ABSTRACT
Maternal smoking in pregnancy (MSP) has been associated with DNA methylation in specific CpG sites (CpGs) in infants and children. We investigated whether MSP, independent of own personal active smoking, was associated with midlife DNA methylation in CpGs that were previously identified in studies of MSP-DNA methylation in children. We used data on MSP collected from pregnant mothers of 89 adult women born in 1959–1964 and measured DNA methylation in blood (granulocytes) collected in 2001–2007 (mean age: 43 years). Seventeen CpGs were differentially methylated by MSP, with multiple CpGs mapping to CYP1A1, MYO1G, AHRR, and GFI1. These associations were consistent in direction with prior studies (e.g., MSP associated with more and less methylation in AHRR and CYP1A1, respectively) and, with the exception of AHRR CpGs, were not substantially altered by adjustment for active smoking. These preliminary results confirm prior prospective reports that MSP influences the offspring DNA methylation, and extends the timeframe to midlife, and suggest that these effects may persist into adulthood, independently of active smoking.
Introduction
Cigarette smoke exposure remains a prevalent and preventable cause of morbidity and mortality.Citation1,2 In the US, an estimated 40 million adults smoke,Citation3 and 58 million nonsmokers are exposed to passive or second hand smoke.Citation4 Despite declines in the prevalence of maternal smoking during pregnancy (MSP), about 12% of US women continue to smoke while pregnant.Citation5 Sufficient evidence suggests a causal role for MSP in a range of outcomes in the offspring including low birth weight, sudden infant death syndrome, reduced fertility, and reduced and impaired lung function.Citation6 Active and passive forms of exposure to cigarette smoke may accumulate over the lifecourse, resulting in cumulatively worse health outcomes. It is also possible that exposure to cigarette smoke in critical windows of susceptibility may have persistent health effects irrespective of similar exposures in later life periods.Citation7-13
Genomic and region specific DNA methylation have been associated with MSP and passive and active cigarette smoke exposure.Citation14,15 More recently, epigenome-wide studies have reported distinct loci-specific DNA methylation patterns by in utero exposure to cigarette smoke in neonates and children.Citation15-22 Other studies focused on DNA methylation in adults have also identified loci associated with exposure to an individual's own cigarette smoking (active smoking), including current and lifetime smoking behavior and cumulative amount of smoke exposure.Citation23-31 There is considerable overlap in differentially methylated genes by prenatal smoke exposure and adult smoke exposures, and in studies of adult health outcomes, these genes have been linked to smoking related diseases, such as certain cancers and chronic pulmonary and neurologic diseases, and relevant biologic processes, such as cell regulation and differentiation, and chemical metabolism and detoxification.Citation32 Limited research has further demonstrated both reversibility and persistence in the associations of DNA methylation with active smoking in adultsCitation29 and with MSP in children and adolescents.Citation16,17,33 Together, these studies support a role for cigarette smoke exposure in altering DNA methylation and provide important information for identifying molecular pathways for the effect of smoke on human development and health. Yet, due to lack of prospective studies of MSP exposure and adult DNA methylation, it is still unclear whether DNA methylation changes associated with MSP exposure persist into adulthood, and whether these associations are independent of associations with exposure to own active smoking. Such information will help clarify whether the effects of cigarette smoke exposure on DNA persists from early life into adulthood independently of similar exposures in later life periods.
A particular challenge in designing a study that can address this limitation is lack of reliable measures of MSP in adult populations, as recall of early life factors, including parental characteristics, have limited accuracy.Citation34,35 In this study, we investigated whether CpG sites previously reported as differentially methylated by prenatal exposure to smoke show similar patterns in midlife by using data on MSP collected prospectively from pregnant mothers of 89 female offspring. The offspring were born in 1959–1964 and followed up as adults in 2001–2006, when we collected data on active smoking and collected a blood sample to extract DNA from granulocytes for methylation measurement.Citation36-38 We focused our analysis on results from 4 published studies that have examined prenatal smoke exposure, including MSP, in relation to DNA methylation changes using lllumina Infinium HumanMethylation450 BeadChip arrays, which have one of the most comprehensive coverages of CpG sites.Citation16-19 Of these 4 studies, DNA methylation was measured at birth in cord blood in 2 studies,Citation18,19 in adolescence in buffy coats in one study,Citation16 and at birth, childhood, and adolescence using repeated measures in cord blood, buffy or blood spots in the remaining study.Citation17 We considered 190 CpG sites in 116 genes identified as being differentially methylated as a result of exposure to maternal smoking in at least one of these studies (see Table S1 for a complete list). We also measured methylation using the Illumina Infinium HumanMethylation450 BeadChip array,Citation39 and used linear regression analyses to estimate the mean methylation level (β values) at each selected CpG site for MSP exposure after accounting for active cigarette smoking.
Results
presents the distribution of key characteristics in the study sample. Thirty-two of 89 women in the sample (36%) had been exposed to MSP. Women born to mothers who smoked during pregnancy were more likely to be ever smokers themselves than women who were born to mothers who did not smoke during pregnancy (72% vs. 42%; ). Duration of smoking was statistically significantly longer in women with MSP exposure than in those without MSP exposure (mean of 21.6 and 15.9 years, respectively).
Table 1. Participant characteristics by maternal smoking status during pregnancy, New York Women's Birth Cohort (n = 89).
Using false discovery rate (FDR) <0.05 for the overall model fit, we found 17 of the previously identified 190 CpG sites to be differentially methylated by MSP exposure. These sites, listed in , mapped to the following genes: FTO (cg03687532), CYP1A1 (cg22549041, cg05549655, cg13570656, cg11924019, cg00213123), MYO1G (cg19089201, cg12803068, cg22132788, cg04180046), AHRR (cg05575921, cg23916896), ANPEP (cg13834112), ZNF536 (cg23458168), and GFI1 (cg12876356, cg09662411, cg09935388).
Table 2. Multivariable linear regression estimates of associations between maternal smoke exposure and DNA methylation by CpG site and gene, New York Women's Birth Cohort.
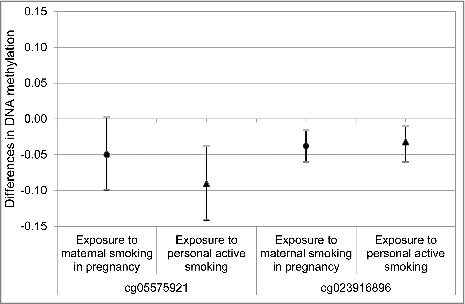
In models adjusted for age only, exposure to MSP was associated with 2–7% more methylation at 12 CpG sites in CYP1A1, FTO, MYO1G, ANPEP, and ZNF536; further adjustment for race/ethnicity and active smoking did not substantially alter the estimates of the association between MSP and DNA methylation at these CpG sites. For the remaining 5 CpG sites in GFI1 and AHRR, MSP exposure was associated with 3–8% less methylation in models adjusted for age and race/ethnicity. We further adjusted for variables capturing active smoking exposure in 3 ways: years of smoking, lifetime smoking status (ever vs. never smoking) and current smoking status (current, former vs. never smoking); results using these different measures were consistent and we present the results of models with years of smoking as a measure of duration of smoking in (model 3). Adjustment for active smoking duration attenuated the association of MSP with DNA methylation from age and race/ethnicity adjusted models for the 2 AHRR CpG sites, and increased the adjusted R2 to 28–29% (model 3) from 7–17% in age and race/ethnicity adjusted models (model 2). We compared the associations of MSP and active smoking with DNA methylation in the 2 AHRR CpG sites. As shown in , for one AHRR CpG site (cg05575921), active smoking status was associated with a lower amount of methylation [β = −0.09; 95% confidence interval (CI): −0.14, −0.04] than MSP status (β = −0.05; 95% CI: −0.10, 0.00). MSP and active smoking status had independent associations that were similar in direction and magnitude for the remaining AHRR CpG site (cg023916896) (β = −0.04; 95% CI: −0.06, −0.02 for MSP – β = −0.03; 95% CI: −0.05, −0.01 for active smoking).
Adjusting for race/ethnicity did not substantially affect the associations between MSP and DNA methylation in any of the 17 CpG sites. However, we noted that African American women had on average 2–5% higher mean DNA methylation on the absolute scale as compared with White and Hispanic women at all 5 CYP1A1 CpG sites (Fig. S1), and that the addition of race/ethnicity to age adjusted models increased the adjusted R2 from 9–19% to 15–31% for these CpG sites ().
Discussion
We examined whether MSP has persistent effects on adult offspring DNA methylation in midlife. We found statistically significant results for 17 CpG sites that were in the same direction and of similar magnitude as the associations in published studies that followed the offspring through childhood and adolescence,Citation16-19 and the majority of these associations were unaffected by accounting for active smoking status and duration. These results confirm prior reports that exposure to MSP is reflected in offspring DNA methylation patterns,Citation15 and add new preliminary evidence that these patterns remain detectable in the offspring 4 decades after the exposure, and persist regardless of the offspring's personal active smoking.
We focused on loci and genes that have been previously reported in peer reviewed publications, as our intent was to examine whether the effect of MSP on methylation patterns of these previously published associations persisted into midlife. These prior studies included recent birth cohorts (born in the 1990s and 2000s) of all white North American or European backgrounds, with relatively low prevalence of prenatal smoke exposure to maternal smoking (13–14% for samples not selected based on smoke exposure). In contrast, our sample included predominantly African American (31%) and Hispanic (42%) women, born in 1959–1963, with 36% of the sample exposed to MSP. Despite these differences, we were able to confirm some of the associations reported in these prior studies. In our study, the identified genes with multiple differentially methylated CpG sites were CYP1A1 (5 sites), MYO1G (4 sites), GFI1 (3 sites), and AHRR (2 sites), which are among the most consistently reported genes associated with tobacco smoke exposures and were identified in all 4 studies considered here. Of the 17 CpG sites identified in our study, 9 were identified in all 4 studies (cg04180046, cg05549655, cg05575921, cg09662411, cg09935388, cg12803068, cg12876356, cg22132788, cg22549041),Citation16-19 2 were identified in 3 studies (cg11924019, cg19089201),Citation16,18,19 2 were identified in 2 studies (cg00213123, cg13570656),Citation16,19 and 4 were identified only in the study by Markunas et al. (cg03687532, cg13834112, cg23458168, cg23916896).Citation19 Furthermore, 13 of the 17 CpG sites in our study were also identified in the 2 studies with a longer duration of follow up into adolescence (age range of 12–18 y at methylation assessment) that also considered participants' active smoking. In these 2 prior studies, adjusting for active smoking in adolescence or exclusion of smokers did not change the overall results; however, the prevalence of active smoking was low (∼5–12%). Nearly 53% of participants in our study reported ever smoking, and with the exception of 2 AHRR CpG sites, we similarly found that active smoking did not substantially affect MSP-methylation patterns nor did it contribute directly to methylation at the majority of CpG sites. Together, these results strengthen the validity of the findings that methylation in genes associated with tobacco metabolism may be susceptible to enduring and independent effects of in utero exposure to maternal smoking. However, the gene expression and functional impact of methylation differences in the range observed in our and prior studies are unknown and merit further research to understand the biologic consequences of these associations.
We considered 190 CpG sites and identified 17 CpG sites that had statistically significant different methylation by MSP exposures after adjusting for multiple comparisons. The more limited number of CpG sites identified as differentially methylation by MSP status in our study than in prior studies may suggest that the majority of prenatal smoke exposure associated methylation observed in early life may not last into midlife. It is important to note that only 35 CpG sites were identified in 2 or more studies, and one study by Markunas et al., based on infants from the Norway Facial Clefts Study, identified a large number of CpG sites, including 154 sites that were not identified in the other 3 studies, each of which identified between 22 and 30 CpG sites. Our small sample size, particularly the small number of women with MSP exposure who themselves were never smoker, may have resulted in unstable estimates, and precluded a sufficiently powered analysis of statistical interaction between the 2 forms of smoke exposures. Although our small sample size may have also led to a failure to detect additional associations with altered methylation, the small sample size should not influence those associations that we did find to be statistically significant. Our study lacked data on DNA methylation from the intervening years between the prenatal period and adult life, and data on the intensity and duration of MSP, which, respectively, limits our ability to determine reversal of DNA methylation patterns over time and presence of dose-response relationship between MSP and DNA methylation in midlife.
The main strengths of our study include the ability to examine DNA methylation in midlife in relation to a highly reliable measure of MSP, which was collected directly from mothers at the time of their pregnancy with the study participants. The MSP data were also collected in a period that preceded the Surgeon General's report on smoking hazards, when smoking behavior was less stigmatized and therefore less likely to be under-reported. Validation of MSP against serum cotinine levels in a subset of women participating in the parent study has shown a high accuracy for self-reported smoking by mothers.Citation40 The similarity of the associations reported in prior study of infants, children, and adolescents, and those observed in our study of adult women in their late 3rd to early 4th decades of life, lend support to the notion that exposure to smoking in utero can exert lasting DNA methylation changes of the offspring that may largely be unaffected by smoke exposure in later life periods.
Materials and methods
Study population
We used data from the New York Women's Birth Cohort, an adult follow-up study of former child participants in the National Collaborative Perinatal Project (NCPP). The New York Women's Birth cohort included 262 females born between 1959 and 1963 at Columbia Presbyterian Hospital in New York City who had follow-up through age 7, and were traced and completed a questionnaire as adults (see for detailsCitation36). We obtained blood samples from 92 (35%) of participants in the follow-up study; 62 participants had their blood drawn by a physician or a local laboratory and shipped the samples to our laboratory, 19 and 11 participants, respectively, provided blood samples through visits to Columbia University Medical Center or during home visits with our study staff.Citation37,38 All adult questionnaires and blood samples were collected between 2001 and 2007 (mean age at blood collection: 43 years—range: 38–46). Participants with blood samples did not differ from those without blood samples (n = 172) in terms of sociodemographic characteristics (age, race, educational level), prenatal and maternal factors and exposures, including maternal smoking during pregnancy (e.g., maternal age, pregnancy weight gain), infant and child height and weight, and adult reproductive and lifestyle factors, including smoking history (e.g., parity, alcohol consumption) (data not shown).
Data collection and key measurements
Prenatal, maternal, and family information were obtained from mothers at the time of mothers' pregnancy with the study participants and their enrollment into the study. Birth, infancy, and early childhood factors were prospectively collected from delivery through age 7 from the offspring (participants in current study) and/or mothers and guardians at regular follow-up appointments. Participants provided questionnaire data on their socio-demographic and risk factors in adolescence and adulthood periods as part of the adult follow-up study. To characterize smoke exposure, we used mothers' reports for maternal smoking during pregnancy (MSP) and participants' reports on their own smoking behaviors (active smoking). Participants were classified as exposed to MSP if their mother reporting smoking any amount during pregnancy, and were classified as an active smoker if they reported ever smoking at least one cigarette per day for at least one month.
Blood collection and methylation analysis
We extracted genomic DNA from granulocyte cells using a salting out procedure. Cells were lysed with SDS in a nuclei lysis buffer and treated with RNase A (final 133 µg/mL) and RNase T1 (final 20 units/mL) to remove RNA. Proteins were co-precipitated with NaCl (330 µL of saturated NaCl added per 1 mL solution) by centrifugation. Genomic DNA was recovered from the supernatant by precipitation with 100% ethanol, washed in 70% ethanol, and dissolved in the Tris-EDTA buffer. Genomic DNA (500 ng) was bisulfite-converted using the EpiTect Bisulfite Kit (Qiagen), as per the manufacturer's instructions. The DNA was resuspended in 20 uL of distilled water and stored at −20ºC until used.
We processed Illumina Infinium HumanMethylation450 array data with the Methylation Module of GenomeStudio software using HumanMethylation450 manifest v1.1. The software calculated methylation levels of CpG sites as β-values {β = intensity of the methylated allele (M)/[intensity of the unmethylated allele (U) + intensity of the methylated allele (M)] x 100}. β-value ranges between 0 (unmethylated, 0%) to 1 (completely methylated, 100%). For quality control (QC), we removed methylation measures with a detection P-value >0.05.
Statistical analysis
We performed linear regression models to examine the association between exposure to maternal smoking during pregnancy and methylation amount/level (β values), at selected CpG sites (previously identified to be differentially methylated according to MSP exposure, see Table S1 for a complete list of CpG sites). We corrected for multiple comparison testing by estimating the false discovery rate (FDR), and identified 17 CpG sites that were statistically significantly associated with MSP at P<0.05. For these 17 CpG sites, we fit a series of models, beginning with an unadjusted model that included only exposure to MSP and ending with more fully adjusted models that included measures of exposure to active smoking, i.e., years of active smoking or smoking status (ever vs. never smoker). There were minimal to no differences between unadjusted and age-adjusted models, likely due to the narrow age range in our study sample (<5 years). Furthermore, results of the models with either one of the measures of exposure to active smoking were similar, we focused the presentation of the results to 3 models that successively adjusted for age (model 1), race/ethnicity (model 2) and years of active smoking (model 3). These models also present the adjusted R2 to assess how much of the variation was explained by MSP vs. inclusion of the other covariates. We used R package and SAS 9.4 to perform statistical analysis.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
KEPI_A_1325065_s02.docx
Download MS Word (35.6 KB)Additional information
Funding
References
- World Health Organization. Global status report on non-communicable diseases 2010, Description of the global burden of NCDs, their risk factors and determinants. World Health Organization, Geneva 2011. Available from: http://www.who.int/nmh/publications/ncd_report2010/en/ (accessed January 18 2015).
- Bauer UE, Briss PA, Goodman RA, Bowman BA. Prevention of chronic disease in the 21st century: Elimination of the leading preventable causes of premature death and disability in the USA. Lancet 2014; 384(9937):45-52; PMID:24996589; https://doi.org/10.1016/S0140-6736(14)60648-6
- Jamal A, Homa DM, O'Connor E, Babb SD, Caraballo RS, Singh T, Hu SS, King BA. Current cigarette smoking among adults - United States, 2005–2014. MMWR Morb Mortal Wkly Rep 2015; 64(44):1233-40; PMID:26562061; https://doi.org/10.15585/mmwr.mm6444a1; https://doi.org/10.15585/mmwr.mm6444a2
- Homa DM, Neff LJ, King BA, Caraballo RS, Bunnell RE, Babb SD, Garrett BE, Sosnoff CS, Wang L, Centers for Disease Control and Prevention (CDC). Vital signs: Disparities in nonsmokers' exposure to secondhand smoke–United States, 1999–2012. MMWR Morb Mortal Wkly Rep 2015; 64(4):103-8; PMID:25654612
- Tong VT, Dietz PM, Morrow B, D'Angelo DV, Farr SL, Rockhill KM, England LJ, Centers for Disease Control and Prevention (CDC). Trends in smoking before, during, and after pregnancy–pregnancy risk assessment monitoring system, United States, 40 sites, 2000–2010. MMWR Surveill Summ 2013; 62(6):1-19; PMID:24196750
- U.S. Department of Health and Human Services. The Health Consequences of Smoking – 50 Years of Progress: A Report of the Surgeon General. Atlanta (GA): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office of Smoking and Health; 2014.
- Tehranifar P, Liao Y, Ferris JS, Terry MB. Life course socioeconomic conditions, passive tobacco exposures and cigarette smoking in a multiethnic birth cohort of U.S. women. Cancer Causes Control 2009; 20(6):867-76; PMID:19238563; https://doi.org/10.1007/s10552-009-9307-1
- Elmasry H, Goodwin RD, Terry MB, Tehranifar P. Early life exposure to cigarette smoke and depressive symptoms among women in midlife. Nicotine Tob Res 2014; 16(10):1298-306; PMID:24776377; https://doi.org/10.1093/ntr/ntu070
- Tawfik H, Kline J, Jacobson J, Tehranifar P, Protacio A, Flom JD, Cirillo P, Cohn BA, Terry MB. Life course exposure to smoke and early menopause and menopausal transition. Menopause 2015; 22(10):1076-83; PMID:25803667; https://doi.org/10.1097/GME.0000000000000444
- Terry MB, Schaefer CA, Flom JD, Wei Y, Tehranifar P, Liao Y, Buka S, Michels KB. Prenatal smoke exposure and mammographic density in mid-life. J Dev Orig Health Dis 2011; 2(6):340-52; PMID:23378890; https://doi.org/10.1017/S2040174411000614
- Neuman A, Hohmann C, Orsini N, Pershagen G, Eller E, Kjaer HF, Gehring U, Granell R, Henderson J, Heinrich J, et al. Maternal smoking in pregnancy and asthma in preschool children: A pooled analysis of eight birth cohorts. Am J Respir Crit Care Med 2012; 186(10):1037-43; PMID:22952297; https://doi.org/10.1164/rccm.201203-0501OC
- Duijts L, Jaddoe VW, van der Valk RJ, Henderson JA, Hofman A, Raat H, Steegers EA, Moll HA, de Jongste JC. Fetal exposure to maternal and paternal smoking and the risks of wheezing in preschool children: The generation R study. Chest 2012; 141(4):876-85; PMID:21960695; https://doi.org/10.1378/chest.11-0112
- Power C, Atherton K, Thomas C. Maternal smoking in pregnancy, adult adiposity and other risk factors for cardiovascular disease. Atherosclerosis 2010; 211(2):643-8; PMID:20400081; https://doi.org/10.1016/j.atherosclerosis.2010.03.015
- Terry MB, Delgado-Cruzata L, Vin-Raviv N, Wu HC, Santella RM. DNA methylation in white blood cells: Association with risk factors in epidemiologic studies. Epigenetics 2011; 6(7):828-37; PMID:21636973; https://doi.org/10.4161/epi.6.7.16500
- Joubert BR, Felix JF, Yousefi P, Bakulski KM, Just AC, Breton C, Reese SE, Markunas CA, Richmond RC, Xu CJ, et al. DNA methylation in newborns and maternal smoking in pregnancy: Genome-wide consortium meta-analysis. Am J Hum Genet 2016; 98(4):680-96; PMID:27040690; https://doi.org/10.1016/j.ajhg.2016.02.019
- Lee KW, Richmond R, Hu P, French L, Shin J, Bourdon C, Reischl E, Waldenberger M, Zeilinger S, Gaunt T, et al. Prenatal exposure to maternal cigarette smoking and DNA methylation: Epigenome-wide association in a discovery sample of adolescents and replication in an independent cohort at birth through 17 years of age. Environ Health Perspect 2015; 123(2):193-9; PMID:25325234; https://doi.org/10.1289/ehp.1408614
- Richmond RC, Simpkin AJ, Woodward G, Gaunt TR, Lyttleton O, McArdle WL, Ring SM, Smith AD, Timpson NJ, Tilling K, et al. Prenatal exposure to maternal smoking and offspring DNA methylation across the lifecourse: Findings from the Avon Longitudinal Study of Parents and Children (ALSPAC). Hum Mol Genet 2015; 24(8):2201-17; PMID:25552657; https://doi.org/10.1093/hmg/ddu739
- Joubert BR, Haberg SE, Nilsen RM, Wang X, Vollset SE, Murphy SK, Huang Z, Hoyo C, Midttun Ø, Cupul-Uicab LA, et al. 450K epigenome-wide scan identifies differential DNA methylation in newborns related to maternal smoking during pregnancy. Environ Health Perspect 2012; 120(10):1425-31; PMID:22851337; https://doi.org/10.1289/ehp.1205412
- Markunas CA, Xu Z, Harlid S, Wade PA, Lie RT, Taylor JA, Wilcox AJ. Identification of DNA methylation changes in newborns related to maternal smoking during pregnancy. Environ Health Perspect 2014; 122(10):1147-53; PMID:24906187; https://doi.org/10.1289/ehp.1307892
- Breton CV, Byun HM, Wenten M, Pan F, Yang A, Gilliland FD. Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation. Am J Respir Crit Care Med 2009; 180(5):462-7; PMID:19498054; https://doi.org/10.1164/rccm.200901-0135OC
- Maccani JZ, Koestler DC, Houseman EA, Marsit CJ, Kelsey KT. Placental DNA methylation alterations associated with maternal tobacco smoking at the RUNX3 gene are also associated with gestational age. Epigenomics 2013; 5(6):619-30; PMID:24283877; https://doi.org/10.2217/epi.13.63
- Suter M, Ma J, Harris A, Patterson L, Brown KA, Shope C, Showalter L, Abramovici A, Aagaard-Tillery KM. Maternal tobacco use modestly alters correlated epigenome-wide placental DNA methylation and gene expression. Epigenetics 2011; 6(11):1284-94; PMID:21937876; https://doi.org/10.4161/epi.6.11.17819
- Breitling LP, Yang R, Korn B, Burwinkel B, Brenner H. Tobacco-smoking-related differential DNA methylation: 27K discovery and replication. Am J Hum Genet 2011; 88(4):450-7; PMID:21457905; https://doi.org/10.1016/j.ajhg.2011.03.003
- Wan ES, Qiu W, Baccarelli A, Carey VJ, Bacherman H, Rennard SI, Agusti A, Anderson W, Lomas DA, Demeo DL. Cigarette smoking behaviors and time since quitting are associated with differential DNA methylation across the human genome. Hum Mol Genet 2012; 21(13):3073-82; PMID:22492999; https://doi.org/10.1093/hmg/dds135
- Shenker NS, Ueland PM, Polidoro S, van Veldhoven K, Ricceri F, Brown R, Flanagan JM, Vineis P. DNA methylation as a long-term biomarker of exposure to tobacco smoke. Epidemiology 2013; 24(5):712-6; PMID:23867811; https://doi.org/10.1097/EDE.0b013e31829d5cb3
- Elliott HR, Tillin T, McArdle WL, Ho K, Duggirala A, Frayling TM, Davey Smith G, Hughes AD, Chaturvedi N, Relton CL. Differences in smoking associated DNA methylation patterns in South Asians and Europeans. Clin Epigenetics 2014; 6(1):4; PMID:24485148; https://doi.org/10.1186/1868-7083-6-4
- Sun YV, Smith AK, Conneely KN, Chang Q, Li W, Lazarus A, Smith JA, Almli LM, Binder EB, Klengel T, et al. Epigenomic association analysis identifies smoking-related DNA methylation sites in African Americans. Hum Genet 2013; 132(9):1027-37; PMID:23657504; https://doi.org/10.1007/s00439-013-1311-6
- Dogan MV, Shields B, Cutrona C, Gao L, Gibbons FX, Simons R, Monick M, Brody GH, Tan K, Beach SR, et al. The effect of smoking on DNA methylation of peripheral blood mononuclear cells from African American women. BMC Genomics 2014; 15:151; PMID:24559495; https://doi.org/10.1186/1471-2164-15-151
- Zeilinger S, Kuhnel B, Klopp N, Baurecht H, Kleinschmidt A, Gieger C, Weidinger S, Lattka E, Adamski J, Peters A, et al. Tobacco smoking leads to extensive genome-wide changes in DNA methylation. PLoS One 2013; 8(5):e63812; PMID:23691101; https://doi.org/10.1371/journal.pone.0063812
- Tsaprouni LG, Yang TP, Bell J, Dick KJ, Kanoni S, Nisbet J, Viñuela A, Grundberg E, Nelson CP, Meduri E, et al. Cigarette smoking reduces DNA methylation levels at multiple genomic loci but the effect is partially reversible upon cessation. Epigenetics 2014; 9(10):1382-96; PMID:25424692; https://doi.org/10.4161/15592294.2014.969637
- Zaghlool SB, Al-Shafai M, Al Muftah WA, Kumar P, Falchi M, Suhre K. Association of DNA methylation with age, gender, and smoking in an Arab population. Clin Epigenetics 2015; 7(1):6; PMID:25663950; https://doi.org/10.1186/s13148-014-0040-6
- Lee KW, Pausova Z. Cigarette smoking and DNA methylation. Front Genet 2013; 4:132; PMID:23882278; https://doi.org/10.3389/fgene.2013.00239; https://doi.org/10.3389/fgene.2013.00132
- Breton CV, Siegmund KD, Joubert BR, Wang X, Qui W, Carey V, Nystad W, Håberg SE, Ober C, Nicolae D, et al. Prenatal tobacco smoke exposure is associated with childhood DNA CpG methylation. PLoS One 2014; 9(6):e99716; PMID:24964093; https://doi.org/10.1371/journal.pone.0099716
- Tehranifar P, Liao Y, Flom JD, Terry MB. Validity of self-reported birth weight by adult women: Sociodemographic influences and implications for life-course studies. Am J Epidemiol 2009; 170(7):910-7; PMID:19748903; https://doi.org/10.1093/aje/kwp205
- Batty GD, Lawlor DA, Macintyre S, Clark H, Leon DA. Accuracy of adults' recall of childhood social class: Findings from the Aberdeen children of the 1950s study. J Epidemiol Community Health 2005; 59(10):898-903; PMID:16166367; https://doi.org/10.1136/jech.2004.030932
- Terry MB, Flom J, Tehranifar P, Susser E. The role of birth cohorts in studies of adult health: The New York women's birth cohort. Paediatr Perinat Epidemiol 2009; 23(5):431-45; PMID:19689494; https://doi.org/10.1111/j.1365-3016.2009.01061.x
- Flom JD, Ferris JS, Liao Y, Tehranifar P, Richards CB, Cho YH, Gonzalez K, Santella RM, Terry MB. Prenatal smoke exposure and genomic DNA methylation in a multiethnic birth cohort. Cancer Epidemiol Biomarkers Prev 2011; 20(12):2518-23; PMID:21994404; https://doi.org/10.1158/1055-9965.EPI-11-0553
- Terry MB, Ferris JS, Pilsner R, Flom JD, Tehranifar P, Santella RM, Gamble MV, Susser E. Genomic DNA methylation among women in a multiethnic New York City birth cohort. Cancer Epidemiol Biomarkers Prev 2008; 17(9):2306-10; PMID:18768498; https://doi.org/10.1158/1055-9965.EPI-08-0312
- Dedeurwaerder S, Defrance M, Calonne E, Denis H, Sotiriou C, Fuks F. Evaluation of the infinium methylation 450K technology. Epigenomics 2011; 3(6):771-84; PMID:22126295; https://doi.org/10.2217/epi.11.105
- Klebanoff MA, Mednick BR, Schulsinger C, Secher NJ, Teasdale TW, Baker RL, Berendes HW. Second generation follow-up of the Danish perinatal study women: Study design and factors affecting response. Paediatr Perinat Epidemiol 1993; 7(1):9-22; PMID:8426835; https://doi.org/10.1111/j.1365-3016.1993.tb00596.x