ABSTRACT
We investigated the impact of maternal smoking during pregnancy on placental DNA methylation and how this may mediate the association between maternal smoking and pro-inflammatory proteins in cord blood. The study population consisted of 27 individuals exposed to maternal smoking throughout pregnancy, 32 individuals exposed during a proportion of the pregnancy, and 61 unexposed individuals. Methylation of 11 regions within 6 genes in placenta tissue was assessed by pyrosequencing. Levels of 7 pro-inflammatory proteins in cord blood were assessed by electrochemiluminescence. Differential methylation was observed in the CYP1A1 promoter and AHRR gene body regions between women who smoked throughout pregnancy and non-smokers on the fetal-side of the placenta and in the GFI1 promoter between women who quit smoking while pregnant and non-smokers on the maternal-side of the placenta. Maternal smoking resulted in elevated levels of IL-8 protein in cord blood, which was not mediated by DNA methylation of our candidate regions at either the maternal or the fetal side of the placenta. Placental DNA methylation was associated with levels of inflammatory proteins in cord blood. Our observations suggest that maternal smoking during pregnancy affects both placental DNA methylation and the neonate's immune response.
Introduction
Several studies have reported adverse health outcomes in children exposed to maternal smoking during pregnancy, including low birth weight, early respiratory illnesses, childhood and adult obesity, impaired behavioral development, anxiety and depression, cancer, and impaired reproductive health.Citation1-9 These impacts on childhood health may be mediated by epigenetic programming. A major epigenetic mark influencing gene expression is DNA methylation. DNA methylation contributes to the regulation of some key biologic processes important during early life development, such as imprinting, X-chromosome inactivation, cellular differentiation, and pluripotency.Citation10-12 Maternal smoking during pregnancy has been reported to impact differential DNA methylation in cord blood and these changes can be maintained into childhood.Citation13-21 A recently published meta-analysis performed in the PACE consortium reported that CpG loci methylation in cord blood associated with maternal smoking remained associated with maternal smoking in older children, demonstrating the persistence of findings at birth into later childhood.Citation21
To date, few studies have investigated the influence of maternal smoking on placental DNA methylation.Citation22-25 The placenta plays an important role in fetal tissue growth, vascularization, and hormone production. Additionally, it facilitates the supply of nutrients from mother to child and filters harmful substances to prevent embryo exposure.Citation26 Maternal smoking during pregnancy can induce abnormal development of the placenta, by influencing the placental vasculature, causing DNA damage and impairing DNA repair.Citation27-29 Some of these smoking-induced changes may be mediated by DNA methylation, which may in turn play a role in the adverse health outcomes observed in neonates exposed to maternal smoking.
To investigate this hypothesis, we analyzed the impact of maternal smoking during pregnancy on human placental DNA methylation in genes that were previously identified to exhibit differential methylation in cord blood, maternal blood or placenta tissue.Citation13-16 Placenta tissue from both the maternal and the fetal side of the placenta was included to explore whether the impact of maternal smoking on placental DNA methylation may be modified by placenta physiology. Subsequently, specific pro-inflammatory and cardiovascular injury proteins, which were previously reported to be elevated in newborns who had inflammatory lesions of the placenta,Citation30 were analyzed in the plasma of cord blood to investigate the impact of maternal smoking during pregnancy on the presence of these inflammation-related proteins in neonates and the possible relation between these inflammation-related proteins and placental DNA methylation of our target genes.
Results
Locus-specific differential DNA methylation in placentas exposed to maternal smoking during pregnancy compared with unexposed placentas
We analyzed the DNA methylation status of 11 regions within 6 genes, previously reported to be associated with maternal smoking in cord blood, maternal blood, or placenta. These candidate genes included CYP1A1, AHRR, GALNT2, GFI1, RUNX3, and TSLP and among 1 to 10 CpG loci were analyzed per region. The median methylation levels of the assayed loci in the promoters of GALNT2, GFI1, RUNX3, and TSLP was low, ranging from 0.80 to 8.23%. In contrast, the median methylation levels in the promoters of CYP1A1 and AHRR were much higher, ranging from 16.71 to 62.52%. Gene body methylation varied greatly between and within each gene (Supplementary Table 1). The correlation in methylation levels between the placenta tissues isolated from the maternal and fetal side of the placenta varied greatly between regions and even within the same region. The methylation levels of 49 out of the 51 CpG loci included in our analyses were significantly correlated between the maternal and the fetal side of the placenta (P ≤ 0.05)(Supplementary Table 1).
Among placenta samples extracted from the fetal side, we did not detect any significant differences in methylation between the mothers that quit smoking during pregnancy and mother that never smoked. However, fetal side DNA methylation was significantly lower among placentas exposed to maternal smoking throughout pregnancy compared with the unexposed placentas for all 3 CpG loci analyzed in the AHRR gene body and at one CpG locus in the CYP1A1 promoter (, P < 0.05). These changes in DNA methylation were relatively small; methylation was 4.70% lower [95% confidence interval (CI): −7.74%, −1.64%] at the CpG locus in the CYP1A1 promoter among exposed placentas (). The shift in methylation was similar in the AHRR gene body; the decrease in methylation ranged from 4.18% (95% CI: −8.00%, −0.35%) to 3.86% (95% CI: −6.66%, −1.09%; ), after adjusting for maternal age, BMI, and infant gender.
Figure 1. DNA methylation levels of the placentas from non-smoking mothers compared with the placentas from smoking mothers. Differential methylation observed in the placentas exposed to maternal smoking is shown relative to the unexposed placentas. The model was adjusted for maternal age, BMI category, and infant gender. The placentas of mothers who quit smoking while pregnant are indicated by the red color, the placentas exposed to maternal smoking during the entire duration of the pregnancy are indicated by the blue color. On the fetal side, significant decrease in methylation was observed in the mothers that smoked throughout pregnancy at 3 CpG loci in the AHRR gene body and one CpG locus in the CYP1A1 promoter. On the maternal side of the placenta, one CpG locus in the GFI1 promoter was significantly reduced in placentas of mothers who smoked and quit (P < 0.05). None of the other genes and loci included in this study reached statistical significant differences between our exposure groups for either the maternal or the fetal side of the placenta (Supplementary Figure 1)
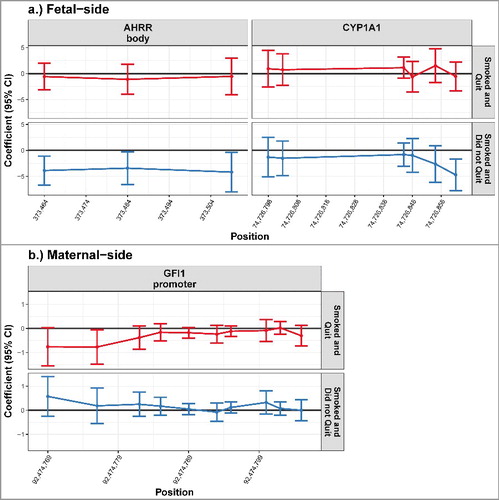
Table 1. Estimated association between unexposed placentas and placentas exposed to maternal smoking during pregnancy. Significantly lower levels of methylation were observed in the placentas from mothers who smoked throughout pregnancy compared with non-smoking mothers in all 3 CpG loci located in the AHRR gene body and in 1 CpG loci located in the CYP1A1 promoter on the fetal side of the placenta (P < 0.05). On the maternal side of the placenta were significantly lower levels of methylation observed in placentas from mothers who quit smoking while pregnant compared with mothers who did not smoke (P < 0.05), after adjusting for maternal age, BMI category, and infant gender.
Conversely, there were no significant differences in DNA methylation between placentas exposed to maternal smoking throughout pregnancy and those that were not exposed among samples obtained from the maternal side of the placenta. However, there was a very small, but statistically significant, reduction in DNA methylation at one locus in the GFI1 promoter in the placentas of mothers who quit smoking while pregnant, compared with unexposed placentas (95% CI: −1.48, −0.06; ), after adjusting for maternal age, BMI, and infant gender.
These disparities in the association between maternal smoking and placental DNA methylation by sampling side suggest tissue side-specific impacts within the placenta.
Locus-specific placental DNA methylation and inflammation-related protein levels in cord blood
Levels of 7 pro-inflammatory-related proteins—4 pro-inflammatory cytokines, the interleukins IL-6, IL-8, and IL1β, and tumor necrosis factor α (TNFα)—and 3 acute-phase proteins—intercellular adhesion molecule 1 (ICAM-1), serum amyloid A (SAA), and C-reactive protein (CRP)—were assayed in cord blood. All 7 proteins are major acute phase reactants and play an important role in the early response to infection. They were previously reported to be elevated in newborns with inflammatory lesions of the placenta.
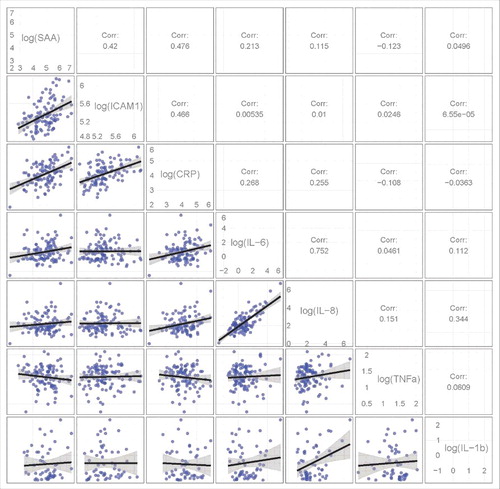
Moderate to strong pairwise correlations were detected between several of these proteins (P < 0.001; ). Significant positive correlations were observed between the proteins levels of ICAM1, SAA, and CRP, with correlations ranging from 0.420 to 0.476. The strongest correlation was between the protein levels of IL-8 with IL-6 (R = 0.752).
Figure 2. Correlations between several pro-inflammatory proteins levels. The lower triangle contains pair-wise pro-inflammatory protein scatterplots. The upper triangle shows the Pearson correlation coefficient between the pro-inflammatory proteins. Significant positive correlations were observed between the proteins levels of ICAM1, SAA and CRP and between the protein levels of IL-8 with IL-6 (P < 0.001).
We investigated the interaction between these pro-inflammatory-related proteins in cord blood and the DNA methylation levels in placenta of the CpG loci analyzed in this study. Each of the pro-inflammatory proteins assessed was significantly associated with placental DNA methylation levels of at least one of the CpG loci examined. The interactions between placental DNA methylation and cord blood protein levels largely varied by placenta side; only ICAM1 was consistent in the interaction with DNA methylation status of a single CpG locus on both sides of the placenta. A 5% increase in fetal side DNA methylation at this locus in the GFI1 body was accompanied by a small increase in log(pg/mL) ICAM1 [0.03 (95% CI: 0.00, 0.006)] and a similar increase in ICAM1 was detected with an 5% increase in maternal side DNA methylation at this locus [0.02 (95% CI: 0.00, 0.05); Supplementary Table 2]. All other significant interactions between locus-specific DNA methylation and pro-inflammatory protein levels were restricted to one side of the placenta. For example, on the maternal-side of the placenta, an increase in DNA methylation of CpG loci in the GFI1 promoter were accompanied by an increase of CRP, SAA, and IL-6 levels, while on the fetal side of the placenta, an increase of DNA methylation in other CpG loci in the GFI1 promoter region were accompanied by reduced levels of the CRP and IL-1β proteins (P < 0.05). Additional significant interactions between DNA methylation and pro-inflammatory proteins are included in Supplementary Table 2.
Generally, these results suggest that levels of inflammation-related proteins in cord blood are associated with locus- and placenta-side-specific differential DNA methylation.
IL-8 protein levels (pg/mL) in cord blood were associated with maternal smoking
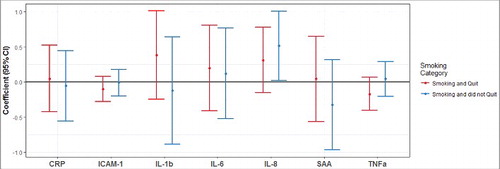
Maternal smoking during pregnancy was significantly associated with IL-8 protein levels in cord blood plasma (P < 0.05, ). Among neonates whose mothers smoked throughout pregnancy, cord blood IL-8 was 0.52 log(pg/mL) higher (95% CI: 0.02, 1.01) compared with neonates whose mothers did not smoke () after adjusting for maternal age, BMI category, and infant gender. None of the other pro-inflammatory proteins included in this study were significantly affected after exposure to maternal smoking during pregnancy (). Additionally, there were no significant differences in cord blood pro-inflammatory protein levels between neonates of mothers that smoked and quit during pregnancy and mothers that never smoked.
Figure 3. Maternal smoking during pregnancy and IL-8 protein levels (pg/mL) in cord blood plasma. Differential levels of cord blood inflammatory proteins observed in neonates exposed to maternal smoking during pregnancy is shown relative to the neonates unexposed to maternal smoking during pregnancy. The model was adjusted for maternal age, BMI category, and infant gender. The cord blood samples of neonates with mothers who quit smoking while pregnant are indicated by the red color, the cord blood samples of neonates exposed to maternal smoking during the entire duration of the pregnancy are indicated by the blue color. Cord blood plasma IL-8 was 0.52 log(pg/mL) higher in neonates whose mother smoked during the entire duration of the pregnancy compared with neonates of mothers who did not smoke.
Mediation analysis was used to estimate a possible natural indirect effect of maternal smoking on IL-8 protein levels through DNA methylation of the candidate loci. Methylation of the CpG loci interacting with IL-8 protein levels (2 CpG loci located in the GFI1 gene body and 1 CpG locus located in the GLANT2 gene body; Supplementary Table 2) did not significantly mediate the impact of maternal smoking (Supplementary Table 3). Accordingly, the natural direct effect of smoking throughout pregnancy on cord blood IL-8 levels compared with neonates of mothers that did not smoke was very similar to the estimated effect of maternal smoking on this inflammatory protein (Supplementary Table 3). This association was also not mediated by methylation at any of the other assayed CpG loci within either the maternal side or fetal side of the placenta samples.
These results suggest that maternal smoking during pregnancy affects IL-8 protein levels in the neonates largely independently of placental DNA methylation of the CpG loci analyzed in this study.
Discussion
This project was designed to investigate the influence of maternal smoking during pregnancy on placental DNA methylation of selected target genes and the interaction between those methylation patterns and inflammation-related proteins levels in cord blood. The genes included in this study were previously reported to be differentially methylated after smoking exposure in cord blood, adult peripheral blood lymphocytes (PBL), or placenta. In some cases, the effects of maternal smoking on peripheral blood methylation in the offspring persisted to age 7 and 17, respectively.Citation13,31-34 These target genes are suspected to play an important role in cell growth, immune response, and metabolic processes. Our study revealed that very few of the loci previously associated with maternal smoking during pregnancy in cord blood or maternal PBL were also affected in the placenta.
The placenta is a heterogeneous tissue comprising several cell types, each with potentially different patterns of DNA methylation and distinct morphologies, and cell composition is also different between the maternal and the fetal side of the placenta. Maternal smoking during pregnancy may influence placental DNA methylation either by directly influencing the methylation levels of certain cytosines of specific cell types or by modifying the distribution of cell types within the placenta, possibly by impacting pluripotent cell fate early in placental development. Our current study does not allow us to determine the pathway by which maternal smoking alters placental DNA methylation but, either way, a significant change in DNA methylation suggests that maternal smoking modifies placental function. However, differences in placental DNA methylation associated with exposure to maternal smoking were relatively small and smoking-induced alterations in placental DNA methylation were observed to be specific to the placenta sampling location.
Maternal smoking only significantly decreased DNA methylation at one CpG locus in the GFI1 promoter on the maternal side of the placenta; on the fetal side, methylation was lower at one CpG locus in the CYP1A1 promoter, as well as 3 CpG loci in the AHRR gene body. Suter et al. reported differential DNA methylation in the placenta of the CYP1A1 gene, similar to our observations, and they reported that this differential methylation was accompanied by an increase in placenta CYP1A1 expression.Citation35 Novakovic et al. analyzed placental AHRR methylation but, in contrast to us, they did not observe differential methylation after exposure to maternal smoking.Citation34 This difference may be due to the small number of placentas included in their study or by the way they collected the placenta tissue. Our results suggest that maternal smoking during pregnancy affects primarily the fetal side of placenta, so analyses of another part of the placenta or from a full thickness biopsy will likely result in different observations. Differential methylation between exposed and unexposed placentas was previously reported for RUNX3,Citation36 an observation that could not be reproduced in our study even though our primers were designed to analyze the same CpG locus. Compared to mothers who did not smoke, differential DNA methylation was only observed in the placentas exposed to maternal smoking throughout pregnancy for the affected CpG loci in the CYP1A1 and AHRR genes, while the CpG locus in the GFI1 promoter was differentially methylated in the placentas of mothers who quit smoking while pregnant. Other studies have reported that the degree of AHRR and GFI1 gene methylation in adult PBL is strongly associated with degree of smoking, serum cotinine levels, and years of smoking.Citation31,37 We did not measure the serum cotinine levels, so we were unable to investigate whether the degree of placental DNA methylation was associated with degree of smoking. However, this might explain why differential DNA methylation for the affected loci in the AHRR and CYP1A1 genes were only observed in the placentas exposed to maternal smoking throughout the entire pregnancy and not in the placentas of mothers who quit smoking during pregnancy. It also suggests that smoking induced alterations in placental DNA methylation reflect maternal smoking during pregnancy rather than a long-term smoking history.
The gene with multiple fetal side placental CpG loci associated with maternal smoking, AHRR, was previously reported to mediate toxic effects to environmental contaminants, including cigarette smoke, and can initiate the transcription of many genes, including the cytochrome P450 enzymes.Citation38,39 The cytochrome P450 enzymes have been reported to play a role in the mediation of metabolic pathways for nicotine metabolismCitation40 and some members of the cytochrome P450 superfamily are encoded by CYP1A1. As a result, the observed differential methylation of both AHRR and CYP1A1 in the placenta might affect the placenta's ability to protect the fetus from nicotine exposure. Future studies may address whether this impaired nicotine filter may mediate an elevated susceptibility to nicotine dependence in the child. Most of the genes included in this study were reported to be differentially methylated in cord blood between neonates exposed to maternal smoking during pregnancy and unexposed neonates. Our analyses revealed similarities in differential DNA methylation in placenta and cord blood for AHRR and GFI1. Reduced levels of methylation were observed in those exposed to maternal smoking during pregnancy in both cord blood and placenta. For the AHRR gene, studies in both cord blood and placenta reported that these altered methylation patterns were most profound in those exposed to maternal smoking throughout pregnancy.Citation33 CYP1A1 methylation levels in cord blood were reported to increase after exposure to maternal smoking,Citation33 while our analyses revealed reduced levels of placenta DNA methylation. Nevertheless, our results suggest that, in most cases, differential DNA methylation after exposure to maternal smoking, as observed in cord blood, is not accompanied by differential DNA methylation in the placenta. This raises the question of how cord blood DNA methylation can be affected without perturbations in the placental epigenome, especially because one of the main functions of the placenta is to act as a filter to prevent harmful substances to enter the fetus. One potential explanation may be the potential of certain cells to cross the placental barrier without affecting the placental methylome. Several studies have reported that cells from both the mother and the fetus can cross the placenta during pregnancy and can enter the blood stream or certain tissues.Citation41 Immunoglobulin A and G (IgA and IgG) diffusion or active transport of maternal IgA to the child was observed during pregnancy through currently not fully appreciated mechanisms.Citation42 These phenomena might contribute to the observed differential methylation in cord blood after maternal smoking exposure, without necessarily interfering with the placentas methylome.
To further investigate potential effects of maternal smoking during pregnancy on the neonates' health and immune systems, we determined the levels of a subset of inflammation-related proteins in cord blood. Both pro-inflammatory proteins as well as vascular injury proteins were included. Only one of the proteins, IL-8, revealed elevated protein levels in cord blood after exposure to maternal smoking throughout pregnancy. IL-8 is an important mediator of the immune reaction in the innate immune system response. It has been reported to increase after exposure to oxidative stress and plays a role in the bronchus allergic inflammation.Citation43-45 None of the other inflammation-related proteins analyzed in cord blood were observed to be affected by maternal smoking during pregnancy in our study, even though a negative association was previously reported between cord blood IL-6 protein levels and maternal smoking.Citation46 Furthermore, our study observed a significant association of IL-8 protein levels with placental DNA methylation of CpG loci located in the GLANT2 and GFI1 promoters. However, further analyses suggested that the impact of maternal smoking during pregnancy on IL-8 protein levels in the neonates is not mediated by placental DNA methylation of any of the genes included in our study. Our study only included a small number of CpG loci, spanning 6 genes, and it is possible that other genes in the placental epigenome can mediate IL-8 protein levels in neonates. For example, placental methylation of the IL-8 gene might influence cord blood IL-8 protein levels. Especially since IL-8 belongs to the class of pro-inflammatory chemokines that is produced by placental cells, among other cells. IL-8 is often produced after an infectious process originated in the uterus and placental inflammation has been reported to influence IL-8 protein levels in cord blood, such that newborns with inflammatory lesions of the placenta were much more likely than their peers to have elevated blood concentrations of IL-830. In line with these observations, it is well possible that placental DNA methylation of other genes in the epigenome may affect IL-8 protein levels. However, further research is required to investigate this in greater detail.
Further analyses of possible interactions between placental DNA methylation and pro-inflammatory protein levels in cord blood suggest that locus-specific placental DNA methylation is associated with the levels of specific inflammation-related proteins in cord blood, independently of the effect of maternal smoking. We included 7 proteins that were previously reported to be elevated in newborns with inflammatory lesions of the placentaCitation30 and we observed several interactions between placental DNA methylation and inflammation-related proteins in cord blood. This was primarily dependent on placental side; only ICAM1 protein levels were positively interacting with placental DNA methylation levels of one CpG locus in the GFI1 body on both sides of the placenta. Interestingly, the only pro-inflammatory protein not interacting with differential methylation of any of the CpG loci in the GFI1 gene on either side of the placenta was TNFα, all other proteins were found to be related to the DNA methylation levels of at least one CpG locus in either the body or the promoter region of GFI1. On the maternal side, SAA protein levels were even accompanying an increase in DNA methylation of 3 CpG loci in the GFI1 promoter and increased levels of IL-8 and IL-1β protein levels were observed with increasing levels of DNA methylation of 2 CpG loci in the GFI1 gene body. GFI1 is thought to play a role as regulator of cell fate choice in innate as well as adaptive immune systems, and GFI1 knockout studies suggest that GFI1 can limit the inflammatory immune response.Citation47,48,49 The effect of GFI1 methylation on the inflammatory immune response is further supported by the observation that differential GFI1 methylation in the placenta was accompanied by differential levels of several pro-inflammatory proteins in cord blood.
All pro-inflammatory proteins included in this study interacted with differential DNA methylation in at least one CpG locus. This suggests that locus-specific placental DNA methylation may affect the levels of pro-inflammatory proteins in cord blood and it may provide a pathway in which placental DNA methylation affects the immune response of the child. However, another potential explanation for this relation may be that both DNA methylation levels in the placenta and the inflammatory protein levels in cord blood are bystander effects caused by an inflammatory response in the placenta. Levels of pro-inflammatory proteins in the placenta were not measured in this study and therefore this possibility cannot be excluded.
In conclusion, differential DNA methylation of CpG loci located in the genes AHRR, CYP1A1 and GFI1 was observed after exposure to maternal smoking during pregnancy, although this was dependent on placental side. AHRR and CYP1A1 are important mediators in the nicotine metabolism pathway, while GFI1 facilitates inflammatory immune response. Maternal smoking also resulted in elevated levels of IL-8. These observations suggest that maternal smoking during pregnancy affects both placental DNA methylation and the neonate's early immune response. In addition, several interactions between pro-inflammatory proteins in cord blood and placental DNA methylation were observed, suggesting an association between placental DNA methylation and levels of pro-inflammatory proteins in cord blood.
Material and methods
Study population
Placenta tissue and participant information were collected as part of the Harvard Epigenetic Birth Cohort (HEBC). Details of the study protocol have been described previously.Citation50 This study included 120 mother-child dyads, of which 59 mothers smoked during pregnancy. Twenty-seven of these mothers smoked during the entire duration of the pregnancy, while 32 mothers quit smoking while pregnant. The other 61 mothers did not smoke during pregnancy. Dyads of smoking and non-smoking mothers were matched based on infant sex, ethnicity, parity, alcohol consumption during pregnancy (ever/never), and maternal age. Smoking behavior was self-reported by the mother.
The use of illicit drugs during pregnancy was an exclusion criterion. The study was restricted to singleton births.
Cord blood plasma was available for a subset of individuals. A total of 107 cord blood samples were included: 54 unexposed to maternal smoking during pregnancy, 25 exposed during the entire duration of the pregnancy, and 28 samples came from neonates whose mothers quit smoking while pregnant. A complete list of participant details can be found in .
Table 2. (A) Characteristics of mother-child dyads selected for the placental DNA methylation study. (B) Characteristics of mother-child dyads selected for the pro-inflammatory protein study.
This study was approved by the Institutional Review Board of the Brigham and Women's Hospital in Boston, MA, USA.
Placenta sample preparation
A 2 cm incision was made in the amnion to access the placenta tissue. Placenta tissue was then collected from the area near to the umbilical cord, from both the fetal and maternal side of the placenta. Placenta tissue was snap-frozen and stored in liquid nitrogen until further use. Genomic DNA was extracted from fresh frozen placenta tissue using the DNA Easy Blood and Tissue Kit (Qiagen, Cat. No. 69504), according to manufacturer's recommendation. Subsequently, genomic DNA was treated with bisulphite salt using the EZ DNA Methylation Gold kit (Zymo Research, Cat. No. D5007), according to manufacturer's recommendation.
DNA methylation analysis
DNA was amplified in 20 µl PCR reactions, containing 10 µl of Hot StarTaq Master Mix Kit (Qiagen, Cat. No. 203446), 150 ng of each primer and ∼20 ng modified DNA. PCR was performed with one cycle of 95°C for 15 min, 40 cycles of 95°C for 30 sec, 57–63°C for 30 sec and 72°C for 30 sec, followed by one cycle of 72°C for 10 min. For each set of primers one of the primers included a 5′-biotin label to allow subsequent analysis by pyrosequencing. An overview of primer sequences can be found in .
Table 3. Primer information.
Pyrosequencing was performed on a PyroMark Q24 MD pyrosequencer (Qiagen, Cat. No. 9001514) according to manufacturer's recommendations. The primers were designed using the primer design program ‘PSQ assay’ (Biotage), using gene sequences that were obtained from the GenBank entry on NCBI. The first set of primers were designed to cover CpG loci that were reported previously by methylation arrays to exhibit differential methylation between those exposed to maternal smoking during pregnancy vs. controls in either cord blood, maternal blood or placenta.Citation13-16,36 These CpG loci were located in the gene body for the genes AHRR, GFI1, GLANT2, RUNX3, and TSLP1. A second set of primers included CpG islands near the transcriptional start site in the same set of genes, plus CYP1A1. All of these genes were reported to play a role in cell growth, immune response, and/or metabolic processes. More detailed information about the primer sequence positions can be found in .
Assay validation was performed on samples of known methylation status, using the EpiTect Control DNA and Control DNA Set (Qiagen, Cat. No. 59568). All pyrosequencing analyses were performed in duplicate.
Biomarker analysis
Plasma aliquots of cord blood were collected at birth by gradient centrifugation and stored in liquid nitrogen or at −80°C until further implication. Protein biomarker levels in cord blood plasma were quantified using Meso Scale Discovery electrochemiluminescence (ECL) multiplex immunoassays, according to manufacturer's recommendation (MSD, Cat. No. K15228N-2 and K15229N-2). ECL multiplex assays were optimized to allow detection of each biomarker within the linearity concentration range. This resulted in a 1:500 dilution for the proteins of the vascular injury panel, while no dilution was used for the proteins in the pro-inflammatory panel.
Seven inflammation-related proteins were included in this study, the pro-inflammatory markers IL-1β, IL-6, IL-8, and TNFα; and the vascular injury markers SAA, CRP, and ICAM1. All samples were measured in duplicate. Measurements below the standard curve were excluded from subsequent analysis.
Statistical analysis
For each candidate region, multivariable linear models were used to investigate the association between continuous locus-specific placental methylation and self-reported maternal smoking category (non-smoker, smoked and quit during pregnancy, smoked throughout pregnancy), stratifying by placenta side. Using dummy variables, samples from mothers who smoked throughout pregnancy and those that smoked and quit were compared with non-smokers. Additionally, we modeled log transformed cord blood inflammatory biomarkers as a function of maternal smoking. Independently, the association between each log transformed biomarker and locus-specific methylation was modeled. Influential pro-inflammatory protein outliers were removed from these associations; associations with SAA were restricted to samples with log(pg/mL)<7.5, associations with ICAM1 were restricted to samples with log(pg/mL)>4.5, associations with CRP were restricted to samples with log(pg/mL)<6, and associations with TNFα were restricted to samples with log(pg/mL)>0.
All models were adjusted for maternal age, BMI category (< 18.5, 18.5–24.9, 25–29.9, ≥30 kg/m2), and infant gender. Wald tests were used to determine the significance of each association, using an α-level of 0.05. Finally, we performed mediation analyses to estimate the natural indirect effect of maternal smoking behavior on inflammatory biomarker levels through locus-specific DNA methylation changes. Models were adjusted by the covariates listed above, and robust standard errors were estimated to calculate significance and 95% confidence intervals (CIs). All analyses were conducted using R version 3.2.2 software and visualized using the ggplot2 package.
Author contributions
K.B. Michels and S.D van Otterdijk designed the research; S.D van Otterdijk performed the research; A.M. Binder and S.D van Otterdijk analyzed the data; all authors have contributed to the final version of the manuscript.
The authors declare they have no competing financial interests or conflict of interest.
2017EPI0126R-s01.docx
Download MS Word (688.2 KB)Acknowledgments
The Epigenetic Birth Cohort was funded by the National Cancer Institute, National Institute of Health under research grant number R21CA128382. We thank the participants of the Harvard Epigenetic Birth Cohort and all staff who helped collect and process the samples at the Brigham and Women's Hospital in Boston. We are grateful to Dr. Raina Fichorova and her laboratory team for technical advice on the ECL analyses.
Additional information
Funding
References
- Harrod CS, Reynolds RM, Chasan-Taber L, Fingerlin TE, Glueck DH, Brinton JT, Dabelea D. Quantity and timing of maternal prenatal smoking on neonatal body composition: the Healthy Start study. J Pediatr. 2014;165:707-12. doi:10.1016/j.jpeds.2014.06.031. PMID:25063722
- Gilliland FD, Li YF, Peters JM. Effects of maternal smoking during pregnancy and environmental tobacco smoke on asthma and wheezing in children. Am J Respir Crit Care Med. 2001;163:429-36. doi:10.1164/ajrccm.163.2.2006009. PMID:11179118
- Fuentes-Leonarte V, Estarlich M, Ballester F, Murcia M, Esplugues A, Aurrekoetxea JJ, Basterrechea M, Fernández-Somoano A, Morales E, Gascón M, et al. Pre- and postnatal exposure to tobacco smoke and respiratory outcomes during the first year. Indoor Air. 2015;25:4-12. doi:10.1111/ina.12128. PMID:24810295
- Ino T. Maternal smoking during pregnancy and offspring obesity: Meta-analysis. Pediatr Int Off J Jpn Pediatr Soc. 2010;52:94-9. doi:10.1111/j.1442-200X.2009.02883.x
- Gaysina D, Fergusson DM, Leve LD, Horwood J, Reiss D, Shaw DS, Elam KK, Natsuaki MN, Neiderhiser JM, Harold GT. Maternal smoking during pregnancy and offspring conduct problems: evidence from 3 independent genetically sensitive research designs. JAMA Psychiatry. 2013;70:956-63. doi:10.1001/jamapsychiatry.2013.127. PMID:23884431
- Moylan S, Gustavson K, Øverland S, Karevold EB, Jacka FN, Pasco JA, Berk M. The impact of maternal smoking during pregnancy on depressive and anxiety behaviors in children: the Norwegian Mother and Child Cohort Study. BMC Med. 2015;13:24. doi:10.1186/s12916-014-0257-4. PMID:25644294
- Håkonsen LB, Ernst A, Ramlau-Hansen CH. Maternal cigarette smoking during pregnancy and reproductive health in children: a review of epidemiological studies. Asian J Androl. 2014;16:39-49. doi:10.4103/1008-682X.122351. PMID:24369132
- Stavrou EP, Baker DF, Bishop JF. Maternal smoking during pregnancy and childhood cancer in New South Wales: a record linkage investigation. Cancer Causes Control CCC. 2009;20:1551-8. doi:10.1007/s10552-009-9400-5. PMID:19609689
- Harris HR, Willett WC, Michels KB. Parental smoking during pregnancy and risk of overweight and obesity in the daughter. Int J Obes 2005. 2013;37:1356-63.
- Fedoriw A, Mugford J, Magnuson T. Genomic imprinting and epigenetic control of development. Cold Spring Harb Perspect Biol. 2012;4:a008136. doi:10.1101/cshperspect.a008136. PMID:22687277
- Li Y, Tan T, Zong L, He D, Tao W, Liang Q. Study of methylation of histone H3 lysine 9 and H3 lysine 27 during X chromosome inactivation in three types of cells. Chromosome Res. 2012;20:769-78. doi:10.1007/s10577-012-9311-2. PMID:22956184
- Wu H, Sun YE. Epigenetic regulation of stem cell differentiation. Pediatr Res. 2006;59:21R-5R. doi:10.1203/01.pdr.0000203565.76028.2a. PMID:16549544
- Joubert BR, Håberg SE, Nilsen RM, Wang X, Vollset SE, Murphy SK, Huang Z, Hoyo C, Midttun Ø, Cupul-Uicab LA, et al. 450K epigenome-wide scan identifies differential DNA methylation in newborns related to maternal smoking during pregnancy. Env Health Perspect. 2012;120:1425-31. doi:10.1289/ehp.1205412
- Lee KW, Richmond R, Hu P, French L, Shin J, Bourdon C, Reischl E, Waldenberger M, Zeilinger S, Gaunt T, et al. Prenatal Exposure to Maternal Cigarette Smoking and DNA Methylation: Epigenome-Wide Association in a Discovery Sample of Adolescents and Replication in an Independent Cohort at Birth through 17 Years of Age. Env Health Perspect. 2015;123:193-9.
- Wang IJ, Chen SL, Lu TP, Chuang EY, Chen PC. Prenatal smoke exposure, DNA methylation, and childhood atopic dermatitis. Clin Exp Allergy. 2013;43:535-43. doi:10.1111/cea.12108. PMID:23600544
- Markunas CA, Xu Z, Harlid S, Wade PA, Lie RT, Taylor JA, Wilcox AJ. Identification of DNA methylation changes in newborns related to maternal smoking during pregnancy. Env Health Perspect. 2014;122:1147-53.
- Breton CV, Byun HM, Wenten M, Pan F, Yang A, Gilliland FD. Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation. Am J Respir Crit Care Med. 2009;180:462-7. doi:10.1164/rccm.200901-0135OC. PMID:19498054
- Breton CV, Siegmund KD, Joubert BR, Wang X, Qui W, Carey V, Nystad W, Håberg SE, Ober C, Nicolae D, et al. Prenatal tobacco smoke exposure is associated with childhood DNA CpG methylation. PLoS One. 2014;9:e99716. doi:10.1371/journal.pone.0099716. PMID:24964093
- Ivorra C, Fraga MF, Bayón GF, Fernández AF, Garcia-Vicent C, Chaves F, Redon J, Lurbe E. DNA methylation patterns in newborns exposed to tobacco in utero. J Transl Med. 2015;13:25. doi:10.1186/s12967-015-0384-5. PMID:25623364
- Patil VK, Holloway JW, Zhang H, Soto-Ramirez N, Ewart S, Arshad SH, Karmaus W. Interaction of prenatal maternal smoking, interleukin 13 genetic variants and DNA methylation influencing airflow and airway reactivity. Clin Epigenetics. 2013;5:22. doi:10.1186/1868-7083-5-22. PMID:24314122
- Joubert BR, Felix JF, Yousefi P, Bakulski KM, Just AC, Breton C, Reese SE, Markunas CA, Richmond RC, Xu C-J, et al. DNA Methylation in Newborns and Maternal Smoking in Pregnancy: Genome-wide Consortium Meta-analysis. Am J Hum Genet. 2016;98:680-96. doi:10.1016/j.ajhg.2016.02.019. PMID:27040690
- Banister CE, Koestler DC, Maccani MA, Padbury JF, Houseman EA, Marsit CJ. Infant growth restriction is associated with distinct patterns of DNA methylation in human placentas. Epigenetics. 2011;6:920-7. doi:10.4161/epi.6.7.16079. PMID:21758004
- Koukoura O, Sifakis S, Spandidos DA. DNA methylation in the human placenta and fetal growth (review). Mol Med Rep. 2012;5:883-9. PMID:22294146
- Frost JM, Moore GE. The importance of imprinting in the human placenta. PLoS Genet. 2010;6:e1001015. doi:10.1371/journal.pgen.1001015. PMID:20617174
- Robinson WP, Price EM. The Human Placental Methylome. Spring Harb Perspect Med. 2015;5:a023044. doi:10.1101/cshperspect.a023044
- Gude NM, Roberts CT, Kalionis B, King RG. Growth and function of the normal human placenta. Thromb Res. 2004;114:397-407. doi:10.1016/j.thromres.2004.06.038. PMID:15507270
- Larsen LG, Clausen HV, Jønsson L. Stereologic examination of placentas from mothers who smoke during pregnancy. Am J Obstet Gynecol. 2002;186:531-7. doi:10.1067/mob.2002.120481. PMID:11904619
- Slatter TL, Park L, Anderson K, Lailai-Tasmania V, Herbison P, Clow W, Royds JA, Devenish C, Hung NA. Smoking during pregnancy causes double-strand DNA break damage to the placenta. Hum Pathol. 2014;45:17-26. doi:10.1016/j.humpath.2013.07.024. PMID:24125744
- Demir R, Demir AY, Yinanc M. Structural changes in placental barrier of smoking mother. A quantitative and ultrastructural study. Pathol Res Pr. 1994;190:656-67.
- Hecht JL, Fichorova RN, Tang VF, Allred EN, McElrath TF, Leviton A, Elgan Study Investigators. Relationship Between Neonatal Blood Protein Concentrations and Placenta Histologic Characteristics in Extremely Low GA Newborns. Pediatr Res. 2011;69:68-73. doi:10.1203/PDR.0b013e3181fed334. PMID:20921924
- Philibert RA, Beach SRH, Lei M-K, Brody GH. Changes in DNA methylation at the aryl hydrocarbon receptor repressor may be a new biomarker for smoking. Clin Epigenetics. 2013;5:19. doi:10.1186/1868-7083-5-19. PMID:24120260
- Allione A, Marcon F, Fiorito G, Guarrera S, Siniscalchi E, Zijno A, Crebelli R, Matullo G. Novel epigenetic changes unveiled by monozygotic twins discordant for smoking habits. PloS One. 2015;10:e0128265. doi:10.1371/journal.pone.0128265. PMID:26043106
- Richmond RC, Simpkin AJ, Woodward G, Gaunt TR, Lyttleton O, McArdle WL, Ring SM, Smith AD, Timpson NJ, Tilling K, et al. Prenatal exposure to maternal smoking and offspring DNA methylation across the lifecourse: findings from the Avon Longitudinal Study of Parents and Children (ALSPAC). Hum Mol Genet. 2015;24:2201-17. doi:10.1093/hmg/ddu739. PMID:25552657
- Novakovic B, Ryan J, Pereira N, Boughton B, Craig JM, Saffery R. Postnatal stability, tissue, and time specific effects of AHRR methylation change in response to maternal smoking in pregnancy. Epigenetics. 2014;9:377-86. doi:10.4161/epi.27248. PMID:24270552
- Suter M, Abramovici A, Showalter L, Hu M, Shope CD, Varner M, Aagaard-Tillery K. In utero tobacco exposure epigenetically modifies placental CYP1A1 expression. Metabolism. 2010;59:1481-90. doi:10.1016/j.metabol.2010.01.013. PMID:20462615
- Maccani JZ, Koestler DC, Houseman EA, Marsit CJ, Kelsey KT. Placental DNA methylation alterations associated with maternal tobacco smoking at the RUNX3 gene are also associated with gestational age. Epigenomics. 2013;5:619-30. doi:10.2217/epi.13.63. PMID:24283877
- Wang X, Pittman GS, Su D, Adamski KN, Campbell MR, Joubert BR, Huang ZY, Hoyo C, Murphy SK, London SA, et al. Abstract 3647: Dose-dependent alteration of CpG methylation in AHRR and GFI1 in mononuclear cell DNA of smokers. Cancer Res. 2013;73:3647-3647. doi:10.1158/1538-7445.AM2013-3647
- Denison MS, Heath-Pagliuso S. The Ah receptor: a regulator of the biochemical and toxicological actions of structurally diverse chemicals. Bull Environ Contam Toxicol. 1998;61:557-68. doi:10.1007/PL00002973. PMID:9841714
- Baglole CJ, Maggirwar SB, Gasiewicz TA, Thatcher TH, Phipps RP, Sime PJ. The aryl hydrocarbon receptor attenuates tobacco smoke-induced cyclooxygenase-2 and prostaglandin production in lung fibroblasts through regulation of the NF-kappaB family member RelB. J Biol Chem. 2008;283:28944-57. doi:10.1074/jbc.M800685200. PMID:18697742
- Hukkanen J, Jacob P, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79-115. doi:10.1124/pr.57.1.3. PMID:15734728
- Dawe GS, Tan XW, Xiao Z-C. Cell Migration from Baby to Mother. Cell Adhes Migr. 2007;1:19-27. doi:10.4161/cam.4082
- Borte S, Janzi M, Pan-Hammarström Q, von Döbeln U, Nordvall L, Winiarski J, Fasth A, Hammarström L. Placental transfer of maternally-derived IgA precludes the use of guthrie card eluates as a screening tool for primary immunodeficiency diseases. PloS One. 2012;7:e43419. doi:10.1371/journal.pone.0043419. PMID:22916257
- Charles A Janeway J, Travers P, Walport M, Shlomchik MJ. Induced innate responses to infection. 2001 [cited 2015 Nov 10]; Available from: http://www.ncbi.nlm.nih.gov/books/NBK27122/.
- Jeanson L, Kelly M, Coste A, Guerrera IC, Fritsch J, Nguyen-Khoa T, Baudouin-Legros M, Papon J-F, Zadigue P, Prulière-Escabasse V, et al. Oxidative stress induces unfolding protein response and inflammation in nasal polyposis. Allergy. 2012;67:403-12. doi:10.1111/j.1398-9995.2011.02769.x. PMID:22188019
- Liu G, Zhu R, Li B. TNF-alpha and IL-8 of the patients with allergic asthma. J Huazhong Univ Sci Technol Med Sci Hua Zhong Ke Ji Xue Xue Bao Yi Xue Ying Wen Ban Huazhong Keji Daxue Xuebao Yixue Yingdewen Ban. 2005;25:274-5, 309. doi:10.1007/BF02828140
- Latzin P, Frey U, Armann J, Kieninger E, Fuchs O, Röösli M, Schaub B. Exposure to moderate air pollution during late pregnancy and cord blood cytokine secretion in healthy neonates. PloS One. 2011;6:e23130. doi:10.1371/journal.pone.0023130. PMID:21826232
- Karsunky H, Zeng H, Schmidt T, Zevnik B, Kluge R, Schmid KW, Dührsen U, Möröy T. Inflammatory reactions and severe neutropenia in mice lacking the transcriptional repressor Gfi1. Nat Genet. 2002;30:295-300. doi:10.1038/ng831. PMID:11810106
- Zeng H, Yücel R, Kosan C, Klein-Hitpass L, Möröy T. Transcription factor Gfi1 regulates self-renewal and engraftment of hematopoietic stem cells. EMBO J. 2004;23:4116-25. doi:10.1038/sj.emboj.7600419. PMID:15385956
- Spooner CJ, Cheng JX, Pujadas E, Laslo P, Singh H. A recurrent network involving the transcription factors PU.1 and Gfi1 orchestrates innate and adaptive immune cell fates. Immunity. 2009;31:576-86. doi:10.1016/j.immuni.2009.07.011. PMID:19818654
- Michels KB, Harris HR, Barault L. Birthweight, maternal weight trajectories and global DNA methylation of LINE-1 repetitive elements. PLoS One. 2011;6:e25254. doi:10.1371/journal.pone.0025254. PMID:21980406
