Abstract
Insights on active DNA demethylation disproved the original assumption that DNA methylation is a stable epigenetic modification. Interestingly, mammalian DNA methyltransferases 3A and 3B (DNMT-3A and -3B) have also been reported to induce active DNA demethylation, in addition to their well-known function in catalyzing methylation. In situations of extremely low levels of S-adenosyl methionine (SAM), DNMT-3A and -3B might demethylate C-5 methyl cytosine (5mC) via deamination to thymine, which is subsequently replaced by an unmodified cytosine through the base excision repair (BER) pathway. Alternatively, 5mC when converted to 5- hydroxymethylcytosine (5hmC) by TET enzymes, might be further modified to an unmodified cytosine by DNMT-3A and -3B under oxidized redox conditions, although exact pathways are yet to be elucidated. Interestingly, even direct conversion of 5mC to cytosine might be catalyzed by DNMTs. Here, we summarize the evidence on the DNA dehydroxymethylase and demethylase activity of DNMT-3A and -3B. Although physiological relevance needs to be demonstrated, the current indications on the 5mC- and 5hmC-modifying activities of de novo DNA C-5 methyltransferases shed a new light on these enzymes. Despite the extreme circumstances required for such unexpected reactions to occur, we here put forward that the chromatin microenvironment can be locally exposed to extreme conditions, and hypothesize that such waves of extremes allow enzymes to act in differential ways.
Abbreviations
| 5caC | = | 5-carboxylcytosine |
| 5fC | = | 5-formylcytosine |
| 5hmC | = | 5 hydroxymethylcytosine |
| 5mC | = | 5-methylcytosine |
| AID | = | activation-induced cytidine deaminase |
| APOBEC | = | apolipoprotein B mRNA editing enzyme catalytic polypeptide-like |
| BER | = | base excision and repair |
| C | = | cytosine |
| CGI | = | CpG islands |
| DNMT | = | DNA methyltransferase |
| GADD45 | = | growth arrest and DNA-damage-inducible protein 45 |
| RARE | = | retinoic acid response element |
| SAM | = | S-adenosyl methionine |
| TDG | = | thymine DNA glycosylase |
| TET | = | ten-eleven translocation |
Introduction
DNA methylation is the first and best studied epigenetic modification and, as no mammalian enzymes were known to actively remove this mark, DNA methylation was classically considered to be a stable mark. Mammalian DNA methylation is regulated by 3 DNA (cytosine-5)-methyltransferases: DNMT-1, -3A, and -3B and underlies a wide variety of processes in the body including cognition. In general, DNA methylation takes place at CpG sites and, especially when around transcription start sites, is often associated with gene repression. Despite this generally accepted association, many studies have documented that increased DNMT levels correlate with increased gene expression.
Aging-associated cognitive deficits, for example, were associated with decreased expression of Dnmt3a2 in the hippocampus in mice, and the deficits could be rescued by transfection of Dnmt3a2.Citation1 Remarkably, induced expression of Dnmt3a2 in rescued mice was associated with increased expression of early activity genes arc and bdnf.Citation1 Another example of increased DNMT3 expression and increased expression of certain genes is “first-time-event” fear, (e.g. a student appearing for his first viva voce exam). Subsequent exposures to similar events reduce the sensation of fear due to habitual conditioning.Citation2 The molecular mechanisms of such fear conditioning have been investigated and differential DNA methylation is among the changes observed for hippocampal neurons: Increased expression of the de novo DNA methyltransferases DNMT-3A and -3B was observed, while the DNA methylation status of the Reelin promoter was reduced.Citation3
To explain such seemingly contradictory observations, recent reports have suggested a direct DNA demethylase and hydroxymethylase activity of DNMT-3A and -3B in vitro.Citation4,5 We here examine cellular context requirements that would allow these enzymes to function in the process of active DNA demethylation.
Known Players in DNA Demethylation
Active DNA demethylation refers to the enzymatic removal or modification of the methyl group from 5mC, eventually resulting in an unmodified cytosine (C).Citation6 In this respect, ten-eleven translocation (TET) methylcytosine dioxygenases, activation-induced cytidine deaminase (AID), growth arrest and DNA-damage-inducible protein 45 α (Gadd45a), and thymine DNA glycosylase (TDG) are some of the factors described to play a role in this process of active DNA demethylation.Citation7-15
The enzymatic action of TET enzymes results in 5hmC,Citation13,16 a modification that is observed in many tissuesCitation17 and which can be further oxidized by TET to 5-formylcytosine (5fC) and 5-carboxylcytosine (5caC).Citation18,19 5caC can be further decarboxylatedCitation20 or excised by TDG,Citation19,21,22 which can also remove 5fC. In addition, 5hmC, 5fC, and 5caC are also considered DNA demethylation intermediates, as these can undergo replication-mediated dilution leading to passive DNA demethylation.Citation23,24 Alternatively, 5hmC can be deaminated to 5hmU by AID, followed by excision by TDG and replacement by an unmodified cytosine by the base excision and repair (BER) mechanism.Citation25
A role for DNMT-3A and -3B in the active DNA demethylation process?
In addition to the above described mechanisms of DNA demethylation, 3 possible alternative pathways of active DNA demethylation, involving DNMT-3A and -3B, have been proposed,Citation4,5,26 although their physiological relevance is under debate. Interestingly, 2 of these pathways concern BER-independent conversion of 5mC or 5hmC to unmodified cytosine (DNA demethylase and DNA dehydryoxymethylase activity, respectively). BER-independent conversion of methylated cytosines would provide a safer alternative to BER-mediated replacement as the TDG/BER pathway acts via DNA cleavage: its localized action on multiple methylated cytosines in a given region likely causes instability of the particular locus.Citation4,5 In addition to DNMT3s, also DNMT1 was found to contain strong DNA demethylating activity,Citation5 although its DNA dehydroxymethylase activity did not exceed background levels.Citation4
Role in deamination-induced DNA demethylation
Representative bacterial DNA methyltransferases, like M.HpaII and M.SssI, can convert C to uracil (U) in double stranded DNA in the absence of SAM.Citation27,28 During the process of methylation of C to 5mC in the presence of SAM, methyltransferases initiate a flipping of C out of the DNA helix, followed by the formation of a covalent bond between a cysteine in the methyltransferase and the C6 carbon of the flipped C.Citation29 This covalent interaction with the C6 carbon forms the activated cytosine which is then converted into an intermediate, namely 5,6-dihydrocytidine.Citation28-30 Dependent on the context, the cytosine intermediate can follow one of 2 possible routes: a) in the presence of SAM, it will accept the methyl group to form 5mC, and b) in the absence of SAM, the cytosine intermediate would be deaminated into U.Citation28 In line with bacterial methyltransferases, also mammalian DNMT-3A and -3B could deaminate C to U and importantly, 5mC to thymine (T).Citation26 The resulting T:G mismatch recruits TDG and other BER proteins which induce completion of the demethylation process. These observations tempted researchers to speculate on dual roles of DNMT-3A and -3B in living cells: as a demethylator at the beginning of the transcription cycle to induce expression of pS2, and as a methylator at the end of the cycle to stop the expression.Citation26,31 Indeed, the cyclical methylation of pS2 is abolished upon inhibition of DNMT-3A and -3B. In the above studies, however, it is possible that the, at that time unidentified, TET enzymes may have functioned as the active DNA demethylases as suggested by other studies.Citation32,33
Role in redox-dependent DNA demethylation
In the absence of SAM, M.HpaII and M.SssI, as well as murine Dnmt1 (albeit weakly), are able to catalyze the addition of formaldehyde to C forming 5hmC.Citation34 Interestingly, the authors also demonstrated that formaldehyde can be released from 5hmC which is then reconverted to an unmodified C in a reverse reaction catalyzed by these bacterial DNA methyltransferases.Citation34 DNA dehydryoxymethylase activity was also shown for purified mammalian DNMT-3A and -3B in the absence of SAM, although the exact mechanism has not been uncovered.Citation4 Importantly, the DNMT-3A and -3B-mediated dehydryoxymethylase activity depended on the redox state: an oxidizing agent (H2O2) enhanced dehydryoxymethylase activity, while suppressing the DNA methylation activity; reducing agents (DTT, β-mercaptoethanol) inhibited the dehydryoxymethylase activity while slightly enhancing DNA methylation.Citation4 When the cysteine residue in the methylation-active center of DNMT-3A or -3B was replaced by a serine residue, both enzymes exhibited reduced 5hmC dehydroxymethylase activity. Interestingly, oxidative post-translational modifications of particular cysteine residues have the potential to modulate protein activity.Citation35 Possibly, a similar mechanism might be in place for DNMT-3A and -3B. Despite such observations, another study, although confirming a direct 5hmC dehydryoxymethylase activity for human DNMT-3A (when in combination with the catalytically inactive DNMT3L), did not observe an effect of H2O2.Citation36
In the absence of SAM, DNMT3s could also directly convert 5mC to C in a non-reducing environment.Citation5 However, supraphysiological concentrations of Ca2+ are required for this reaction to occur (1 mM Ca2+ is optimal for demethylation; the minimum concentration is 10 µM). Apart from a direct action on 5mC and 5hmC, again in the absence of SAM, DNMT-3A and -3B can also directly convert 5caC, but not 5fC, into unmodified C.Citation36 These results indicate the intriguing possibility that active DNA demethylation can proceed via the reduction of oxidized products of 5mC without the need for BER, although these processes may serve as complementary pathways to BER.
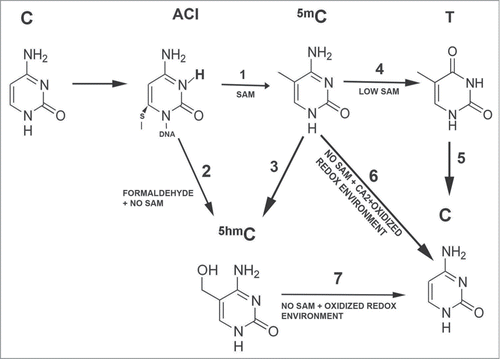
The above evidence can be summarized in the following scheme depicting how and when DNMT-3A and -3B could contribute to active DNA demethylation (). In the presence of SAM, DNMT-3A and -3B transfer a methyl group to the C5 of the activated cytosine to form 5mC (1). Whereas in the absence of SAM, DNMT1 (DNMT3s have not been analyzed) can couple a formaldehyde to the activated C to form 5hmC directly (2). When 5mC is formed, DNMT-mediated active DNA demethylation can, depending on the environment, proceed via 3 different pathways: Independently of the DNMTs, TET enzymes can directly convert 5mC to 5hmC (3). When SAM levels are low or completely depleted, DNMT-3A and -3B can contribute to the active DNA demethylation process. In the case of low SAM levels, DNMT-3A and -3B catalyze the deamination of 5mC to T (4). T is in turn replaced by an unmodified C via the BER pathway (5). In the case of absence of SAM combined with high levels of Ca2+ and an oxidized redox environment, DNMT-3A and -3B can convert 5mC to an unmodified C (6). Also the conversion of 5hmC to an unmethylated C might be catalyzed by DNMT-3A and -3B. This reaction can take place when SAM depletion is combined with an oxidizing redox environment (7).
Figure 1. Involvement of DNMTs in the active DNA methylation process. In the presence of SAM, DNMT-3A and -3B transfer a methyl group to the C5 of the activated cytosine to form 5mC (1). Whereas in the absence of SAM, DNMT1 (DNMT3s have not been analyzed) can couple a formaldehyde to the activated C to form 5hmC directly (2). When 5mC is formed, DNMT-mediated active DNA demethylation can, depending on the environment, proceed via 3 different pathways: Independently of the DNMTs, TET enzymes can directly convert 5mC to 5hmC (3). When SAM levels are low or completely depleted, DNMT-3A and -3B can contribute to the active DNA demethylation process. In the case of low SAM levels, DNMT-3A and -3B catalyze the deamination of 5mC to T (4). T is in turn replaced by an unmodified C via the BER pathway (5). In the case of absence of SAM combined with high levels of CA2+ and an oxidized redox environment, DNMT-3A and -3B can convert 5mC to an unmodified C (6). Also the conversion of 5hmC to an unmethylated C might be catalyzed by DNMT-3A and -3B. This reaction can take place when SAM depletion is combined with an oxidizing redox environment (7).
DNMT-3A and -3B as Demethylating Enzymes: Implications
Aberrant DNA hypomethylation is associated with various clinical phenotypes and can be explained by low SAM substrate availability or increased active DNA demethylation. Increased active DNA demethylation can be caused by deregulated activity of enzymes known to be involved in the demethylation pathway, including TET family members. As an alternative mechanism, we are here concerned with the processes of active DNA demethylation that involve DNMTs. However, specific circumstances are required for these activities to occur. In this respect, the calcium-dependent character of DNMT-induced 5mC demethylation requires extremely high levels of Ca2+ (>10 μM), which prevented this mechanism from reaching broad acceptance for physiological relevance.Citation5,37 Also the oxidizing conditions, potentially required for both the DNA demethylase as well as the DNA dehydroxylase activity of DNMT3, are unlikely to occur on a cellular level: 1 mM H2O2 is extremely toxic to cells. Below, we advocate that it is conceivable that substrate availability and redox conditions are not steady throughout the nucleus;Citation38 Extreme microenvironmental chromatin conditions might enable the cell to locally generate conditions that are required for DNMTs to act in the process of active DNA demethylation without the high cytotoxicity that is associated with total cell exposure. Here, we point out mechanisms that enable the cell to generate such local chromatin microenvironments, enabling the DNMTs to act differently on the local level.
The epigenetic landscape is continuously maintained/adjusted by re-inforcing epigenetic enzymes acting on histones and on the DNA molecule. The enzymatic reactions are associated with transient local changes in the microenvironment, as described (for example, histone demethylases). Currently, there are 2 known classes of histone demethylases: the Flavin-dependent histone demethylases and the Jumonji-containing histone demethylases. The first class, containing lysine-specific histone demethylase 1 and 2 (LSD1, 2), is involved in modulating gene expression through demethylation of either mono- or di-methyllysine residues of H3K4Citation38-41 or H3K9Citation42-44 resulting in gene repression or gene activation, respectively.Citation45 During this histone demethylation process, H2O2 is produced,Citation46 which has been proposed to be involved in signaling processes.Citation47 In the case of H3K9 demethylase activity, and thus gene activation, the H2O2 produced by LSDs might modulate the microenvironmental chromatin conditions in such a way that DNMT3s can re-inforce re-expression by acting in the process of active DNA demethylation.
The mechanism as proposed above might be especially relevant to rapidly “ultradian” oscillitating genes, in which gene expression is regulated in a rhytmic pattern of cyclical demethylation-remethylation events, with time periods of the order of hours.Citation48 It is plausible that the cell uses the ability of DNMT3s to act both in the process of DNA methylation and DNA demethylation, in order to enable a quick switch from demethylation to remethylation that is required to regulate the fast oscillitation of these genes. Promoters that follow such a fast oscillitating behavior are for example, the estrogen-sensitive pS2 promoterCitation26,31 and promoters containing a retinoic acid response element (RARE).Citation49 The latter study by Zuchegna et al. revealed that the dual action of the histone demethylases LSD1 and JMJD2A resulted in histone demethylation, local DNA oxidation, attraction of base (BER) and nucleotide excision repair (NER) enzymes and chromatin looping, and all this together was essential for and directly contributed to RA-induced transcription.Citation49 Moreover, this study clearly showed that the observed effects were strictly localized to the micro-chromatin environment of the RA-dependent genes, as the chromatin of neighboring RA-independent genes was unaffected. Similarly, LSD1 is also involved in the demethylation-remethylation cycles of the pS2 promoterCitation50 and therefore, the above described mechanism could be a more general feature of “ultradian” oscillitating genes.
If such described local changes in redox state would also occur for Ca2+ and/or SAM levels, such fluctuations might thus alter the function of DNMT-3A and -3B. To identify such situations in physiological situations, the targeting of DNMTs to pre-determined genomic loci by epigenetic editing, as described by us and others previouslyCitation51-55 provides an interesting tool to test and to eventually exploit DNA demethylation activities of DNMTs in vivo.
Apart from such extreme microenvironmental chromatin conditions, clinical situations exist where substrate conditions might allow DNMTs to act as demethylators: excess of formaldehyde is associated with aging, and has been put forward to explain memory deficits.Citation56 Moreover, in certain congenital diseases like Down syndrome, SAM levels are found to be decreased while oxidation status is increased.Citation57 Methionine adenosyl transferases (MATs), the enzymes that catalyze the conversion of methionine into SAM, are decreased in cirrhosis which is associated with genomic instability and an increased susceptibility of hepatocytes to oxidative stress-induced carcinogenesis (presumably due to impaired glutathione metabolism).Citation58 Preneoplastic changes seen in the liver of rats fed on a folate-deficient diet, resulting in decreased levels of SAM, are associated with global hypomethylation despite an increase in DNMT-1 and DNMT-3A.Citation59 These conditions exemplify situations where DNMT-3A could be actually functioning as a DNA demethylase in the context of SAM deficiency.
Conclusion
In vitro studies have suggested that DNMT-3A and -3B, known to methylate DNA de novo, can function as players in the active DNA demethylation pathway in certain situations. In conditions of low SAM concentrations, DNMT-3A and -3B can catalyze the deamination of 5mC to T, which could then be replaced by an unmodified C by the BER pathway. In an oxidizing redox chromatin microenvironment and in the absence of SAM, DNMT-3A and -3B can even catalyze the direct conversion of 5mC or 5hmC to an unmodified C, although the exact pathways are yet to be defined. Further cellular studies are now required to investigate the physiological relevance of DNMT-3A and -3B as players in active DNA demethylation. Epigenetic editing toolsCitation51-55 offer exciting possibilities to address differential effects of epigenetic enzymes in various chromatin contexts in living cells.Citation60
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Raman Kutty, Medical College of Wisconsin, Milwaukee, for critical reading of the manuscript.
Funding
MV received a talent grant for outstanding students from the department of natural sciences, Rijksuniversiteit Groningen. This work was supported by The Netherlands Organization for Scientific Research (NWO) through a CHEMTHEM grant, No 728.011.101.
References
- Oliveira AM, Hemstedt TJ, Bading H. Rescue of aging-associated decline in Dnmt3a2 expression restores cognitive abilities. Nat Neurosci 2012; 15:1111-3; PMID:22751036; http://dx.doi.org/10.1038/nn.3151
- Kandel ER, Schwartz JH. Principles of Neural Science. 5th ed. New York, NY etc.: McGraw-Hill, 2013
- Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron 2007; 53:857-69; PMID:17359920; http://dx.doi.org/10.1016/j.neuron.2007.02.022
- Chen CC, Wang KY, Shen CK. The mammalian de novo DNA methyltransferases DNMT3A and DNMT3B are also DNA 5-hydroxymethylcytosine dehydroxymethylases. J Biol Chem 2012; 287:33116-21; PMID:22898819; http://dx.doi.org/10.1074/jbc.C112.406975
- Chen CC, Wang KY, Shen CK. DNA 5-methylcytosine demethylation activities of the mammalian DNA methyltransferases. J Biol Chem 2013; 288:9084-91; PMID:23393137; http://dx.doi.org/10.1074/jbc.M112.445585
- Rots M, Petersen-Mahrt S. Conference Scene A symphony on C: orchestrating DNA repair for gene expression via cytosine modification. Epigenomics 2013; 5:1-4; PMID:23414310; http://dx.doi.org/10.2217/epi.12.73
- Cortellino S, Xu J, Sannai M, Moore R, Caretti E, Cigliano A, Le Coz M, Devarajan K, Wessels A, Soprano D, et al. Thymine DNA glycosylase is essential for active DNA demethylation by linked deamination-base excision repair. Cell 2011; 146:67-79; PMID:21722948; http://dx.doi.org/10.1016/j.cell.2011.06.020
- Barreto G, Schafer A, Marhold J, Stach D, Swaminathan SK, Handa V, Doderlein G, Maltry N, Wu W, Lyko F, et al. Gadd45a promotes epigenetic gene activation by repair-mediated DNA demethylation. Nature 2007; 445:671-5; PMID:17268471; http://dx.doi.org/10.1038/nature05515
- Fritz EL, Papavasiliou FN. Cytidine deaminases: AIDing DNA demethylation? Genes Dev 2010; 24:2107-14; PMID:20889711; http://dx.doi.org/10.1101/gad.1963010
- Gu TP, Guo F, Yang H, Wu HP, Xu GF, Liu W, Xie ZG, Shi L, He X, Jin SG, et al. The role of Tet3 DNA dioxygenase in epigenetic reprogramming by oocytes. Nature 2011; 477:606-10; PMID:21892189; http://dx.doi.org/10.1038/nature10443
- Bhutani N, Brady JJ, Damian M, Sacco A, Corbel SY, Blau HM. Reprogramming towards pluripotency requires AID-dependent DNA demethylation. Nature 2010; 463:1042-7; PMID:20027182; http://dx.doi.org/10.1038/nature08752
- Guo JU, Su Y, Zhong C, Ming GL, Song H. Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain. Cell 2011; 145:423-34; PMID:21496894; http://dx.doi.org/10.1016/j.cell.2011.03.022
- Ito S, D'Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y. Role of tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature 2010; 466:1129-33; PMID:20639862; http://dx.doi.org/10.1038/nature09303
- Schomacher L. Mammalian DNA demethylation: multiple faces and upstream regulation. Epigenetics 2013; 8:679-84; PMID:23803967; http://dx.doi.org/10.4161/epi.24977
- Wu H, Zhang Y. Reversing DNA methylation: mechanisms, genomics, and biological functions. Cell 2014; 156:45-68; PMID:24439369; http://dx.doi.org/10.1016/j.cell.2013.12.019
- Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 2009; 324:930-5; PMID:19372391; http://dx.doi.org/10.1126/science.1170116
- Globisch D, Munzel M, Muller M, Michalakis S, Wagner M, Koch S, Bruckl T, Biel M, Carell T. Tissue distribution of 5-hydroxymethylcytosine and search for active demethylation intermediates. PLoS One 2010; 5:e15367; PMID:21203455; http://dx.doi.org/10.1371/journal.pone.0015367
- Ito S, Shen L, Dai Q, Wu SC, Collins LB, Swenberg JA, He C, Zhang Y. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science 2011; 333:1300-3; PMID:21778364; http://dx.doi.org/10.1126/science.1210597
- He YF, Li BZ, Li Z, Liu P, Wang Y, Tang Q, Ding J, Jia Y, Chen Z, Li L, et al. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science 2011; 333:1303-7; PMID:21817016; http://dx.doi.org/10.1126/science.1210944
- Schiesser S, Hackner B, Pfaffeneder T, Muller M, Hagemeier C, Truss M, Carell T. Mechanism and stem-cell activity of 5-carboxycytosine decarboxylation determined by isotope tracing. Angew Chem Int Ed Engl 2012; 51:6516-20; PMID:22644704; http://dx.doi.org/10.1002/anie.201202583
- Zhang L, Lu X, Lu J, Liang H, Dai Q, Xu GL, Luo C, Jiang H, He C. Thymine DNA glycosylase specifically recognizes 5-carboxylcytosine-modified DNA. Nat Chem Biol 2012; 8:328-30; PMID:22327402; http://dx.doi.org/10.1038/nchembio.914
- Shen L, Wu H, Diep D, Yamaguchi S, D'Alessio AC, Fung HL, Zhang K, Zhang Y. Genome-wide analysis reveals TET- and TDG-dependent 5-methylcytosine oxidation dynamics. Cell 2013; 153:692-706; PMID:23602152; http://dx.doi.org/10.1016/j.cell.2013.04.002
- Inoue A, Shen L, Dai Q, He C, Zhang Y. Generation and replication-dependent dilution of 5fC and 5caC during mouse preimplantation development. Cell Res 2011; 21:1670-6; PMID:22124233; http://dx.doi.org/10.1038/cr.2011.189
- Inoue A, Zhang Y. Replication-dependent loss of 5-hydroxymethylcytosine in mouse preimplantation embryos. Science 2011; 334:194; PMID:21940858; http://dx.doi.org/10.1126/science.1212483
- Cortellino S, Xu J, Sannai M, Moore R, Caretti E, Cigliano A, Le Coz M, Devarajan K, Wessels A, Soprano D, et al. Thymine DNA glycosylase is essential for active DNA demethylation by linked deamination-base excision repair. Cell 2011; 146:67-79; PMID:21722948; http://dx.doi.org/10.1016/j.cell.2011.06.020
- Metivier R, Gallais R, Tiffoche C, Le Peron C, Jurkowska RZ, Carmouche RP, Ibberson D, Barath P, Demay F, Reid G, et al. Cyclical DNA methylation of a transcriptionally active promoter. Nature 2008; 452:45-50; PMID:18322525; http://dx.doi.org/10.1038/nature06544
- Shen JC, Rideout WM 3rd, Jones PA. High frequency mutagenesis by a DNA methyltransferase. Cell 1992; 71:1073-80; PMID:1473145; http://dx.doi.org/10.1016/S0092-8674(05)80057-1
- Zingg JM, Shen JC, Yang AS, Rapoport H, Jones PA. Methylation inhibitors can increase the rate of cytosine deamination by (cytosine-5)-DNA methyltransferase. Nucleic Acids Res 1996; 24:3267-75; PMID:8774911; http://dx.doi.org/10.1093/nar/24.16.3267
- Gerasimaite R, Merkiene E, Klimasauskas S. Direct observation of cytosine flipping and covalent catalysis in a DNA methyltransferase. Nucleic Acids Res 2011; 39:3771-80; PMID:21245034; http://dx.doi.org/10.1093/nar/gkq1329
- Gabbara S, Sheluho D, Bhagwat AS. Cytosine methyltransferase from escherichia coli in which active site cysteine is replaced with serine is partially active. Biochemistry 1995; 34:8914-23; PMID:7612633; http://dx.doi.org/10.1021/bi00027a044
- Kangaspeska S, Stride B, Metivier R, Polycarpou-Schwarz M, Ibberson D, Carmouche RP, Benes V, Gannon F, Reid G. Transient cyclical methylation of promoter DNA. Nature 2008; 452:112-5; PMID:18322535; http://dx.doi.org/10.1038/nature06640
- Hashimoto H, Liu Y, Upadhyay AK, Chang Y, Howerton SB, Vertino PM, Zhang X, Cheng X. Recognition and potential mechanisms for replication and erasure of cytosine hydroxymethylation. Nucleic Acids Res 2012; 40:4841-9; PMID:22362737; http://dx.doi.org/10.1093/nar/gks155
- de la Rica L, Rodriguez-Ubreva J, Garcia M, Islam AB, Urquiza JM, Hernando H, Christensen J, Helin K, Gomez-Vaquero C, Ballestar E. PU.1 target genes undergo Tet2-coupled demethylation and DNMT3b-mediated methylation in monocyte-to-osteoclast differentiation. Genome Biol 2013; 14:R99; PMID:24028770; http://dx.doi.org/10.1186/gb-2013-14-9-r99
- Liutkeviciute Z, Lukinavicius G, Masevicius V, Daujotyte D, Klimasauskas S. Cytosine-5-methyltransferases add aldehydes to DNA. Nat Chem Biol 2009; 5:400-2; PMID:19430486; http://dx.doi.org/10.1038/nchembio.172
- Lindahl M, Mata-Cabana A, Kieselbach T. The disulfide proteome and other reactive cysteine proteomes: analysis and functional significance. Antioxid Redox Signal 2011; 14 (12): 2581-2642; PMID:21275844; http://dx.doi.org/10.1089/ars.2010.3551
- Liutkeviciute Z, Kriukiene E, Licyte J, Rudyte M, Urbanaviciute G, Klimasauskas S. Direct decarboxylation of 5-carboxylcytosine by DNA C5- methyltransferases. J Am Chem Soc 2014; PMID:24716540; http://dx.doi.org/10.1021/ja5019223
- Wang KY, Chen CC, Shen CK. Active DNA demethylation of the vertebrate genomes by DNA methyltransferases: deaminase, dehydroxymethylase or demethylase? Epigenomics 2014; 6:353-63; PMID:25111488; http://dx.doi.org/10.2217/epi.14.21
- Lee MG, Wynder C, Cooch N, Shiekhattar R. An essential role for CoREST in nucleosomal histone 3 lysine 4 demethylation. Nature 2005; 437:432-5; PMID:16079794
- Amente S, Bertoni A, Morano A, Lania L, Avvedimento EV, Majello B. LSD1-mediated demethylation of histone H3 lysine 4 triggers myc-induced transcription. Oncogene 2010; 29:3691-702; PMID:20418916; http://dx.doi.org/10.1038/onc.2010.120
- Ouyang H, Qin Y, Liu Y, Xie Y, Liu J. Prox1 directly interacts with LSD1 and recruits the LSD1/NuRD complex to epigenetically co-repress CYP7A1 transcription. PLoS One 2013; 8:e62192; PMID:23626788; http://dx.doi.org/10.1371/journal.pone.0062192
- Pan D, Mao C, Wang YX. Suppression of gluconeogenic gene expression by LSD1-mediated histone demethylation. PLoS One 2013; 8:e66294; PMID:23755305; http://dx.doi.org/10.1371/journal.pone.0066294
- Laurent B, Ruitu L, Murn J, Hempel K, Ferrao R, Xiang Y, Liu S, Garcia B, Wu H, Wu F, et al. A specific LSD1/KDM1A isoform regulates neuronal differentiation through H3K9 demethylation. Mol Cell 2015; 57:957-70; PMID:25684206; http://dx.doi.org/10.1016/j.molcel.2015.01.010
- Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA, Shi Y. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 2004; 119:941-53; PMID:15620353; http://dx.doi.org/10.1016/j.cell.2004.12.012
- Metzger E, Wissmann M, Yin N, Muller JM, Schneider R, Peters AH, Gunther T, Buettner R, Schule R. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature 2005; 437:436-9; PMID:16079795
- Shin J, Ming GL, Song H. Molecular toggle switch of histone demethylase LSD1. Mol Cell 2015; 57:949-50; PMID:25794611; http://dx.doi.org/10.1016/j.molcel.2015.03.007
- Karytinos A, Forneris F, Profumo A, Ciossani G, Battaglioli E, Binda C, Mattevi A. A novel mammalian flavin-dependent histone demethylase. J Biol Chem 2009; 284:17775-82; PMID:19407342; http://dx.doi.org/10.1074/jbc.M109.003087
- Forneris F, Binda C, Battaglioli E, Mattevi A. LSD1: oxidative chemistry for multifaceted functions in chromatin regulation. Trends Biochem Sci 2008; 33:181-9; PMID:18343668; http://dx.doi.org/10.1016/j.tibs.2008.01.003
- Isomura A, Kageyama R. Ultradian oscillations and pulses: coordinating cellular responses and cell fate decisions. Development 2014; 141:3627-36; PMID:25249457; http://dx.doi.org/10.1242/dev.104497
- Zuchegna C, Aceto F, Bertoni A, Romano A, Perillo B, Laccetti P, Gottesman ME, Avvedimento EV, Porcellini A. Mechanism of retinoic acid-induced transcription: histone code, DNA oxidation and formation of chromatin loops. Nucleic Acids Res 2014; 42:11040-55; PMID:25217584; http://dx.doi.org/10.1093/nar/gku823
- Perillo B, Ombra MN, Bertoni A, Cuozzo C, Sacchetti S, Sasso A, Chiariotti L, Malorni A, Abbondanza C, Avvedimento EV. DNA oxidation as triggered by H3K9me2 demethylation drives estrogen-induced gene expression. Science 2008; 319:202-6; PMID:18187655; http://dx.doi.org/10.1126/science.1147674
- Siddique AN, Nunna S, Rajavelu A, Zhang Y, Jurkowska RZ, Reinhardt R, Rots MG, Ragozin S, Jurkowski TP, Jeltsch A. Targeted methylation and gene silencing of VEGF-A in human cells by using a designed Dnmt3a-Dnmt3L single-chain fusion protein with increased DNA methylation activity. J Mol Biol 2013; 425:479-91; PMID:23220192; http://dx.doi.org/10.1016/j.jmb.2012.11.038
- Rivenbark AG, Stolzenburg S, Beltran AS, Yuan X, Rots MG, Strahl BD, Blancafort P. Epigenetic reprogramming of cancer cells via targeted DNA methylation. Epigenetics 2012; 7:350-60; PMID:22419067
- de Groote ML, Verschure PJ, Rots MG. Epigenetic editing: targeted rewriting of epigenetic marks to modulate expression of selected target genes. Nucleic Acids Res 2012; 40:10596-613; PMID:23002135; http://dx.doi.org/10.1093/nar/gks863
- Chen H, Kazemier HG, de Groote ML, Ruiters MH, Xu GL, Rots MG. Induced DNA demethylation by targeting ten-eleven translocation 2 to the human ICAM-1 promoter. Nucleic Acids Res 2014; 42:1563-74; PMID:24194590; http://dx.doi.org/10.1093/nar/gkt1019
- Maeder ML, Angstman JF, Richardson ME, Linder SJ, Cascio VM, Tsai SQ, Ho QH, Sander JD, Reyon D, Bernstein BE, et al. Targeted DNA demethylation and activation of endogenous genes using programmable TALE-TET1 fusion proteins. Nat Biotechnol 2013; 31:1137-42; PMID:24108092; http://dx.doi.org/10.1038/nbt.2726
- Tong Z, Han C, Luo W, Li H, Luo H, Qiang M, Su T, Wu B, Liu Y, Yang X, et al. Aging-associated excess formaldehyde leads to spatial memory deficits. Sci Rep 2013; 3:1807; PMID:23657727
- Dekker AD, De Deyn PP, Rots MG. Epigenetics: the neglected key to minimize learning and memory deficits in down syndrome. Neurosci Biobehav Rev 2014; 45C:72-84; http://dx.doi.org/10.1016/j.neubiorev.2014.05.004
- Lu SC, Mato JM. S-adenosylmethionine in liver health, injury, and cancer. Physiol Rev 2012; 92:1515-42; PMID:23073625; http://dx.doi.org/10.1152/physrev.00047.2011
- Ghoshal K, Li X, Datta J, Bai S, Pogribny I, Pogribny M, Huang Y, Young D, Jacob ST. A folate- and methyl-deficient diet alters the expression of DNA methyltransferases and methyl CpG binding proteins involved in epigenetic gene silencing in livers of F344 rats. J Nutr 2006; 136:1522-7; PMID:16702315
- Jurkowski TP, Ravichandran M, Stepper P. Synthetic epigenetics-towards intelligent control of epigenetic states and cell identity. Clin Epigenetics 2015; 7 (1):18; PMID:25628764; http://dx.doi.org/10.1186/s13148-015-0044-x
