ABSTRACT
Downregulation of miR26A1 has been reported in various B-cell malignancies; however, the mechanism behind its deregulation remains largely unknown. We investigated miR26A1 methylation and expression levels in a well-characterized series of chronic lymphocytic leukemia (CLL) and mantle cell lymphoma (MCL). From 450K methylation arrays, we first observed miR26A1 (cg26054057) as uniformly hypermethylated in MCL (n = 24) (all >75%), while CLL (n = 18) showed differential methylation between prognostic subgroups. Extended analysis using pyrosequencing confirmed our findings and real-time quantitative PCR verified low miR26A1 expression in both CLL (n = 70) and MCL (n = 38) compared to normal B-cells. Notably, the level of miR26A1 methylation predicted outcome in CLL, with higher levels seen in poor-prognostic, IGHV-unmutated CLL. Since EZH2 was recently reported as a target for miR26A1, we analyzed the expression levels of both miR26A1 and EZH2 in primary CLL samples and observed an inverse correlation. By overexpression of miR26A1 in CLL and MCL cell lines, reduced EZH2 protein levels were observed using both Western blot and flow cytometry. In contrast, methyl-inhibitor treatment led to upregulated miR26A1 expression with a parallel decrease of EZH2 expression. Finally, increased levels of apoptosis were observed in miR26A1-overexpressing cell lines, further underscoring the functional relevance of miR26A1. In summary, we propose that epigenetic silencing of miR26A1 is required for the maintenance of increased levels of EZH2, which in turn translate into a worse outcome, as shown in CLL, highlighting miR26A1 as a tumor suppressor miRNA.
Introduction
MicroRNAs (miRNAs) belong to the class of small (∼22 nucleotides) noncoding RNAs, known to play critical regulatory roles in normal cell development as well as during tumorigenesis.Citation1-4 The importance of miRNAs in tumor development was first documented for the miR-15a/16-1 family in chronic lymphocytic leukemia (CLL), where the deletion of this cluster on chromosome 13q14 was reported in over two-thirds of CLL patients.Citation5 A number of studies have subsequently revealed the biological and clinical relevance of different miRNAs in various types of cancer, including CLL and the clinically more aggressive mantle cell lymphoma (MCL).Citation6-11
One of these miRNAs, miR26A1, was reported to be deregulated in several cancer types, such as thyroid anaplastic carcinoma, Burkitt lymphoma, and rhabdomyosarcoma, and was proposed to function as a tumor suppressor miRNA.Citation12-14 miR26A1 is known to be involved in several key signaling pathways (e.g., p53, TGF-β signaling) and to regulate several transformation-related targets, such as SMAD1Citation15 and EZH2.Citation13 The latter is a member of the polycomb group (PcG) and a histone methyltransferase catalyzing H3K27me3 methylation, which is known to be overexpressed in different cancer types, including CLL and MCL, and linked to poor outcome.Citation16-18 Recently, the direct effect of miR26A1 on EZH2 regulation was indicated in lung cancer and nasopharyngeal carcinoma, where miR26A1 overexpression led to downregulation of EZH2, inhibition of cell proliferation, and induction of apoptosis by blockage of the G1/S phase transition.Citation13,19
In CLL and MCL, miR26A1 expression was shown to be noticeably lower in patient samples compared to normal B-cells.Citation20-22 However, the role of miR26A1 and the mechanism behind its deregulation remains unknown. To gain insight into this issue, we investigated the methylation status and gene expression of miR26A1 in a well-characterized CLL (70 patients) and MCL (65 patients) material. While MCL uniformly showed high levels of miR26A methylation and low miR26A expression, CLL patients showed a more varying methylation spectrum that correlated with outcome. By overexpressing miR26A1 in CLL and MCL cell lines, we showed downregulation of EZH2, supporting a direct regulatory role of miR26A1.
Results
DNA hypermethylation of miR26A1 in CLL and MCL
From our recent 450K DNA methylation array analysis in CLL,Citation23 we observed a differentially methylated CpG site in miR26A1 (cg26054057) between prognostic subgroups based on IGHV gene mutational status,Citation24,25 with IGHV-unmutated cases displaying a significantly higher methylation level than IGHV-mutated patients (median 53.0% vs. 83.7%, P = 0.006; ). In MCL, we performed 450K array analysis on 24 MCL cases [12 high proliferative (HP) + 12 low proliferative (LP)] and miR26A1 was found to be homogeneously hypermethylated in all MCL samples (median 96.0% and 96.6% in LP and HP samples, respectively; ).
Figure 1. 450K methylation array and pyrosequencing data. Box plots showing miR26A1 methylation levels for CpG site cg26054057 in IGHV-mutated (n = 9) and IGHV-unmutated (n = 9) CLL (A) and MCL (B) samples based on 450K methylation array data. Box plots showing percentage of DNA methylation levels of miR26A1, as assessed by pyrosequencing in CLL (C) and MCL (D) primary samples along with normal B-cell controls.
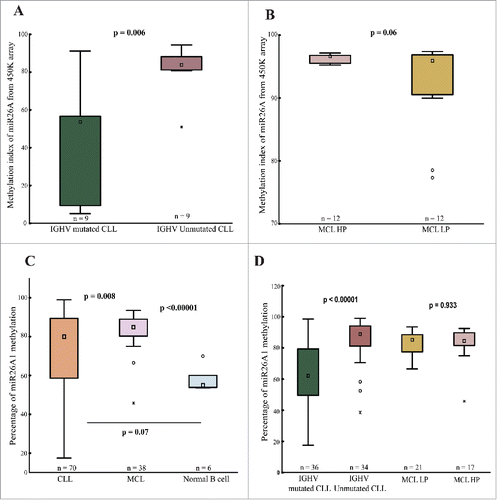
In order to extend these observations, we performed pyrosequencing to analyze the methylation status of miR26A1 (average of 4 CpG sites) in an additional 38 MCL and 70 CLL samples. Compared to normal controls, we observed that all CLL and MCL samples were hypermethylated (). In MCL, all samples showed hypermethylation with high median percentage of miR26A1 methylation (84.8%; range: 80.2-89.0%) (), with no difference between HP and LP cases, which is in line with our array data. Similarly, the CLL samples showed a wider range of miR26A1 methylation (80%; range: 58.6-89.3%). Again, IGHV-unmutated cases showed significantly higher methylation levels (median 88.9%), similar to MCL samples, as compared to IGHV-mutated samples (median 62%, P < 0.00001; ).
DNA methylation status of miR26A1 predicts survival in CLL
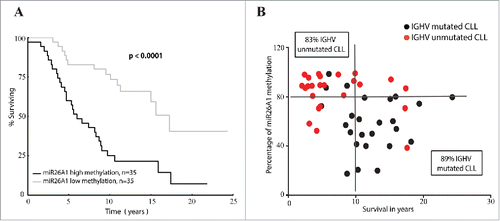
CLL patients were divided into cases with high or low miR26A1 methylation based on median value as cutoff (80%). Indeed, CLL patients with high methylation levels showed a poorer overall survival compared to the patients with low methylation levels (median survival 6.0 vs 17.2 years, P < 0.0001; ). Interestingly, when we correlated the percentage of methylation to survival time in years (), we observed that most of the IGHV-mutated cases (17 out of 19 cases, 89%) with long survival times (>10 years) showed lower methylation levels. On the other hand, most IGHV-unmutated cases (15 out of 18 cases, 83%) with survival times below 10 y showed higher methylation levels ().
Figure 2. miR26A1 DNA methylation levels predicts overall survival in CLL. Kaplan-Meier curves for CLL patients with high and low DNA methylation (A). Scatter plots showing methylation levels of miR26A1 plotted against survival data in years (B). Black dots represent IGHV-mutated samples and red dots IGHV-unmutated samples. This scatter plot is again divided into 4 quartiles based on the median survival time in CLL (which is 10 years) and the median methylation percentage levels (which is 80% based on the pyrosequencing data).
Deregulated expression of miR26A1 in CLL and MCL
Using real-time quantitative PCR (RQ-PCR), we determined the miR26A1 expression levels in 38 MCL and 70 CLL patient samples and compared to CD19+ sorted B-cells from 6 healthy, age-matched controls. As previously reported,Citation20,22 both MCL and CLL samples uniformly showed several-fold lower miR26A1 expression compared to normal B-cell controls (Supplementary Fig. 1A). Hence, no differential miR26A1 expression was observed between IGHV-mutated/unmutated CLL samples or between HP and LP MCL samples (Supplementary Fig. 1B). Accordingly, no differences in survival were detected in relation to miR26A1 expression, based on the median value as cutoff level (0.000676) (P = 0.88; Supplementary Fig. 1C). Nevertheless, most IGHV-mutated CLL cases showed high miR26A1 expression and long overall survival (8 out of 11 cases, 72%) and vice versa for IGHV-unmutated CLL (13 out of 16 cases, 81%; Supplementary Fig. 1D).
miR26A1 targets EZH2 in CLL and MCL
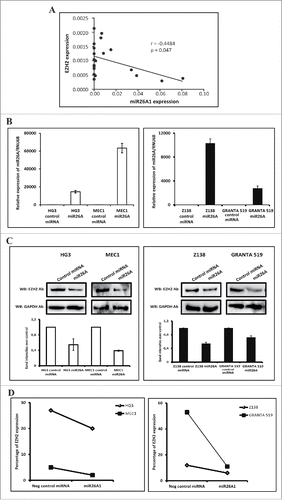
Recently, miR26A1 was shown to target EZH2 and reduce its expression in nasopharyngeal and hepatocellular carcinomas.Citation26,27 To examine this potential link further, we first selected primary CLL samples exhibiting high (10 samples) and low (10 samples) miR26A1 expression and analyzed the EZH2 mRNA expression levels using RQ-PCR. As shown in , a significant negative correlation (P = 0.04, r = −0.45) was observed. Next, we overexpressed miR26A1 in 4 CLL and MCL cell lines (HG3, MEC1, Z138 and GRANTA519) using Amaxa nucleofection: all 4 cell lines showed a significant overexpression of miR26A1 with a few thousand-fold increase after transfection (). Even though no significant decrease in EZH2 mRNA expression levels was seen (Supplementary Fig. 2A-B), we observed a significant downregulation of EZH2 protein levels compared to the negative control using 2 independent methods, i.e., Western blot analysis () and flow cytometry ( and Supplementary Fig. 2C), hence suggesting that that EZH2 is a target for miR26A1 in both CLL and MCL.
Figure 3. Correlation between miR26A1 and EZH2 expression. Scatter plot showing inverse correlation between EZH2 and miR26A1 expression levels in CLL patient samples (P < 0.05 and r = −0.4484) (A). Relative expression levels of miR26A1 in negative control mimic miRNA and miR26A1 mimic miRNA transfected cell lines. Left panel shows data in HG3 and MEC1 CLL cell lines and right panel the corresponding data in Z138 and GRANTA519 MCL cell lines (B). Western blot analysis of EZH2 expression levels in miR26A1 and control mimic miRNA transfected HG3/MEC1 CLL cell lines (left panel) and Z138/GRANTA519 MCL cell lines (right panel). GAPDH was used as internal loading controls. Histograms below Western blots show calculated band intensities based on 2 independent transfections (C). Percentage of EZH2 protein expression using FACS analysis in CLL (left) and MCL (right) cell lines (D).
Loss of DNA methylation results in upregulation of miR26A1 expression
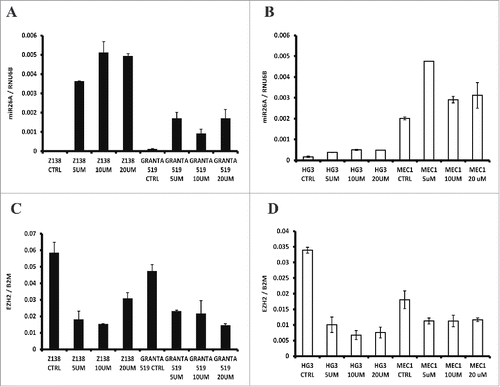
To investigate the direct role of DNA methylation in regulating miR26A1 expression, we treated the 4 MCL/CLL cell lines with increasing concentrations of the methyl inhibitor DAC. As shown in , a corresponding increase of miR26A1 expression was demonstrated for all 4 cell lines with different DAC concentrations. Since miR26A1 was negatively correlated with EZH2 expression, we also analyzed EZH2 expression in these DAC treated cell lines, showing a corresponding reduction of EZH2 with increasing miR26A1 ().
Figure 4. Effect on miR26A1 and EZH2 expression levels after DAC treatment using CLL and MCL cell lines. Relative expression levels of miR26A1 in Z138 and GRANTA519 cell lines (left side) and HG3 and MEC1 cell lines (right side) after DAC treatment using increasing concentrations (A-B). Relative EZH2 mRNA expression for the same MCL (left side) and CLL (right side) DAC treated cell lines (C-D). All samples were treated for 3 d; DAC was changed every 24 h.
Overexpression of miR26A1 leads to increased apoptosis
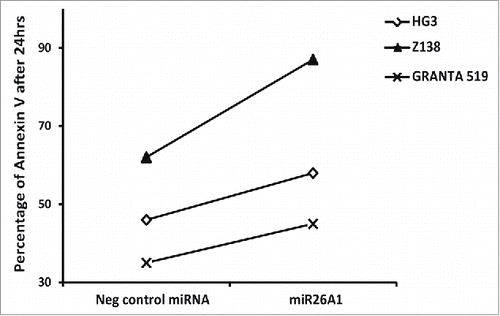
Finally, to study the role of miR26A1 in inducing apoptosis, we performed Annexin V staining in cells overexpressing miR26A1 demonstrating that increased levels of miR26A1 coincided with increased apoptosis in all 3 leukemic cell lines investigated (HG3, Z138, and GRANTA 519) ( and Supplementary Fig. 3).
Figure 5. Overexpression of miR26A1 induces apoptosis in CLL and MCL cell lines. The percentage of Annexin V-positive cells analyzed using FACS for control and miR26A1 mimic miRNA transfected cell lines. The percentage of Annexin V positive cells show the average of 2 independent sets. The Annexin V levels were measured after 24, 48, and 72 h.
Discussion
miR26A1 was recently suggested to act as a tumor suppressor miRNA, and studies of CLL and MCL have revealed generally lower miR26A1 expression levels compared to normal B-cells.Citation28-30 However, little is known about the mechanism leading to deregulation of miR26A1 in lymphoid malignancies. From preliminary observations based on 450K methylation array analysis, we noted that miR26A1 was hypermethylated in most CLL and MCL samples, though with differential methylation between IGHV-mutated and unmutated CLL, while all MCL samples appeared to be more uniformly hypermethylated. This initial finding pointed to a potential involvement of aberrant DNA methylation in regulating miR26A1 expression.
To study this further, we decided to investigate larger series of CLL and MCL patients using a more quantitative technology, i.e., pyrosequencing. Indeed, miR26A1 was found to be differentially methylated between favorable-prognostic IGHV-mutated and poor-prognostic IGHV-unmutated CLL cases, where the latter subgroup displayed significantly higher methylation levels, well in line with recent high-throughput studies showing differential methylation between these subgroups.Citation23,28,31,32 The degree of miR26A1 methylation was also associated with a worse outcome in CLL, with significantly shorter overall survival in patients with high miR26A1 methylation levels. Notably, the CLL cohort investigated did not contain any 11q- or 17p-deleted case (which are already known to render a poor outcome) to avoid biased results. In contrast, in MCL, miR26A1 was highly methylated in almost all patient samples, independently of whether they were judged as high or low proliferative, indicating a more uniform silencing in MCL.
Next, we analyzed the miR26A1 expression in primary CLL and MCL samples using RQ-PCR, overall demonstrating, as expected, very low expression levels in both malignancies (including both IGHV-mutated/unmutated CLL and HP/LP MCL) compared to normal B-cells. To scrutinize the direct role of DNA methylation in regulating miR26A1 expression, we treated 4 CLL and MCL cell lines with the methyl inhibitor DAC and observed a corresponding increase of miR26A1 expression. From these data, we conclude that epigenetic silencing of miR26A1 through DNA methylation appears to represent an important event in the pathobiology of both CLL and MCL. This was further underscored by our findings that miR26A1 overexpression in cell lines led to induction of apoptosis, supporting its tumor suppressor properties, now also in these 2 B-cell malignancies.
Overexpression of EZH2, a well-studied chromatin modifier, is known to be associated with tumor invasion, tumor progression, and poor prognosis in many different cancer types, including CLL and MCL.Citation16,33,34 A key role of EZH2 in MCL pathobiology was recently suggested from studies showing that i) upregulated EZH2 conferred reduced expression of the tumor suppressor miR29A, and ii) epigenetic silencing of HOX genes in MCL was directed by EZH2.Citation35 Since EZH2 most recently was indicated as a direct target of miR26A1 in other cancer types,Citation26 we decided to investigate the potential consequence of miR26A1 deregulation on EZH2 expression. To do this, we overexpressed miR26A1 in CLL and MCL cell lines, which all showed reduced protein expression levels of EZH2 using both Western blot and FACS analysis. Importantly, this negative correlation was also seen in primary CLL samples, where EZH2 expression was significantly higher in samples with low miR26A1 expression and vice versa. In addition, in our re-induction experiments of miR26A1 expression using the methyl inhibitor treatment, we observed a corresponding decrease in EZH2 expression. Hence, our data strongly suggests that silenced miR26A1 expression is a necessity for maintaining high levels of EZH2 in CLL and MCL, which renders these cells a proliferative and survival advantage.
In summary, this study highlights the importance of epigenetic silencing of miR26A1 miRNA in CLL and MCL. Indeed, overexpression of miR26A1 resulted in decreased levels of EZH2, which in turn led to induction of apoptosis, further supporting miR26A1 as an important tumor suppressor micro-RNA. While hypermethylation was a uniform finding for all MCL samples, a more varying degree of miR26A1 methylation was seen in CLL, with high methylation predicting significantly shorter survival, a finding that deserves further investigation. Our novel observations further reinforces that EZH2 could be one of the therapeutic targets with clinical impact in CLL.
Methods
Patient materials
Peripheral blood mononuclear cells (PBMCs) from 70 CLL cases (36 were IGHV-mutated and 34 IGHV-unmutated), collected from the Biobank at Uppsala University Hospital, Sweden, and University Hospital Brno, Czech Republic, were included in the study. All CLL cases were diagnosed according to recently revised iwCLL criteriaCitation36 and tumor samples were obtained before treatment and had a high tumor percentage ≥70 %. The MCL samples used for 450K array analysis (i.e., 12 HP + 12 LP lymph node samples; all FACS sorted) were diagnosed according to the WHO classificationCitation37 and collected from the Biobank at Karolinska University Hospital, Huddinge, Sweden. For validation, an additional 38 MCL samples (17 HP + 21 LP lymph node samples; tumor percentage ≥70 %) were collected from the Department of Molecular Medicine, University of Pavia, Italy. All MCL samples were classified as HP and LP based on the percentage of Ki-67 staining using 25% as cutoff. Clinicobiological characteristics of the CLL and MCL cohorts are summarized in Supplementary Table 1. All patients provided informed consent in accordance with the Helsinki Declaration and the study was approved by the local ethics review boards. As normal healthy controls, CD19+ sorted B-cells were isolated from 6 age-matched healthy donors (range: 62–75 years).
Methylation arrays
Bisulfite-converted DNA prepared from sorted MCL samples was hybridized to the HumanMethylation450 (450K) BeadChip Arrays (Illumina, San Diego, CA, USA) and processed as previously detailed.Citation23
Pyrosequencing
The methylation status for the miR26A1 target region (127 bp) containing 4 CpG sites was assessed using pyrosequencing; this includes the CpG site which was shown to be differentially methylated in primary CLL and MCL samples using 450K arrays (ILMNID: cg26054057; Index: 152130). Genomic DNA was bisulfite converted using the EZ DNA Methylation Gold kit (D5005, Zymo Research, Irvine, USA) according to manufacturer's protocol.Citation38 The PyroMark™ software (Qiagen) was applied to design pyrosequencing primer sets and the primer sequences are as follows; forward primer (5′-GGTTTTAGGGTTGGGGTTAGAA-3′), reverse primer (Biotin 5′-ACCTACACAACCTATCCTAAATTACT-3′) and sequencing primer (5′-GTTGGGGTTAGAAATTTT-3′). The pyrosequencing was performed according to the manufacturer's instructions using the PyroMark™ Q24 Advanced pyrosequencer (Qiagen). The average percentage of methylation for all 4 CpG sites was calculated.
miR26A1 expression analysis using real-time quantitative PCR
Total RNA was extracted using miRNeasy Mini Kit (217004, Qiagen, Hilden, Germany) according to the manufacturer's protocol. Reverse transcription was performed using the TaqMan micro RNA transcription kit (4366596, Applied Biosystems, Foster City, USA). Real-time PCR was performed using the 7900HT Fast Real-time PCR System instrument (Applied Biosystems, Warrington, UK) using TaqMan gene expression assay for miR-26A1 (has-miR-26a1, PN 4427975, Applied Biosystems, Foster City, USA) according to manufacturer's instructions. RNU6B (PN 4440887, Applied Biosystems, Foster City, USA) was used as internal control. All samples were run in triplicates. The relative expression of miR-26A1 to RNU6B (the standard reference control miRNA) was calculated using the ΔCt method.
Cell lines and culture conditions
Four B-cell lymphoma cell lines, 2 CLL-derived (HG3Citation39 and MEC1Citation40) and 2 MCL-derived (Z138Citation41 and GRANTA 519Citation42) were cultured in RPMI 1640 (R0883, Sigma-Aldrich, St. Louis, USA) supplemented with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, USA), 1× penicillin/streptomycin (P4333, Sigma-Aldrich, St. Louis, USA) and 100 mM and 200 mM L-Glutamine (G7513, Sigma-Aldrich, St. Louis, USA) for CLL and MCL cell lines, respectively.
Overexpression of miR26A1
miR26A1 transfection was performed on CLL and MCL cell lines using custom designed miR26A1 oligo (has-miR-26a1, 4464066, Life technologies, Carlsbad, USA). The miR mimic negative (mirVana miRNA mimic negative control, 4464058, Life technologies, Carlsbad, USA) was used as negative control. Transient transfection was carried out on an Amaxa Nucleofection Device II (Nucleofector 2b device, Lonza group AG, Basel, Switzerland) according to the manufacturer's instruction. In brief, cells were split at a density of 1×106/mL in medium without antibiotics 24 h before transfection. Thereafter, 2×106 cells were collected and resuspended in 100 μL Amaxa Cell Line Nucleofector Kit V with 100 pmol of miR26A1 mimic or miR mimic negative control using the X-001 electroporation program and cells were harvested after 24-72 h of transfection depending on the downstream assay used.
DNA methyl inhibitor treatment
To investigate the effect of CpG methylation on gene expression, cells were treated with the 5'-Aza-2'-deoxycytidine (DAC) methyl inhibitor using CLL and MCL cell lines cultured in RPM1 media as described previously.Citation28 Briefly, the cultured cells were checked for viability (>98 %) and split to contain 1×106/mL. Cells were subsequently cultured over 3 d in supplemented RPMI media treated with increasing concentrations of DAC (0, 5, 10, and 20 μM DAC; A3656, Sigma-Aldrich, St. Louis, USA). DAC was changed every 24 h during treatment. Control cells were cultured in similar way but with no drugs added. We performed at least 2 independent experiments for every treatment condition on all cell lines. DNA and RNA were extracted after harvesting the cells to analyze miR26A1 methylation and expression levels by RQ-PCR and pyrosequencing, respectively.
Western blot analysis
Western blot analysis was performed using nuclear extracts of transfected CLL and MCL cell lines, which were extracted using the NE-PER Nuclear and Cytoplasmic Extraction Kit (Thermo Scientific, Rockford, USA) according to manufacturer's protocol. Equal amounts of nuclear lysates were loaded on NUPAGE 10% Bis-Tris gels (Invitrogen, Carlsbad, USA) and transferred to membranes (Amersham Hybond ECL; GE Health Care Life Sciences, Sweden). After blocking in TBS with the addition of 5% BSA, the membranes were incubated with the appropriate primary and secondary antibodies, followed by washes with TBS containing 0.05% Triton X-100. Blots were visualized with SuperSignal West Dura Extended Duration Substrate (Thermo Scientific, Rockford, USA) using the ChemiDoc XRS+ (Bio-Rad) instrument. Primary antibodies used for western blotting were: EZH2 (3147, Cell Signaling Technology, Danvers, USA) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (SC-25778; Santa Cruz Biotechnology, Dallas, USA), while the secondary antibodies were anti-mouse IgG (Cell Signaling Technology, Danvers, USA) and anti-rabbit IgG (Cell Signaling Technology, Danvers, USA).
Annexin V and EZH2 expression levels using FACS analysis
Apoptosis was measured by flow cytometry using a BD FACSAria cell sorter and analyzed using the FACSDiva version 6 software (BD Biosciences). The FITC Annexin V Apoptosis Detection Kit I (556547, BD Biosciences, New Jersey, USA) and 7-AAD (A1310, Thermo Scientific, Rockford, USA was used to measure apoptosis and cell death for transfected cells according to the manufacturer's instructions. To analyze EZH2 protein levels, cells were permeabilized with solution B (Intrasure kit, 641778, BD Biosciences, New Jersey, USA) followed by incubation with diluted EZH2 primary antibody (PA5-24594, Thermo Scientific, Rockford, USA) and APC conjugated goat anti-rabbit IgG secondary antibody (A-10931, Thermo Scientific, Rockford, USA).
Statistical analysis
Differences in gene expression and methylation levels between groups were assessed using 2-tailed Student's t-test. Patients were classified as low or high expressing and low or high methylated based on the median value. Overall survival was calculated from time of diagnosis until date of death or last follow-up. Kaplan-Meier analysis was performed to construct survival curves and log-rank test was applied to evaluate differences between subgroups. Correlation of expression level of miR26A1 and EZH2 was assessed by Spearman's rank correlation coefficient. All statistical analyses were carried out using Statistica Software version 12 (Stat Soft, Tulsa, OK, USA) and GraphPad Prism version 6 (GraphPad Software, La Jolla California, USA).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Authorship Contributions
PK and SB contributed equally to this study, performed research, analyzed the data and wrote the paper. LA, KP, SP, AW and GC performed research. LM, BS, and MP analyzed data. RR analyzed data and wrote the paper. MK was the principle investigator, performed research, analyzed data and wrote the paper.
KEPI_A_1164375_s02.pdf
Download PDF (1 MB)Acknowledgments
This study was supported by the Swedish Research Council, the Swedish Cancer Society, AGFond, “FoU Västra Götalandsregionen,” Lion's Cancer Foundation in Uppsala, the Ministry of Education, Youth and Sports of the Czech Republic under the project CEITEC 2020 (LQ1601) and the Horizon2020 Program Twinning (MEDGENET/2016-2018/no.692298).
References
- Tashkandi H, Shah N, Patel Y, Chen H. Identification of new miRNA biomarkers associated with HER2-positive breast cancers. Oncoscience 2015; 2:924-9; PMID:26697527
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009; 136:215-33; PMID:19167326; http://dx.doi.org/10.1016/j.cell.2009.01.002
- Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer research 2005; 65:7065-70; PMID:16103053; http://dx.doi.org/10.1158/0008-5472.CAN-05-1783
- Omrane I, Kourda N, Stambouli N, Privat M, Medimegh I, Arfaoui A, Uhrhammer N, Bougatef K, Baroudi O, Bouzaienne H, et al. MicroRNAs 146a and 147b biomarkers for colorectal tumor's localization. Biomed Res Int 2014; 2014:584852; PMID:24800242; http://dx.doi.org/10.1155/2014/584852
- Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A 2002; 99:15524-9; PMID:12434020; http://dx.doi.org/10.1073/pnas.242606799
- Calin GA, Liu CG, Sevignani C, Ferracin M, Felli N, Dumitru CD, Shimizu M, Cimmino A, Zupo S, Dono M, et al. MicroRNA profiling reveals distinct signatures in B cell chronic lymphocytic leukemias. Proc Natl Acad Sci U S A 2004; 101:11755-60; PMID:15284443; http://dx.doi.org/10.1073/pnas.0404432101
- Calin GA, Ferracin M, Cimmino A, Di Leva G, Shimizu M, Wojcik SE, Iorio MV, Visone R, Sever NI, Fabbri M, et al. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med 2005; 353:1793-801; PMID:16251535; http://dx.doi.org/10.1056/NEJMoa050995
- Fulci V, Chiaretti S, Goldoni M, Azzalin G, Carucci N, Tavolaro S, Castellano L, Magrelli A, Citarella F, Messina M, et al. Quantitative technologies establish a novel microRNA profile of chronic lymphocytic leukemia. Blood 2007; 109:4944-51; PMID:17327404; http://dx.doi.org/10.1182/blood-2006-12-062398
- Zhang J, Jima DD, Jacobs C, Fischer R, Gottwein E, Huang G, Lugar PL, Lagoo AS, Rizzieri DA, Friedman DR, et al. Patterns of microRNA expression characterize stages of human B-cell differentiation. Blood 2009; 113:4586-94; PMID:19202128; http://dx.doi.org/10.1182/blood-2008-09-178186
- Deng SY, Guo XX, Wang N, Wang KH, Wang S. Network analysis of microRNAs, genes and their regulation in mantle cell lymphoma. Asian Pac J Cancer Prev 2015; 16:457-63; PMID:25684471; http://dx.doi.org/10.7314/APJCP.2015.16.2.457
- Husby S, Geisler C, Gronbaek K. MicroRNAs in mantle cell lymphoma. Leuk Lymphoma 2013; 54:1867-75; PMID:23339447; http://dx.doi.org/10.3109/10428194.2013.766731
- Visone R, Pallante P, Vecchione A, Cirombella R, Ferracin M, Ferraro A, Volinia S, Coluzzi S, Leone V, Borbone E, et al. Specific microRNAs are downregulated in human thyroid anaplastic carcinomas. Oncogene 2007; 26:7590-5; PMID:17563749; http://dx.doi.org/10.1038/sj.onc.1210564
- Sander S, Bullinger L, Klapproth K, Fiedler K, Kestler HA, Barth TF, Moller P, Stilgenbauer S, Pollack JR, Wirth T. MYC stimulates EZH2 expression by repression of its negative regulator miR-26a. Blood 2008; 112:4202-12; PMID:18713946; http://dx.doi.org/10.1182/blood-2008-03-147645
- Ciarapica R, Russo G, Verginelli F, Raimondi L, Donfrancesco A, Rota R, Giordano A. Deregulated expression of miR-26a and Ezh2 in rhabdomyosarcoma. Cell Cycle 2009; 8:172-5; PMID:19106613; http://dx.doi.org/10.4161/cc.8.1.7292
- Luzi E, Marini F, Sala SC, Tognarini I, Galli G, Brandi ML. Osteogenic differentiation of human adipose tissue-derived stem cells is modulated by the miR-26a targeting of the SMAD1 transcription factor. J Bone Miner Res 2008; 23:287-95; PMID:18197755; http://dx.doi.org/10.1359/jbmr.071011
- Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt RG, Otte AP, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 2002; 419:624-9; PMID:12374981; http://dx.doi.org/10.1038/nature01075
- Kleer CG, Cao Q, Varambally S, Shen R, Ota I, Tomlins SA, Ghosh D, Sewalt RG, Otte AP, Hayes DF, et al. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci U S A 2003; 100:11606-11; PMID:14500907; http://dx.doi.org/10.1073/pnas.1933744100
- Heyn H, Esteller M. EZH2: An Epigenetic Gatekeeper Promoting Lymphomagenesis. Cancer Cell 2013; 23:563-5; PMID:23680141; http://dx.doi.org/10.1016/j.ccr.2013.04.028
- Lu J, He ML, Wang L, Chen Y, Liu X, Dong Q, Chen YC, Peng Y, Yao KT, Kung HF, et al. MiR-26a inhibits cell growth and tumorigenesis of nasopharyngeal carcinoma through repression of EZH2. Cancer Res 2011; 71:225-33; PMID:21199804; http://dx.doi.org/10.1158/0008-5472.CAN-10-1850
- Dang X, Ma A, Yang L, Hu H, Zhu B, Shang D, Chen T, Luo Y. MicroRNA-26a regulates tumorigenic properties of EZH2 in human lung carcinoma cells. Cancer Genet 2012; 205:113-23; PMID:22469510; http://dx.doi.org/10.1016/j.cancergen.2012.01.002
- Zhao JJ, Lin J, Lwin T, Yang H, Guo J, Kong W, Dessureault S, Moscinski LC, Rezania D, Dalton WS, et al. microRNA expression profile and identification of miR-29 as a prognostic marker and pathogenetic factor by targeting CDK6 in mantle cell lymphoma. Blood 2010; 115:2630-9; PMID:20086245; http://dx.doi.org/10.1182/blood-2009-09-243147
- Zhang X, Zhao X, Fiskus W, Lin J, Lwin T, Rao R, Zhang Y, Chan JC, Fu K, Marquez VE, et al. Coordinated silencing of MYC-mediated miR-29 by HDAC3 and EZH2 as a therapeutic target of histone modification in aggressive B-Cell lymphomas. Cancer Cell 2012; 22:506-23; PMID:23079660; http://dx.doi.org/10.1016/j.ccr.2012.09.003
- Cahill N, Bergh AC, Kanduri M, Goransson-Kultima H, Mansouri L, Isaksson A, Ryan F, Smedby KE, Juliusson G, Sundstrom C, et al. 450K-array analysis of chronic lymphocytic leukemia cells reveals global DNA methylation to be relatively stable over time and similar in resting and proliferative compartments. Leukemia 2013; 27:150-8; PMID:22922567; http://dx.doi.org/10.1038/leu.2012.245
- Damle RN, Wasil T, Fais F, Ghiotto F, Valetto A, Allen SL, Buchbinder A, Budman D, Dittmar K, Kolitz J, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood 1999; 94:1840-7; PMID:10477712
- Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood 1999; 94:1848-54; PMID:10477713
- Yu L, Lu J, Zhang B, Liu X, Wang L, Li SY, Peng XH, Xu X, Tian WD, Li XP. miR-26a inhibits invasion and metastasis of nasopharyngeal cancer by targeting EZH2. Oncol Lett 2013; 5:1223-8; PMID:23599767; http://dx.doi.org/10.3892/ol.2013.1173
- Wang G, Sun Y, He Y, Ji C, Hu B, Sun Y. miR-26a promoted by interferon-α inhibits hepatocellular carcinoma proliferation and migration by blocking EZH2. Genet Test Mol Biomarkers 2015; 19:30-6; PMID:25494962; http://dx.doi.org/10.1089/gtmb.2014.0245
- Kanduri M, Cahill N, Goransson H, Enstrom C, Ryan F, Isaksson A, Rosenquist R. Differential genome-wide array-based methylation profiles in prognostic subsets of chronic lymphocytic leukemia. Blood 2010; 115:296-305; PMID:19897574; http://dx.doi.org/10.1182/blood-2009-07-232868
- Kanduri M, Marincevic M, Halldorsdottir AM, Mansouri L, Junevik K, Ntoufa S, Kultima HG, Isaksson A, Juliusson G, Andersson PO, et al. Distinct transcriptional control in major immunogenetic subsets of chronic lymphocytic leukemia exhibiting subset-biased global DNA methylation profiles. Epigenetics 2012; 7:1435-42; PMID:23154584; http://dx.doi.org/10.4161/epi.22901
- Halldorsdottir AM, Kanduri M, Marincevic M, Mansouri L, Isaksson A, Goransson H, Axelsson T, Agarwal P, Jernberg-Wiklund H, Stamatopoulos K, et al. Mantle cell lymphoma displays a homogenous methylation profile: a comparative analysis with chronic lymphocytic leukemia. Am J Hematol 2012; 87:361-7; PMID:22374828; http://dx.doi.org/10.1002/ajh.23115
- Kulis M, Heath S, Bibikova M, Queiros AC, Navarro A, Clot G, Martinez-Trillos A, Castellano G, Brun-Heath I, Pinyol M, et al. Epigenomic analysis detects widespread gene-body DNA hypomethylation in chronic lymphocytic leukemia. Nat Genet 2012; 44:1236-42; PMID:23064414; http://dx.doi.org/10.1038/ng.2443
- Oakes CC, Seifert M, Assenov Y, Gu L, Przekopowitz M, Ruppert AS, Wang Q, Imbusch CD, Serva A, Koser SD, et al. DNA methylation dynamics during B cell maturation underlie a continuum of disease phenotypes in chronic lymphocytic leukemia. Nat Genet 2016; PMID:26780610; http://dx.doi.org/10.1038/ng.3488
- Bracken AP, Pasini D, Capra M, Prosperini E, Colli E, Helin K. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J 2003; 22:5323-35; PMID:14532106; http://dx.doi.org/10.1093/emboj/cdg542
- Yu J, Rhodes DR, Tomlins SA, Cao X, Chen G, Mehra R, Wang X, Ghosh D, Shah RB, Varambally S, et al. A polycomb repression signature in metastatic prostate cancer predicts cancer outcome. Cancer Res 2007; 67:10657-63; PMID:18006806; http://dx.doi.org/10.1158/0008-5472.CAN-07-2498
- Kanduri M, Sander B, Ntoufa S, Papakonstantinou N, Sutton LA, Stamatopoulos K, Kanduri C, Rosenquist R. A key role for EZH2 in epigenetic silencing of HOX genes in mantle cell lymphoma. Epigenetics 2013; 8:1280-8; PMID:24107828; http://dx.doi.org/10.4161/epi.26546
- Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, Hillmen P, Keating MJ, Montserrat E, Rai KR, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008; 111:5446-56; PMID:18216293; http://dx.doi.org/10.1182/blood-2007-06-093906
- Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood 2011; 117:5019-32; PMID:21300984; http://dx.doi.org/10.1182/blood-2011-01-293050
- Martinelli S, Kanduri M, Maffei R, Fiorcari S, Bulgarelli J, Marasca R, Rosenquist R. ANGPT2 promoter methylation is strongly associated with gene expression and prognosis in chronic lymphocytic leukemia. Epigenetics 2013; 8:720-9; PMID:23803577; http://dx.doi.org/10.4161/epi.24947
- Rosen A, Bergh AC, Gogok P, Evaldsson C, Myhrinder AL, Hellqvist E, Rasul A, Bjorkholm M, Jansson M, Mansouri L, et al. Lymphoblastoid cell line with B1 cell characteristics established from a chronic lymphocytic leukemia clone by in vitro EBV infection. Oncoimmunology 2012; 1:18-27; PMID:22720208; http://dx.doi.org/10.4161/onci.1.1.18400
- Stacchini A, Aragno M, Vallario A, Alfarano A, Circosta P, Gottardi D, Faldella A, Rege-Cambrin G, Thunberg U, Nilsson K, et al. MEC1 and MEC2: two new cell lines derived from B-chronic lymphocytic leukaemia in prolymphocytoid transformation. Leukemia Res 1999; 23:127-36; PMID:10071128; http://dx.doi.org/10.1016/S0145-2126(98)00154-4
- Estrov Z, Talpaz M, Ku S, Harris D, Van Q, Beran M, Hirsch-Ginsberg C, Huh Y, Yee G, Kurzrock R. Z-138: a new mature B-cell acute lymphoblastic leukemia cell line from a patient with transformed chronic lymphocytic leukemia. Leukemia Res 1998; 22:341-53; PMID:9669839; http://dx.doi.org/10.1016/S0145-2126(97)00191-4
- Jadayel DM, Lukas J, Nacheva E, Bartkova J, Stranks G, De Schouwer PJ, Lens D, Bartek J, Dyer MJ, Kruger AR, et al. Potential role for concurrent abnormalities of the cyclin D1, p16CDKN2 and p15CDKN2B genes in certain B cell non-Hodgkin's lymphomas. Functional studies in a cell line (Granta 519). Leukemia 1997; 11:64-72; http://dx.doi.org/10.1038/sj.leu.2400555
