polymers 2020, 12, 1627. DOI: 10.3390/polym12081627.
- Aboulkas, A.; El harfi, K.; El Bouadili, A. Thermal Degradation Behaviors of Polyethylene and Polypropylene. Part I: Pyrolysis Kinetics and Mechanisms. Energy Convers. Manag. 2010, 51, 1363–1369. DOI: 10.1016/j.enconman.2009.12.017.
- Richaud, E.; Fayolle, B.; Davies, P. 14 - Tensile Properties of Polypropylene Fibers; In: Handbook of Properties of Textile and Technical Fibres. Bunsell, A. R., Eds.; Woodhead Publishing: Duxford, United Kingdom, 2018; pp. 515–543
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Polyhydroxyalkanoate (PHA): Review of Synthesis, Characteristics, Processing and Potential Applications in Packaging. Express Polym. Lett. 2014, 8, 791–808. DOI: 10.3144/expresspolymlett.2014.82.
- Ugbolue, S. 10 - Testing and Quality Control of Polyolefins; In: Polyolefin Fibres. Ugbolue, S. C. O., Eds.; Woodhead Publishing: Duxford, United Kingdom, 2009; pp. 316–340
- Mather, R. R. 2 - The Structural and Chemical Properties of Polyolefin Fibres; In: Polyolefin Fibres. Ugbolue, S. C. O., Eds.; Woodhead Publishing: Duxford, Uunited Kingdom, 2009; pp. 35–56
- Tokiwa, Y.; Calabia, B. P.; Ugwu, C. U.; Aiba, S. Biodegradability of Plastics. IJMS 2009, 10, 3722–3742. DOI: 10.3390/ijms10093722.
- Hufenus, R.; Reifler, F. 2016 Effect of Stress and Temperature on the Molecular Orientation of Melt-Spun Poly(3-Hydroxybutyrate) Fibers. Fiber Society 2016 Spring Conference: Textile Innovations- Opportunities and Challenges.
- Gooch, J. W. Sonic Modulus; In: Encyclopedic Dictionary of Polymers. Gooch, J. W., Eds.; Springer New York: New York, NY, 2011; pp. 683–683