ABSTRACT
Breastmilk has many documented beneficial effects on the developing human infant, but the components of breastmilk that influence these developmental pathways have not been fully elucidated. Increasing evidence suggests that non-coding RNAs encapsulated in extracellular vesicles (EVs) represent an important mechanism of communication between the mother and child. Long non-coding RNAs (lncRNAs) are of particular interest given their key role in gene expression and development. However, it is not known whether breastmilk EVs contain lncRNAs. We used qRT-PCR to determine whether EVs isolated from human breastmilk contain lncRNAs previously reported to be important for developmental processes. We detected 55 of the 87 screened lncRNAs in EVs from the 30 analyzed breastmilk samples, and CRNDE, DANCR, GAS5, SRA1 and ZFAS1 were detected in >90% of the samples. GAS5, SNHG8 and ZFAS1 levels were highly correlated (Spearman's rho > 0.9; P < 0.0001), which may indicate that the loading of these lncRNAs into breastmilk EVs is regulated by the same pathways. The detected lncRNAs are important epigenetic regulators involved in processes such as immune cell regulation and metabolism. They may target a repertoire of recipient cells in offspring and could be essential for child development and health. Further experimental and epidemiological studies are warranted to determine the impact of breastmilk EV-encapsulated lnRNAs in mother to child signaling.
Abbreviations
| Ct | = | cycle threshold |
| EVs | = | extracellular vesicles |
| lncRNAs | = | long non-coding RNAs |
| miRNAs | = | microRNAs |
| TEM | = | transmission electron microscopy |
Introduction
Breastmilk has many beneficial effects on the developing human infantCitation1,2, including decreased risk of sudden infant death syndrome,Citation3-5 reduced neonatal infections,Citation5-9 more optimal metabolic development throughout childhood,Citation10-13 reduced cancer risk,Citation5,14 and better cognitive outcomes.Citation15 In addition, some epidemiologic studies suggest that breastfeeding protects against asthma and allergies in offspring, especially when there is a family history for allergic rhinitis,Citation16 atopic allergies,Citation17 and asthmaCitation5; although the protective effect is contested in breastfed infants.Citation18,19 The specific components of breastmilk influencing developmental processes in infants are not fully elucidated.
Breastmilk contains a diverse mixture of components including nutrients, milk fat globules, hormones, growth factors, immune component cells, antibodies, cytokines, antimicrobial peptides, and extracellular vesicles (EVs) that may play a role in infant development.Citation1,20, 21 Increasing attention has been given to EVs, which are small double-lipid membrane vesicles that are released into the extracellular environment from a variety of cells, enabling cell-cell communications through specific interaction with target recipient cells.Citation22-24 EVs are found in a number of body fluids including blood, urine, saliva, and amniotic fluid, as well as breastmilk.Citation25 Over 4,400 different proteins, thought to be implicated in intracellular communication, have been identified in EVs.Citation24,26 In addition, EVs contain notable amounts of microRNAs (miRNAs), other small non-coding RNAs, and messenger RNAs (mRNAs).Citation26,27
The RNA cargo in EVs does not simply reflect the RNA composition of the cell of origin, indicating a selective loading of RNAs into EVs.Citation28 Because of this, and the observation that transmitted RNAs can function in the recipient cell, it has been suggested that EV-encapsulated RNAs might act as gene expression regulators in the target cell.Citation27-30 There is emerging evidence showing that EV-encapsulated RNAs can regulate pathways related to cellular growth, division, survival, differentiation, stress responses, and apoptosis.Citation24 In comparison to other body fluids, breastmilk was recently found to contain the highest concentration of total RNAs.Citation31 Studies suggest that bovine and human milk transfer substantial amounts of miRNA of functional importance for infant development by EV transport,Citation32-34 and that immune-related miRNAs are enriched in breastmilk EVs.Citation35,36 This, together with studies demonstrating that the EVs lipid membrane helps to protect milk-derived RNAs against degradation, suggest an important function of EV-encapsulated RNA in cell-cell communication from mother to child.Citation34,37 Although EVs contain large amounts of RNAs, miRNAs and mRNAs have so far been the primary focus of research,Citation27,38,39 recent reports show that long non-coding RNAs (lncRNAs) could also be present in EVs.Citation39-42
LncRNAs belong to a novel, heterogeneous class of non-coding RNAs, defined as transcribed RNA molecules greater than 200 nucleotides in length with little or no protein-coding capability.Citation43 They are often expressed at low levels and generally found to be more cell type specific than the expression of protein-coding genes.Citation44-46 Although lncRNAs are implicated in gene expression regulation during developmental and differentiation processes,Citation46-48 the exact mechanisms are not yet fully understood.Citation43,49-51 To date, only a few functional lncRNAs have been well characterized, and they have been demonstrated to control every level of gene regulation.Citation52 For example, lncRNAs are involved in post-transcriptional gene regulation through controlling processes like protein synthesis, RNA maturation and transport, and transcriptional gene silencing via regulation of the chromatin structure and DNA methylation.Citation49,53-55 However, it is currently unknown whether human breastmilk EVs contain lncRNAs.
The aim of this study was to determine whether EVs isolated from human breastmilk contain lncRNAs previously reported to be important for developmental processes, using a custom real time PCR array focused on 87 developmentally related lncRNAs. We consistently detected 5 of the 87 screened lncRNAs—CRNDE, DANCR GAS5, SRA1, and ZFAS1— in the breastmilk EVs of the 30 participating women, while several of the other measured lncRNAs were detected in some of the samples.
Results
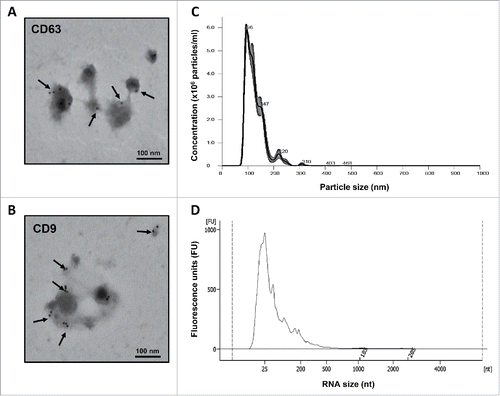
Preparations of intact EVs isolated from breastmilk using the exoEasy Maxi Kit exhibited the characteristic morphology and diameter size under transmission electron microscopy (TEM), and were labeled positive for 2 common surface markers, CD63 and CD9 (). Additional nanoparticle tracking analysis revealed a size distribution consistent with that of EVs with mean diameter size of ∼100 nm and no contamination from particles with >500 nm diameter, such as apoptotic bodies and larger aggregates (). Following total RNA extraction from the isolated EVs, the Bioanalyzer's electropherograms consistently demonstrated the presence of both short (20–35 nucleotides) and long RNAs (>200 nucleotides) and the absence of any cellular RNA contamination ().
Figure 1. Morphological characterization of breastmilk EVs and size distribution of their RNA cargo. (A) EVs immune-gold labeled by anti-CD63 antibody. (B) EVs immune-gold labeled by anti-CD9 antibody. (C) Concentration and size distribution of EVs in breastmilk by nanoparticle tracking analysis using the NanoSight NS300. (D) Size distribution of EV-encapsulated RNA showing the presence of lncRNAs, as measured by Agilent 2100 Bioanalyzer. Transmission electron microscopy images for (A) and (B) were taken by a JEOL 1200EX microscope. Arrows indicate positive staining.
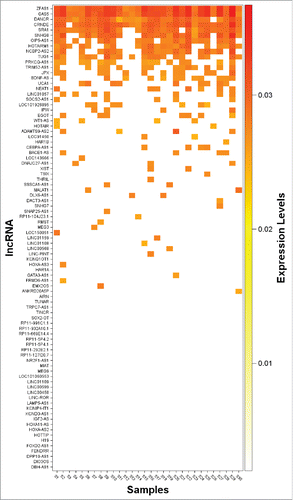
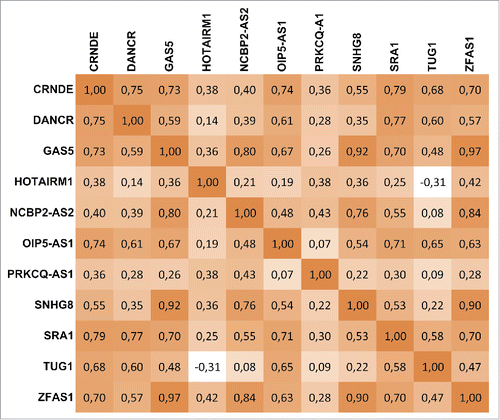
The real-time qRT-PCR analysis revealed the presence of 55 of the 87 screened lncRNAs in EVs from at least one of the analyzed individual breastmilk samples (n = 30). The expression heat map shows all detected lncRNAs in the individual samples (), and 11 lncRNAs—CRNDE, DANCR, GAS5, HOTAIRM1, NCBP2-AS2, OIP5-AS1, PRKCQ-AS1, SNHG8, SRA1, TUG1, and ZFAS1—were present in more than 50% of the samples. Of these, 5—CRNDE, DANCR, GAS5, SRA1, and ZFAS1—were detected in 90–100% of the breastmilk samples (). The cycle threshold (Ct) values ± standard deviation (SD) are shown in Supplemental Table 1. Other notable lncRNAs, such as JPX and NEAT1, were also detected, but in lower frequency (). displays the expression correlations between the 11 most consistently detected lncRNAs. We found that GAS5, SNHG8, and ZFAS1 were highly correlated (Spearman's rho > 0.9; P < 0.0001), and that others such as CRNDE, DANCR, and SRA1 were relatively highly correlated (0.75 < Spearman's rho < 0.79; P < 0.0001). Overall, the technical variability was low, and the coefficient of variance (CV) for the 5 most consistently detected lncRNAs—CRNDE, DANCR, GAS5, SRA1, and ZFAS1—was between 0.65 and 1.53%.
Discussion
To the best of our knowledge, this is the first report demonstrating the presence of lncRNAs in EVs isolated from human breastmilk. This finding raises the exciting possibility that maternal EV-encapsulated lncRNAs might provide signals that regulate gene expression events in the developing infant. Notably, several of the detected lncRNAs have previously been related to diseases that are relevant to children's development and health including allergic disorders, asthma, obesity and autoimmune disease (Supplemental Table 2).
Essentially, all cells release EVs and it is currently unknown whether breastmilk EVs originate from cells present in the milk, epithelial mammary gland cells, or cells present elsewhere in the body. Previous studies have indicated a selective loading of RNA into EVs.Citation28 Therefore, the high correlation between the GAS5, SNHG8, and ZFAS1 levels may indicate that the packing of these lncRNAs into EVs is regulated by the same pathways. One advantage of EVs as mediators of intercellular communication is that the message can be targeted, through specific surface markers or adhesion molecules, to specific, multiple locations that might be widely separated locations within a tissue or between different tissues.Citation24,56, 57 In this regard, EVs are similar to the paracrine and endocrine systems of communication. It has been suggested that milk-derived EVs may pass the intestinal barrier and reach the systemic circulation.Citation34,37, 58 For example, a recent study has shown that humans absorb miRNAs from cow's milk that are sufficient to alter human gene expression in blood mononuclear cells.Citation59 Furthermore, studies in mice have demonstrated systemic uptake and distribution to various organs after intraperitoneal injection of labeled EVs.Citation60 The delivery of lncRNAs through breastmilk EVs could therefore allow for rapid alterations in gene expression in a repertoire of recipient cells and play an important role in infant development (). However, the present study is limited to breastmilk samples from 30 mothers. Further experimental and epidemiological studies are needed to determine the role of the EV-encapsulated lncRNAs in child growth and health.
Figure 4. The role of breastmilk extracellular vesicle (EV) long non-coding RNA (lncRNA) in maternal-infant cell-cell communication: A conceptual model. [Left] Human breastmilk contains a mixture of diverse components, including EVs loaded with maternal lncRNAs. [Right] The EV-encapsulated lncRNAs, e.g. CRNDE, DANCR, GAS5, SRA1, and ZFAS1, which are involved in processes such as cell cycle control, apoptosis, immune cell regulation, steroid hormone signaling, adipogenesis, glucose, and lipid metabolism, can be delivered to the infant and have direct effects in the gastrointestinal tract. Absorption through intestinal epithelial cells is also thought to occur, allowing breastmilk-derived lncRNAs to reach various organs and a repertoire of cells via the systemic circulation of the infant and potentially perform functions, such as developmental programming and immunoprotection.
![Figure 4. The role of breastmilk extracellular vesicle (EV) long non-coding RNA (lncRNA) in maternal-infant cell-cell communication: A conceptual model. [Left] Human breastmilk contains a mixture of diverse components, including EVs loaded with maternal lncRNAs. [Right] The EV-encapsulated lncRNAs, e.g. CRNDE, DANCR, GAS5, SRA1, and ZFAS1, which are involved in processes such as cell cycle control, apoptosis, immune cell regulation, steroid hormone signaling, adipogenesis, glucose, and lipid metabolism, can be delivered to the infant and have direct effects in the gastrointestinal tract. Absorption through intestinal epithelial cells is also thought to occur, allowing breastmilk-derived lncRNAs to reach various organs and a repertoire of cells via the systemic circulation of the infant and potentially perform functions, such as developmental programming and immunoprotection.](/cms/asset/74c9f630-d371-469c-a45d-bd226ffee80b/kepi_a_1216285_f0004_oc.gif)
Research indicates that the lncRNAs GAS5, SRA1, and CRNDE all have important functions in metabolic regulation. GAS5 is induced under conditions of nutrient deprivation and cellular stress, and has pro-apoptotic functions.Citation61-63 GAS5 acts as a glucocorticoid receptor (GR) decoy and increased levels can efficiently inhibit trans-activation of GR-dependent gene promoters.Citation61 In addition, GAS5 appears to repress the effects of other steroid hormone receptors and may have a role in saving energy resources as an adaptive response to starvation by restricting the expression of steroid-responsive genes.Citation61-63 SRA1 serves as a co-activator for a number of nuclear steroid receptors.Citation64,65 Additionally, SRA1 is required for full transactivation of the pro-adipogenic transcription factor peroxisome proliferator-associated receptor gamma (PPARγ) and is therefore important for the differentiation of adipose tissue.Citation66 Microarray analysis reveals hundreds of SRA-responsive genes in adipocytes, including genes involved in the cell cycle, and insulin and TNFα signaling pathways.Citation66 CRNDE interacts with chromatin-modifying complexes and appears to play an important role in regulation of cell differentiation or pluripotency during development.Citation67-69 CRDNE is responsive to insulin and insulin-like growth factor signaling, and regulates genes central for cellular metabolism.Citation67 Hence, the transfer of these 3 lncRNAs, which were detected in EVs from almost all our breastmilk samples, as well as other lncRNAs, from the mother to the infant, could have important implications for the control of infant metabolism and metabolic programming.
Breastmilk also plays an important role in the development of the child's immune system.Citation70,71 A potential role for milk-derived EVs in immune modulation was first suggested by Admyre et al., who found human breastmilk-derived EVs to facilitate regulatory T-cell induction.Citation72 The immune modulatory features of EVs have been suggested to be due to their protein and miRNA contents.Citation36,72,73 Recent publications have shown widespread changes in the expression of lncRNAs during the activation of the innate immune response and T-cell development, differentiation, and activation.Citation74 Our detection of specific lncRNAs in human breastmilk EVs indicates that lncRNAs could also be important for the programming of the neonatal immune system. For instance, GAS5 plays an essential role in apoptosis and normal growth arrest in T-cells,Citation75 while ZFAS1 appears to have an important role in cell cycle control.Citation76 The growth control of lymphocyte populations is crucial to the physiological regulation of the immune system and to the prevention of both leukemia and autoimmune disease. SRA1 may also be important for the infant immune system as it regulates genes in the TNFα signaling pathway.Citation66 In addition, DANCR has been demonstrated to control the expression of IL6 and TNF-α in blood mononuclear cells.Citation77
In summary, lncRNAs are important epigenetic regulators of physiological processes and their presence in human breastmilk EVs provide a novel mechanism to better understand communication between the mother and the child. Our findings may have important implications for early child development, as the identified lncRNAs are known to be involved in processes such as immune cell regulation, adipogenesis, and metabolism. Further experimental and epidemiological studies are warranted to determine factors that influence the lncRNA cargo in EVs, clarify functions and elucidate their impact on infant development and long-term health in children.
Materials and methods
Breastmilk collection
Participants were enrolled as part of the Perinatal Environment & Development Study (PEDS), a prospective pregnancy cohort designed to examine environmental influences on perinatal programming of cardiometabolic, respiratory, and neurodevelopmental outcomes in children. Breastmilk was collected from mothers (n = 30) within the first 2 months postpartum (32.0 ± 14.9 d postpartum, mean ± SD) during their visit at the prenatal clinic at the Mount Sinai Obstetrics & Gynecology Practice in New York City. Almost all the women were from ethnic minorities (16 Hispanic, 13 African American, 1 Caucasian), most had greater than a high school education (n = 20, 66.6%) and were aged 27.3 ± 6.0 y. Research procedures were approved by the human studies committee at the Icahn School of Medicine at Mount Sinai. Written informed consent was obtained from all participants, and the experiments were carried out in accordance with the approved guidelines.
For the collection of breastmilk, participants washed their breast and used a manual pump to collect 3–10 ml of breastmilk before the first morning feed from a single breast. Samples were kept at 4°C until transport to the laboratory the same day. Upon arrival, samples were centrifuged (3,000 × g at 4°C for 15 min) to remove fat globules and cells, and the supernatant was stored in 1 ml aliquots at −80°C until further analysis.
EVs isolation and RNA extraction
At time of assay, aliquots were thawed on ice and centrifuged a second time at 3,000 × g for 15 min at 4°C, to remove residual fat globules and any remaining cell debris. The supernatant was carefully aspirated, mixed with 2 volumes of filtered (0.1-μm membrane) phosphate-buffered saline (PBS), and then filtered using a 0.8-μm membrane unit (Millipore Corp., Bedford, MA) to remove any remaining cell debris and large aggregates. The exoRNeasy Serum/Plasma Maxi Kit (Qiagen, Valencia, CA) was then used to extract EV-encapsulated total RNA following the manufacturer's instructions.Citation78 To evaluate the performance of this kit with breastmilk, we used the exoEasy Maxi Kit (Qiagen, Valencia, CA), which utilizes the same EVs isolation principle, but enables the collection of intact EVs. Upon isolation of intact EVs from 2 replicate aliquots, we used the NanoSight NS300 (Malvern Instruments, Westborough, MA) to measure their size distribution and numbers, and TEM to characterize their morphology. To evaluate the quality, yield, and RNA size distribution of the total RNA extracted from breastmilk EVs, we analyzed all samples by capillary electrophoresis and the Agilent RNA 6000 Nano Kit following manufacturer's protocol (Agilent 2100 Bioanalyzer, Agilent Technologies, Foster City, CA).
Size distribution and concentration of EVs by nanoparticle tracking analysis
The size distribution and concentration of isolated EVs from breastmilk were analyzed using the NanoSight NS300. In brief, EV samples were diluted 1:1000 with PBS and the pump was washed several times with PBS prior to measurement. The settings for the camera were adjusted according to the manufacturer's instructions and samples were analyzed in 5 repeats for 60 seconds.
Transmission electron microscopy
To characterize the morphology of isolated EVs, we used TEM. For negative staining, samples were diluted in water, adsorbed onto formvar/carbon coated grid, and stained with 1% uranyl acetate. Last, samples were dried and immediately examined using TEM. For immune-gold labeling for CD63 and CD9 surface markers, we followed the protocol described by Théry et al.Citation79 A JEOL 1200EX microscope coupled with an AMT 2 k CCD camera was used for imaging, and all work was performed at the Harvard Medical School Electron Microscopy Core.
Real-time quantitative reverse transcription PCR
We measured the levels of EV-encapsulated lncRNAs using a custom 384-well RT2 lncRNA PCR Array (Cat. no. CLAH00013; Qiagen). The panel, consisting of 87 lncRNAs, was based on the human cell development and differentiation RT2 lncRNA PCR array (Cat. no. LAHS-003Z; Qiagen,) that includes 84 differentially expressed lncRNAs during cellular differentiation and organism development, with 3 additional developmentally important lncRNAs: THRIL, PINT, and SRA1. This array allows accurate analysis of the most relevant lncRNAs to cell fate and cell lineage decisions using laboratory-verified SYBR® Green qPCR assays, and includes a set of controls that enable assessment of genomic DNA contamination, reverse transcription performance, and PCR performance. RNA (8.5 ng) was reverse transcribed using the RT2 First Strand Kit (Qiagen, Valencia, CA) according to the provided protocol that includes a genomic DNA elimination step to remove any residual contamination in the samples. Due to lower RNA extraction yield, we used reduced starting amounts for 3 samples (7.0, 8.2, 6.1 ng). The synthesized cDNA was mixed with RT2 SYBR Green Mastermix, and automatically aliquoted to the wells of the lncRNA PCR plate using a Microlab STARlet Liquid Handling Workstation (Hamilton, Franklin, MA). Real-time qRT-PCR was performed on a Bio-Rad CFX384 instrument with an initial HotStartTaq DNA polymerase activation step at 95°C for 10 min, followed by 40 cycles of 15 s at 95°C, and 1 min at 60°C.
Data analysis
Standard descriptive statistics were used to quantify the expression levels of detected lncRNAs in human breastmilk EVs (reported as mean ± SD, min, and max Ct values). Spearman's Rank Correlation test was used to calculate the correlation between lncRNAs that were detected in ≥50 % of the analyzed samples. For the expression heat map, raw Ct values were transformed using the inverse formula 1/Ct and ranked from high to low, based on their transformed expression levels and frequency of detection among all analyzed samples. Statistical analyses were conducted in SAS 9.4 (SAS Institute Inc., Cary, NC).
Disclosure of potential conflicts of interest
The authors declare no conflict of interest or competing financial interests.
KEPI_A_1216285_s02.docx
Download MS Word (32.6 KB)Funding
This initiative in the Perinatal Environment & Development Study (PEDS) was funded by an Empire Clinical Research Investigator Program (ECRIP) Award (R.J.W., R.O.W.); and protocol development for breastmilk collection was funded by P30 ES023515 (R.O.W., R.J.W.). The study was also supported by grants from the Swedish Research Council Formas, Fredrik & Ingrid Thuring and Facias Foundations (O.K.).
References
- Dieterich CM, Felice JP, O'Sullivan E, Rasmussen KM. Breastfeeding and health outcomes for the mother-infant dyad. Pediatr Clin North Am 2013; 60:31-48; PMID:23178059; http://dx.doi.org/10.1016/j.pcl.2012.09.010
- Goldman AS. Evolution of immune functions of the mammary gland and protection of the infant. Breastfeed Med 2012; 7:132-42; PMID:22577734; http://dx.doi.org/10.1089/bfm.2012.0025
- Vennemann MM, Bajanowski T, Brinkmann B, Jorch G, Yucesan K, Sauerland C, Mitchell EA, Ge SIDSG. Does breastfeeding reduce the risk of sudden infant death syndrome? Pediatrics 2009; 123:e406-10; PMID:19254976; http://dx.doi.org/10.1542/peds.2008-2145
- Hauck FR, Thompson JM, Tanabe KO, Moon RY, Vennemann MM. Breastfeeding and reduced risk of sudden infant death syndrome: a meta-analysis. Pediatrics 2011; 128:103-10; PMID:21669892; http://dx.doi.org/10.1542/peds.2010-3000
- Ip S, Chung M, Raman G, Trikalinos TA, Lau J. A summary of the Agency for Healthcare Research and Quality's evidence report on breastfeeding in developed countries. Breastfeed Med 2009; 4 Suppl 1:S17-30; PMID:19827919; http://dx.doi.org/10.1089/bfm.2009.0050
- Kramer MS, Chalmers B, Hodnett ED, Sevkovskaya Z, Dzikovich I, Shapiro S, Collet JP, Vanilovich I, Mezen I, Ducruet T, et al. Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA 2001; 285:413-20; PMID:11242425; http://dx.doi.org/10.1001/jama.285.4.413
- Chien PF, Howie PW. Breast milk and the risk of opportunistic infection in infancy in industrialized and non-industrialized settings. Adv Nutr Res 2001; 10:69-104; PMID:11795054; http://dx.doi.org/10.1007/978-1-4615-0661-4_4
- Bachrach VR, Schwarz E, Bachrach LR. Breastfeeding and the risk of hospitalization for respiratory disease in infancy: a meta-analysis. Arch Pediatr Adolesc Med 2003; 157:237-43; PMID:12622672; http://dx.doi.org/10.1001/archpedi.157.3.237
- Duijts L, Jaddoe VW, Hofman A, Moll HA. Prolonged and exclusive breastfeeding reduces the risk of infectious diseases in infancy. Pediatrics 2010; 126:e18-25; PMID:20566605; http://dx.doi.org/10.1542/peds.2008-3256
- Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Effect of infant feeding on the risk of obesity across the life course: a quantitative review of published evidence. Pediatrics 2005; 115:1367-77; PMID:15867049; http://dx.doi.org/10.1542/peds.2004-1176
- Arenz S, Ruckerl R, Koletzko B, von Kries R. Breast-feeding and childhood obesity–a systematic review. Int J Obes Relat Metab Disor 2004; 28:1247-56; PMID:15314625; http://dx.doi.org/10.1038/sj.ijo.0802758
- Harder T, Bergmann R, Kallischnigg G, Plagemann A. Duration of breastfeeding and risk of overweight: a meta-analysis. Am J Epidemiol 2005; 162:397-403; PMID:16076830; http://dx.doi.org/10.1093/aje/kwi222
- Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Does breastfeeding influence risk of type 2 diabetes in later life? A quantitative analysis of published evidence. Am J Clin Nutr 2006; 84:1043-54; PMID:17093156
- Kwan ML, Buffler PA, Abrams B, Kiley VA. Breastfeeding and the risk of childhood leukemia: a meta-analysis. Public health reports 2004; 119:521-35; PMID:15504444; http://dx.doi.org/10.1016/j.phr.2004.09.002
- Kramer MS, Aboud F, Mironova E, Vanilovich I, Platt RW, Matush L, Igumnov S, Fombonne E, Bogdanovich N, Ducruet T, et al. Breastfeeding and child cognitive development: new evidence from a large randomized trial. Arch Gen Psychiatry 2008; 65:578-84; PMID:18458209; http://dx.doi.org/10.1001/archpsyc.65.5.578
- Mimouni Bloch A, Mimouni D, Mimouni M, Gdalevich M. Does breastfeeding protect against allergic rhinitis during childhood? A meta-analysis of prospective studies. Acta Paediatr 2002; 91:275-9; PMID:12022298; http://dx.doi.org/10.1111/j.1651-2227.2002.tb01714.x
- Gdalevich M, Mimouni D, David M, Mimouni M. Breast-feeding and the onset of atopic dermatitis in childhood: a systematic review and meta-analysis of prospective studies. J Am Acad Dermatol 2001; 45:520-7; PMID:11568741; http://dx.doi.org/10.1067/mjd.2001.114741
- Sears MR, Greene JM, Willan AR, Taylor DR, Flannery EM, Cowan JO, Herbison GP, Poulton R. Long-term relation between breastfeeding and development of atopy and asthma in children and young adults: a longitudinal study. Lancet 2002; 360:901-7; PMID:12354471; http://dx.doi.org/10.1016/S0140-6736(02)11025-7
- Wegienka G, Ownby DR, Havstad S, Williams LK, Johnson CC. Breastfeeding history and childhood allergic status in a prospective birth cohort. Ann Allergy Asthma Immunol 2006; 97:78-83; PMID:16892786; http://dx.doi.org/10.1016/S1081-1206(10)61374-9
- Savino F, Liguori SA. Update on breast milk hormones: leptin, ghrelin and adiponectin. Clin Nutr 2008; 27:42-7; PMID:17950501; http://dx.doi.org/10.1016/j.clnu.2007.06.006
- Armogida SA, Yannaras NM, Melton AL, Srivastava MD. Identification and quantification of innate immune system mediators in human breast milk. Allergy Asthma Proc 2004; 25:297-304; PMID:15603202
- Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 2009; 9:581-93; PMID:19498381; http://dx.doi.org/10.1038/nri2567
- Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics 2010; 73:1907-20; PMID:20601276; http://dx.doi.org/10.1016/j.jprot.2010.06.006
- Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta 2012; 1820:940-8; PMID:22503788; http://dx.doi.org/10.1016/j.bbagen.2012.03.017
- Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell vesicles 2015; 4:27066; PMID:25979354; http://dx.doi.org/10.3402/jev.v4.27066
- Mathivanan S, Simpson RJ. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics 2009; 9:4997-5000; PMID:19810033; http://dx.doi.org/10.1002/pmic.200900351
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007; 9:654-9; PMID:17486113; http://dx.doi.org/10.1038/ncb1596
- Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr., Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 2008; 10:1470-6; PMID:19011622; http://dx.doi.org/10.1038/ncb1800
- Lotvall J, Valadi H. Cell to cell signalling via exosomes through esRNA. Cell Adh Migr 2007; 1:156-8; PMID:19262134; http://dx.doi.org/10.4161/cam.1.3.5114
- Ramachandran S, Palanisamy V. Horizontal transfer of RNAs: exosomes as mediators of intercellular communication. Wiley Interdiscip Rev RNA 2012; 3:286-93; PMID:22012863; http://dx.doi.org/10.1002/wrna.115
- Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, Galas DJ, Wang K. The microRNA spectrum in 12 body fluids. Clin Chem 2010; 56:1733-41; PMID:20847327; http://dx.doi.org/10.1373/clinchem.2010.147405
- Hata T, Murakami K, Nakatani H, Yamamoto Y, Matsuda T, Aoki N. Isolation of bovine milk-derived microvesicles carrying mRNAs and microRNAs. Biochem Biophys Res Commun 2010; 396:528-33; PMID:20434431; http://dx.doi.org/10.1016/j.bbrc.2010.04.135
- Chen X, Gao C, Li H, Huang L, Sun Q, Dong Y, Tian C, Gao S, Dong H, Guan D, et al. Identification and characterization of microRNAs in raw milk during different periods of lactation, commercial fluid, and powdered milk products. Cell Res 2010; 20:1128-37; PMID:20548333; http://dx.doi.org/10.1038/cr.2010.80
- Izumi H, Kosaka N, Shimizu T, Sekine K, Ochiya T, Takase M. Bovine milk contains microRNA and messenger RNA that are stable under degradative conditions. J Dairy Sci 2012; 95:4831-41; PMID:22916887; http://dx.doi.org/10.3168/jds.2012-5489
- Kosaka N, Izumi H, Sekine K, Ochiya T. microRNA as a new immune-regulatory agent in breast milk. Silence 2010; 1:7; PMID:20226005; http://dx.doi.org/10.1186/1758-907X-1-7
- Zhou Q, Li M, Wang X, Li Q, Wang T, Zhu Q, Zhou X, Wang X, Gao X, Li X. Immune-related microRNAs are abundant in breast milk exosomes. Int J Biol Sci 2012; 8:118-23; PMID:22211110; http://dx.doi.org/10.7150/ijbs.8.118
- Arntz OJ, Pieters BC, Oliveira MC, Broeren MG, Bennink MB, de Vries M, van Lent PL, Koenders MI, van den Berg WB, van der Kraan PM, et al. Oral administration of bovine milk derived extracellular vesicles attenuates arthritis in two mouse models. Mol Nutr Food Res 2015; 59:1701-12; PMID:26047123; http://dx.doi.org/10.1002/mnfr.201500222
- Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res 2012; 40:D1241-4; PMID:21989406; http://dx.doi.org/10.1093/nar/gkr828
- Huang X, Yuan T, Tschannen M, Sun Z, Jacob H, Du M, Liang M, Dittmar RL, Liu Y, Liang M, et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics 2013; 14:319; PMID:23663360; http://dx.doi.org/10.1186/1471-2164-14-319
- van Balkom BW, Eisele AS, Pegtel DM, Bervoets S, Verhaar MC. Quantitative and qualitative analysis of small RNAs in human endothelial cells and exosomes provides insights into localized RNA processing, degradation and sorting. J Extracell Vesicles 2015; 4:26760; PMID:26027894; http://dx.doi.org/10.3402/jev.v4.26760
- Gezer U, Ozgur E, Cetinkaya M, Isin M, Dalay N. Long non-coding RNAs with low expression levels in cells are enriched in secreted exosomes. Cell Biol Int 2014; 38:1076-9; PMID:24798520; http://dx.doi.org/10.1002/cbin.10301
- Song J, Kim D, Han J, Kim Y, Lee M, Jin EJ. PBMC and exosome-derived Hotair is a critical regulator and potent marker for rheumatoid arthritis. Clin Exp Med 2015; 15:121-6; PMID:24722995; http://dx.doi.org/10.1007/s10238-013-0271-4
- Wang Kevin C, Chang Howard Y. Molecular Mechanisms of Long Noncoding RNAs. Mol Cell 2011; 43:904-14; PMID:21925379; http://dx.doi.org/10.1016/j.molcel.2011.08.018
- Ravasi T, Suzuki H, Pang KC, Katayama S, Furuno M, Okunishi R, Fukuda S, Ru K, Frith MC, Gongora MM, et al. Experimental validation of the regulated expression of large numbers of non-coding RNAs from the mouse genome. Genome Res 2006; 16:11-9; PMID:16344565; http://dx.doi.org/10.1101/gr.4200206
- Mercer TR, Dinger ME, Sunkin SM, Mehler MF, Mattick JS. Specific expression of long noncoding RNAs in the mouse brain. Proc Natl Acad Sci U S A 2008; 105:716-21; PMID:18184812; http://dx.doi.org/10.1073/pnas.0706729105
- Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet 2014; 15:7-21; PMID:24296535; http://dx.doi.org/10.1038/nrg3606
- Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Ann Rev Biochem 2012; 81:145-66; PMID:22663078; http://dx.doi.org/10.1146/annurev-biochem-051410-092902
- Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell 2013; 152:1298-307; PMID:23498938; http://dx.doi.org/10.1016/j.cell.2013.02.012
- Bernstein E, Allis CD. RNA meets chromatin. Gen Dev 2005; 19:1635-55; PMID:16024654; http://dx.doi.org/10.1101/gad.1324305
- Bracken AP, Helin K. Polycomb group proteins: navigators of lineage pathways led astray in cancer. Nat Rev Cancer 2009; 9:773-84; PMID:19851313; http://dx.doi.org/10.1038/nrc2736
- Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet 2009; 10:155-9; PMID:19188922; http://dx.doi.org/10.1038/nrg2521
- Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol 2011; 21:354-61; PMID:21550244; http://dx.doi.org/10.1016/j.tcb.2011.04.001
- Whitehead J, Pandey GK, Kanduri C. Regulation of the mammalian epigenome by long noncoding RNAs. Biochim Biophys Acta 2009; 1790:936-47; PMID:19015002; http://dx.doi.org/10.1016/j.bbagen.2008.10.007
- Sun BK, Deaton AM, Lee JT. A transient heterochromatic state in Xist preempts X inactivation choice without RNA stabilization. Mol Cell 2006; 21:617-28; PMID:16507360; http://dx.doi.org/10.1016/j.molcel.2006.01.028
- Mohammad F, Mondal T, Guseva N, Pandey GK, Kanduri C. Kcnq1ot1 noncoding RNA mediates transcriptional gene silencing by interacting with Dnmt1. Development 2010; 137:2493-9; PMID:20573698; http://dx.doi.org/10.1242/dev.048181
- Schorey JS, Bhatnagar S. Exosome function: from tumor immunology to pathogen biology. Traffic 2008; 9:871-81; PMID:18331451; http://dx.doi.org/10.1111/j.1600-0854.2008.00734.x
- Mallegol J, Van Niel G, Lebreton C, Lepelletier Y, Candalh C, Dugave C, Heath JK, Raposo G, Cerf-Bensussan N, Heyman M. T84-intestinal epithelial exosomes bear MHC class II/peptide complexes potentiating antigen presentation by dendritic cells. Gastroenterology 2007; 132:1866-76; PMID:17484880; http://dx.doi.org/10.1053/j.gastro.2007.02.043
- Wolf T, Baier SR, Zempleni J. The Intestinal Transport of Bovine Milk Exosomes Is Mediated by Endocytosis in Human Colon Carcinoma Caco-2 Cells and Rat Small Intestinal IEC-6 Cells. J Nutr 2015; 145:2201-6; PMID:26269243; http://dx.doi.org/10.3945/jn.115.218586
- Baier SR, Nguyen C, Xie F, Wood JR, Zempleni J. MicroRNAs are absorbed in biologically meaningful amounts from nutritionally relevant doses of cow milk and affect gene expression in peripheral blood mononuclear cells, HEK-293 kidney cell cultures, and mouse livers. J Nutr 2014; 144:1495-500; PMID:25122645; http://dx.doi.org/10.3945/jn.114.196436
- Wiklander OP, Nordin JZ, O'Loughlin A, Gustafsson Y, Corso G, Mager I, Vader P, Lee Y, Sork H, Seow Y, et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles 2015; 4:26316; PMID:25899407; http://dx.doi.org/10.3402/jev.v4.26316
- Kino T, Hurt DE, Ichijo T, Nader N, Chrousos GP. Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Science signaling 2010; 3:ra8; PMID:20124551; http://dx.doi.org/10.1126/scisignal.2000568
- Barthel A, Schmoll D. Novel concepts in insulin regulation of hepatic gluconeogenesis. Am J Physiol Endocrinol Metab 2003; 285:E685-92; PMID:12959935; http://dx.doi.org/10.1152/ajpendo.00253.2003
- Mourtada-Maarabouni M, Pickard MR, Hedge VL, Farzaneh F, Williams GT. GAS5, a non-protein-coding RNA, controls apoptosis and is downregulated in breast cancer. Oncogene 2009; 28:195-208; PMID:18836484; http://dx.doi.org/10.1038/onc.2008.373
- Lanz RB, McKenna NJ, Onate SA, Albrecht U, Wong J, Tsai SY, Tsai MJ, O'Malley BW. A steroid receptor coactivator, SRA, functions as an RNA and is present in an SRC-1 complex. Cell 1999; 97:17-27; PMID:10199399; http://dx.doi.org/10.1016/S0092-8674(00)80711-4
- Lanz RB, Razani B, Goldberg AD, O'Malley BW. Distinct RNA motifs are important for coactivation of steroid hormone receptors by steroid receptor RNA activator (SRA). Proc Natl Acad Sci U S A 2002; 99:16081-6; PMID:12444263; http://dx.doi.org/10.1073/pnas.192571399
- Xu B, Gerin I, Miao H, Vu-Phan D, Johnson CN, Xu R, Chen XW, Cawthorn WP, MacDougald OA, Koenig RJ. Multiple roles for the non-coding RNA SRA in regulation of adipogenesis and insulin sensitivity. PLoS One 2010; 5:e14199; PMID:21152033; http://dx.doi.org/10.1371/journal.pone.0014199
- Ellis BC, Graham LD, Molloy PL. CRNDE, a long non-coding RNA responsive to insulin/IGF signaling, regulates genes involved in central metabolism. Biochim Biophys Acta 2014; 1843:372-86; PMID:24184209; http://dx.doi.org/10.1016/j.bbamcr.2013.10.016
- Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Rivea Morales D, Thomas K, Presser A, Bernstein BE, van Oudenaarden A, et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci U S A 2009; 106:11667-72; PMID:19571010; http://dx.doi.org/10.1073/pnas.0904715106
- Ellis BC, Molloy PL, Graham LD. CRNDE: A Long Non-Coding RNA Involved in CanceR, Neurobiology, and DEvelopment. Front Genet 2012; 3:270; PMID:23226159; http://dx.doi.org/10.3389/fgene.2012.00270
- Mosconi E, Rekima A, Seitz-Polski B, Kanda A, Fleury S, Tissandie E, Monteiro R, Dombrowicz DD, Julia V, Glaichenhaus N, et al. Breast milk immune complexes are potent inducers of oral tolerance in neonates and prevent asthma development. Mucosal Immunol 2010; 3:461-74; PMID:20485331; http://dx.doi.org/10.1038/mi.2010.23
- Brandtzaeg P. Mucosal immunity: integration between mother and the breast-fed infant. Vaccine 2003; 21:3382-8; PMID:12850345; http://dx.doi.org/10.1016/S0264-410X(03)00338-4
- Admyre C, Johansson SM, Qazi KR, Filen JJ, Lahesmaa R, Norman M, Neve EP, Scheynius A, Gabrielsson S. Exosomes with immune modulatory features are present in human breast milk. J Immunol 2007; 179:1969-78; PMID:17641064; http://dx.doi.org/10.4049/jimmunol.179.3.1969
- Reinhardt TA, Lippolis JD, Nonnecke BJ, Sacco RE. Bovine milk exosome proteome. J Proteomics 2012; 75:1486-92; PMID:22129587; http://dx.doi.org/10.1016/j.jprot.2011.11.017
- Heward JA, Lindsay MA. Long non-coding RNAs in the regulation of the immune response. Trends Immunol 2014; 35:408-19; PMID:25113636; http://dx.doi.org/10.1016/j.it.2014.07.005
- Mourtada-Maarabouni M, Hedge VL, Kirkham L, Farzaneh F, Williams GT. Growth arrest in human T-cells is controlled by the non-coding RNA growth-arrest-specific transcript 5 (GAS5). J Cell Sci 2008; 121:939-46; PMID:18354083; http://dx.doi.org/10.1242/jcs.024646
- Askarian-Amiri ME, Crawford J, French JD, Smart CE, Smith MA, Clark MB, Ru K, Mercer TR, Thompson ER, Lakhani SR, et al. SNORD-host RNA Zfas1 is a regulator of mammary development and a potential marker for breast cancer. Rna 2011; 17:878-91; PMID:21460236; http://dx.doi.org/10.1261/rna.2528811
- Tong X, Gu PC, Xu SZ, Lin XJ. Long non-coding RNA-DANCR in human circulating monocytes: a potential biomarker associated with postmenopausal osteoporosis. Biosci Biotechnol Biochem 2015; 79:732-7; PMID:25660720; http://dx.doi.org/10.1080/09168451.2014.998617
- Enderle D, Spiel A, Coticchia CM, Berghoff E, Mueller R, Schlumpberger M, Sprenger-Haussels M, Shaffer JM, Lader E, Skog J, et al. Characterization of RNA from Exosomes and Other Extracellular Vesicles Isolated by a Novel Spin Column-Based Method. PLoS One 2015; 10:e0136133; PMID:26317354; http://dx.doi.org/10.1371/journal.pone.0136133
- Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Current protocols in cell biology / editorial board, Juan S Bonifacino [et al] 2006; Chapter 3:Unit 3 22; PMID:18228490; http://dx.doi.org/10.1002/0471143030.cb0322s30