 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Synthetic antidepressants in current use for the complex etiopathogeneses of depression have slow response and remission as well as various unpleasant side effects. As a result, it is imperative to develop new antidepressants with more effectiveness and less severe side effects. Recent studies demonstrated that genipin, the aglycon of geniposide, extracted from Gardenia jasminoides Ellis has antidepressive effects. However, knowledge regarding the molecular mechanisms of its antidepressant effects remains limited. Employing a depression-like mouse model, we confirmed that genipin is capable of correcting depressions-like behaviors induced by prenatal stress in offspring from prenatally stressed dams (defined as PRS mice). In further experiments, we found that the effect of genipin on PRS mice occurs through DNA demethylation by inhibiting DNA methyltransferase 1 (DNMT1), normalizing the expression of reduced brain-derived neurotrophic factor (BDNF) in the hippocampus.
Introduction
Depression and anxiety are common and widespread neuropsychiatric disorders with complex etiopathogeneses. Current synthetic antidepressants used for these disorders have slow response and remission as well as various unpleasant side effects [Citation1–Citation7]. Therefore, it is imperative to develop new antidepressants with more effectiveness and less severe side effects.
Recent advances in studies of mental disorders suggest that exposure to stressful life events during pregnancy exerts profound effects on neurodevelopment and increases the risk for neurodevelopmental disorders, such as depression, anxiety, schizophrenia, bipolar disorder, and autism [Citation8–Citation12]. The indication suggests that epigenetic mechanisms triggered by environmental factors (stress), including aberrant DNA methylation and histone modifications, may contribute to the pathophysiology of psychiatric disorders [Citation13–Citation25]. For that reason, epigenetic modifiers may provide an alternative strategy for depression therapies. Recently it has been reported that genipin, a monoterpenoid compound, produces antidepressive effects in rodents [Citation26–Citation29]. However, the molecular mechanisms by which genipin attenuates depression-like behavior are not well understood.
In this study, we explored the effects of genipin on behavioral deficits in young adult offspring of prenatally stressed dams. As we previously reported [Citation30–Citation32], this model, defined as PRS mice, exhibit depression- and anxiety-like behaviors. Molecularly, the PRS mouse brain is characterized by overexpression of DNA methyltransferase 1 (DNMT1) and downregulation of BDNF due to promoter hypermethylation [Citation30,Citation31]. The phenotypes and molecular changes exhibited by PRS mice are reminiscent of the behavioral and molecular endophenotypes observed in depressive disorders [Citation18–Citation25]. We first determined the antidepressant effects of genipin using a forced swim test (FST) and a tail suspension test (TST). Further, possible molecular mechanism of genipin on PRS mice was explored by examining the transcript and protein expression of hippocampal Bdnf variants, and the altered methylation status of variants using DNA methylation immunoprecipitation (MeDIP). Finally, we studied the effects of genipin on the mRNA and protein expression of DNMT1.
Results
Genipin attenuates the depressive-like behaviors in PRS mice
Consistent with our previous report [Citation31], prenatal stress induces remarkable depression-like behaviors in young adult PRS mice. These behavioral alterations were corrected by treatment with genipin [25 mg/kg intraperitoneally (i.p.), once a day for 7 days] ((A and B)) as compared with their non-stress (NS) counterparts. In this study, the drug dose was chosen on the basis of our preliminary dose-response studies (from 25, 50, and 100 mg/kg) showing that 25 mg/kg was the minimum dose at which genipin elicited an antidepressant effect on PRS mice but not on NS mice in the FST.
Figure 1. Depression-like behaviors in PRS mice are reversed by treatment with genipin. Forty-day-old NS (offspring of non-stressed dams) and PRS (offspring of prenatally stressed dams) male mice were treated i.p. once a day for 7 days, with vehicle (VEH), 25 mg/kg of genipin (GEP). The depression-like behaviors were measured 2 h after the last treatment by means of FST (A) and TST (B). Administration of genipin at the dose above normalizes locomotor (horizontal and vertical) hyperactivity in PRS but not NS mice (C and D). The data are expressed as mean +/- SEM of 10 mice per group. The indices for TST and FST are represented as seconds, while locomotor activities are expressed as counts during 15 min. # P < 0.05 when genipin (GEP)-treated PRS mice are compared to vehicle (VEH)-treated PRS mice *P < 0.05 when PRS mice are compared to vehicle (VEH)- and genipin (GEP)-treated NS mice.
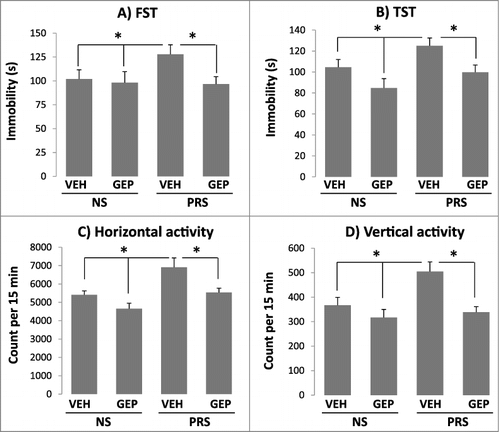
In the present study, we limited the behavioral studies to include FST and TST because the changes in these two tests are reproducible in PRS mice. As we previously reported, prenatal stress also induces locomotor hyperactivity in offspring [Citation30,Citation32]. We found that genipin at a dose that normalized depression-like behaviors (25 mg/kg) also significantly reduced both horizontal and vertical locomotor activities in an open field arena when compared with their NS counterparts ((C and D)). This indicates that genipin possesses an anxiolytic effect and the antidepressant-like effects shown above were not associated with its effect on locomotor activities.
Genipin normalizes reduced hippocampal Bdnf expression in PRS mice
We previously reported that the transcript expressions of BDNF variants, including Bdnf-i, Bdnf-iv, Bdnf-vi, and Bdnf-ix in the hippocampus were decreased significantly [Citation31,Citation32]. The decrease was found to be associated strongly with promoter hypermethylation induced by prenatal stress and correlated positively with depression-like behaviors. Therefore, we next tested whether the antidepressant effects of genipin on PRS mice observed above occur through normalizing the expression of BDNF. The four Bdnf variants: Bdnf-i, Bdnf-iv, Bdnf-vi, and Bdnf-ix were selected because among the other variants no changes were found in PRS mice. Consistent with previous findings [Citation31], prenatal stress induced a significant decrease in the expressions of Bdnf-i, Bdnf-iv, Bdnf-vi, and Bdnf-ix and administration of genipin, increased remarkably the expressions of Bdnf variants above in PRS mice as compared to their NS counterparts ( ). These results were confirmed by immunoblotting assay on BDNF protein expression (). In NS mice, genipin at the dose used failed to alter BDNF expression.
Figure 2. Downregulated expression of Bdnf-i, Bdnf-iv, Bdnf-vi, and Bdnf-ix transcripts in hippocampus of PRS mice are normalized by genipin. Forty-day-old NS (offspring of non-stressed dams) and PRS (offspring of prenatally stressed dams) male mice were treated i.p. once a day for 7 days, with vehicle (VEH), 25 mg/kg of genipin (GEP). Levels of mRNA were measured by qPCR and the data were normalized by β-actin. Bdnf expression was calculated by the ddCt method. Means of mRNA levels are expressed relative to control group. The data are expressed as mean +/- SEM. * P < 0.05 when vehicle (VEH)-treated PRS mice are compared to genipin (GEP)-treated PRS mice, or to vehicle (VEH)- and genipin (GEP)-treated NS mice. n = 10 per group.
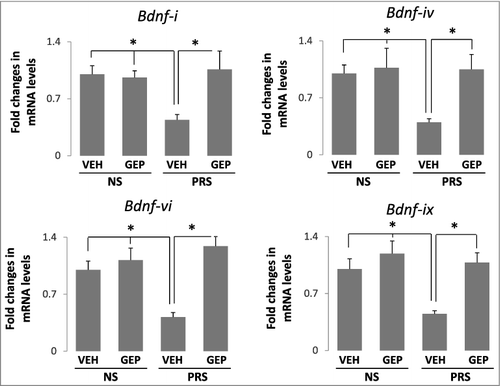
Figure 3. Reduced BDNF protein induced by prenatal stress is normalized by the treatment of genipin. Forty-day-old NS (offspring of non-stressed dams) and PRS (offspring of prenatally stressed dams) male mice were treated i.p. once a day for 7 days, with vehicle (VEH), 25 mg/kg of genipin (GEP). The immunoblots of BDNF protein in hippocampus were examined by Western blot and BDNF immunoblots were normalized by β-actin protein levels. The data are expressed as mean +/- SEM. * P < 0.05 when vehicle (VEH)-treated PRS mice are compared to genipin (GEP)-treated PRS mice, or to vehicle (VEH)- and genipin (GEP)-treated NS mice. n = 5 per group.
Genipin reduces promoter hypermethylation on Bdnf variants in PRS mice
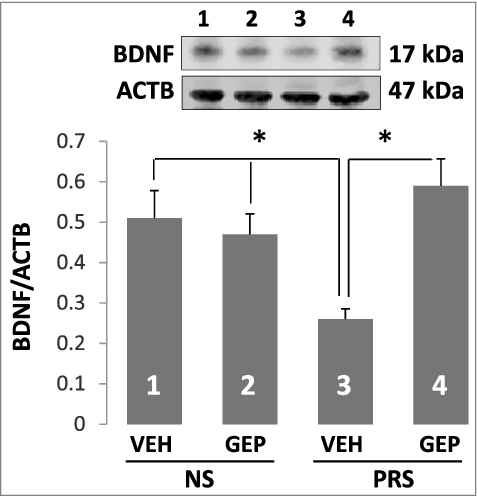
To determine whether the beneficial effects of genipin on BDNF expression observed above are the result of promoter demethylation, we analyzed cytosine methylation status on Bdnf variants using the MeDIP technique with highly specific anti-5-methylcytosine (5mC) antibody. Similar to what we found before [Citation30–Citation32], prenatal stress induces high enrichment of 5mC on the promoter regions of.
Bdnf-i, Bdnf-iv, Bdnf-vi, and Bdnf-ix in the hippocampus (). These 5mC enrichments on Bdnf variants above were consistently and significantly reduced in genipin-treated PRS mice (). There was no effect of genipin on Bdnf promoters in NS mice since the promoters are not hypermethylated.
Figure 4. Enrichment of 5mC on Bdnf-i, Bdnf-iv, Bdnf-vi, and Bdnf-ix promoter regions in the hippocampus of PRS mice are reduced by treatment with genipin. Forty-day-old NS (offspring of non-stressed dams) and PRS (offspring of prenatally stressed dams) male mice were treated i.p. once a day for 7 days, with vehicle (VEH), 25 mg/kg genipin (GEP). The enrichment of 5mC was measured using MeDIP and the enrichments of 5mC were corrected by corresponding input DNA. The data are expressed as mean +/- SEM. * P < 0.05 when vehicle (VEH)-treated PRS samples are compared to genipin (GEP)-treated PRS samples or to vehicle (VEH)- and genipin (GEP)-treated NS mice. n = 10 per group.
Interaction of genipin with promoter methylation mechanisms
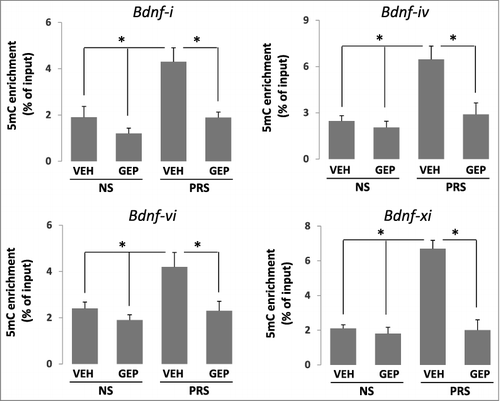
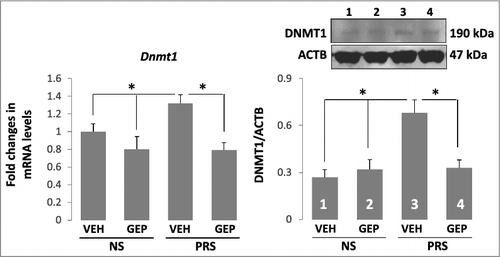
To investigate the mechanism whereby genipin treatment reverts the increased cytosine methylation levels observed at Bdnf promoters in PRS mice above, we focused on the main DNA modifying enzyme, DNMT1, since this enzyme is increased significantly in psychiatric disorders [Citation14] and in PRS mice [Citation30–Citation32]. As expected, prenatal stress induces an approximately 30% increase in DNMT1 mRNA and protein in the offspring hippocampus. With treatment of genipin, the increased DNMT1 expression was abated significantly as compared with their NS counterparts (). These data suggest that genipin possesses inhibitory activity of DNMT1, which may limit DNA methylation either indirectly by reducing the expression of DNMT, and/or more directly by interfering with the DNMT1 DNA-binding domain.
Figure 5. The increased expression of DNMT1 mRNA and protein in the hippocampus of PRS mice is reduced by treatment with genipin. Forty-day-old NS (offspring of non-stressed dams) and PRS (offspring of prenatally stressed dams) male mice were treated i.p. once a day for 7 days, with vehicle (VEH), 25 mg/kg of genipin (GEP). The Dnmt1 transcript was measured by qPCR and data were normalized by β-actin. PCR values were calculated by the Delta-Delta Ct (ddCt) method. Means of mRNA levels are expressed relative to control group (A). (B) DNMT1 protein was accessed by Western blot and the immunoblot of DNMT1 protein was normalized by β-actin protein levels. The data are expressed as mean +/- SEM. * P < 0.05 when vehicle (VEH)-treated PRS samples are compared to genipin (GEP)-treated PRS samples or to vehicle (VEH)- and genipin (GEP)-treated NS mice. n = 10 per group for qPCR and n = 5 per group for Western blot assay.
Discussion
Currently, between 30 and 50% of individuals treated with a given antidepressant do not show a response. Furthermore, there is also an established direct link between suicide, violent behavior, and the use of antidepressants, such as selective serotonin reuptake inhibitors [Citation1–Citation7]. Thus, new antidepressants with more effectiveness and less severe side effects are imperative. Because of the complex etiologies of major depression, the development of new medication for this disease is limited.
DNA methylation is emerging in physiology and pathology as an important epigenetic control mechanism for the regulation of central nervous system function [Citation13,Citation21–Citation23] and growing evidence suggests that aberrant DNA methylation on candidate genes is associated with the pathophysiology of psychotic disorders, including major depression [Citation23,Citation24]. To this end, we directed the focus of our research toward identifying the epigenetic DNA methylation signature found in depressed humans. We found that stress exposure during pregnancy increased DNMT1 expression and decreased the expression of hippocampal BDNF due to promoter methylation [Citation30–Citation32]. These alterations may contribute to depression- and anxiety-like behaviors in adult offspring [Citation30]. BDNF is a crucial factor regulating synaptic plasticity in brain. Decreased BDNF, dendritic spine, and neurogenesis in the hippocampal region are consistently found in depressed humans. Since DNA methylation on candidate genes associated with neurotrophic function seems to affect neurogenesis and synaptic plasticity in the hippocampus, it is possible that hippocampal DNA hypermethylation of such genes may contribute to the stress-induced synaptic plasticity and dysfunction within the region.
Recently, studies reveal that genipin possesses antidepressant effects on rodents [Citation26–Citation29]. Genipin, a compound with monoterpenoid structure, is generated by removing a glucose from geniposide catalyzed by β-glucosidase in the intestine and liver [Citation33,Citation34]. Phytochemical studies show that geniposide is a major ingredient of Gardenia fruit (Gardenia jasminoides Ellis), which, as a traditional medicine, has been used to treat various diseases, including depression, in China for hundreds of years. Besides antidepressant effects, genipin is found to have various biological activities, for instance, anti-inflammatory, anti-angiogenesis, anti-thrombotic, anti-diabetic, antioxidant, protection of neurotrophic activity, inhibition of nitric oxide production, and antitumor, regulation of epinephrine and 5-hydroxytryptamine levels in the hippocampus and energy metabolism [Citation35–Citation41]. These findings prompted us to test the effect of genipin on PRS mice. The results show that administration of genipin can significantly normalize prenatal stress induced depression like behaviors as evaluated by FST and TST tests. In order to explore the mechanisms underlying the effects of genipin on PRS mice, we first measured the expressions of Bdnf variants, including Bdnf-i, Bdnf-iv, Bdnf-vi, and Bdnf-ix, which are significantly decreased in the hippocampus of PRS mice as we reported previously [Citation31]. Genipin treatment remarkably increases reduced expressions of Bdnf variants and protein. These data are consistent with the findings from other groups [Citation26–Citation29] that genipin can increase BDNF expression.
Since downregulation of BDNF in PRS results from promoter hypermethylation, we then examined cytosine methylation statues on Bdnf variants above with and without genipin treatment. Consistent with our previous findings [Citation31], prenatal stress induces hypermethylation on promoters of Bdnf-i, Bdnf-iv, Bdnf-vi, and Bdnf-ix in the hippocampus. With genipin treatment, the enriched 5mC level (DNA methylation) on these promoters is remarkably reduced, indicating that the effect of genipin on enhancing the expression of BDNF is at least in part through reducing cytosine methylation on their promoters in PRS mice.
PRS brain is characterized by overexpression of DNMT1, which is responsible for DNA hypermethylation on genes associated with depression, including BDNF. To obtain further evidence that the beneficial effect of genipin on the behavioral deficits of PRS mice is associated by DNMT1 expression, we measured DNMT1 expression after genipin treatment. It has shown that genipin significantly reduces both mRNA and protein expressions of DNMT1 in hippocampus of PRS mice. This provides evidence that the antidepressant effect of genipin is mediated by inhibiting Dnmt1, decreasing promoter methylation, and favoring demethylation. In future studies, it will be important to establish whether genipin, acting at the Wnt/β-catenin signaling pathway, indirectly interferes with Dnmt1 transcription or with the binding of DNMT1 to the promoter or other regulatory regions of target genes.
An observation of this study is that, despite a potent behavioral and DNA demethylating action in PRS mice, only marginal behavioral and molecular effects of genipin were detected in NS mice. This was because the dose of genipin used in this study was the lowest one required to normalize PRS mice behavior based on our preliminary dose-response experiments. Another reason to be considered is that the dynamic state of DNA methylation may be greatly accelerated in PRS mice compared to NS mice. Thus, the steady state methylation process might be very sensitive to short-term inhibition in PRS mice. This does not necessarily mean that genipin fails to work on the demethylation processes in NS mice.
The present study shows that genipin is capable of correcting behavioral deficits and can induce chromatin remodeling in PRS mice. However, more studies need to be done to further characterize this compound. In the future, we would like to establish whether genipin, because of its unique chromatin remodeling properties, has advantages over antidepressants in current use. Taken together, the findings from this study provide evidence that genipin may serve as a new candidate drug for the treatment of depression.
A reliable animal model of depression should predict responsiveness to antidepressants. The present and previous studies [Citation30–Citation32] support the conclusion that the PRS mouse model has construct face validity as an experimental epigenetic model of vulnerability for depression disorders and can be used to screen potential antidepressants for improved clinical efficacy in acting on altered epigenetic mechanisms.
Materials and methods
Animals and gestation stress procedures
All animal experiments were performed in accordance with the Institutional Animal Care and Use Guidelines of Chongqing Medical University. Pregnant mice (Kunming species) were individually housed with food and water ad libitum. Control dams were left undisturbed throughout gestation with a 12-h light-dark cycle. The gestational-stress dams were housed in a separate room with fluorescent ceiling lights. The stress procedure consisted of restraining the pregnant dam in a transparent tube (12 cm × 3 cm) for 30 minutes three times per day from the fifth day of pregnancy until delivery [Citation31], and 24-h constant light throughout gestation. After weaning [postnatal day (PND) 20], male mice were selected for the study and housed four-to-five per cage, separately by condition. Mice from the same litter were randomly assigned to vehicle or genipin groups. A maximum of one or two male pups was taken from each litter for each measure to remove any litter effects [Citation42,Citation43]. At postnatal day PND 40, the following experiments were performed. Since behavioral tests may have significant effects on gene expression, we had an additional set of mice not subjected to behavioral tests for gene and protein expression and promoter methylation experiments. The behavioral test battery was conducted in the order of increasing invasiveness: locomotor activity, TST, and FST.
Drug treatment
Genipin (MedChemExpress, New Jersey) was dissolved in 2% DMSO. The drug (25 mg/kg) was administered to PRS and NS mice at PND 40 i.p. once a day for seven consecutive days. Vehicle (2% DMSO) was administered to corresponding control groups using a similar regimen. Behavioral tests were conducted on the day at 2 h after the last administration of vehicle (VEH) or genipin (GEP). Where indicated, animals were sacrificed 2 h after the last behavioral test.
Locomotor activity
A computerized Animal Activity Monitoring System with VersaMax software (AccuScan Instruments, Columbus, OH) was used for the quantification and tracking of locomotor activity in mice, as described previously [Citation30,Citation32]. Each activity cage consisted of a Perspex box (20 × 20 × 20 cm divided into quadrants) surrounded by horizontal and vertical infrared sensor beams. The total number of interruptions of the horizontal sensors (counts) was taken as a measure of horizontal activity, whereas that of vertical sensors was used as a measure of vertical activity. Activity was recorded for 15 min.
Tail suspension test
The experiment was carried out according to the method described by us [Citation31]. The mice were individually suspended by the tail taped on a stand above the floor. The duration of the test was 6 min. Immobility was defined as the mouse remaining completely motionless. Results are expressed as the immobility time during the 200-s test period.
Forced swimming test
As previously described [Citation31,Citation44], in the pre-test session, mice were placed individually in a clear container (20 cm diameter, 50 cm height) that contained water (25 ± 1°C) to a depth of 25 cm and forced to swim for 6 min. The water was replaced for each mouse. In the test-session, mice were placed back into the container for 5 min. Immobility was noted if the mouse remained floating without climbing. The time of immobility was recorded during the last 4 min of the 6-min testing period, thus after 2 min of habituation. Results are expressed as the immobility time during the 240-s test period.
Quantitative real-time polymerase chain reaction
Quantitative real-time polymerase chain reaction (qRT-PCR) was performed using SYBR®Premix Ex TaqTMII (TaKaRa RR820A). Total RNA from the hippocampus was isolated using TRIzol reagent (Life Technologies, Grand Island, New York) and was further purified using the QIAGEN RNeasy kit (Qiagen, Valencia, California). cDNA synthesis was performed using PrimeScriptTM RT reagent Kit (TaKaRa RRO37A). The primer sequences for the genes analyzed are summarized in Table 1 in Supplement 1. Each sample was run in duplicate and repeated twice. For normalizing messenger RNA expression, housekeeping gene (β-actin) was chosen as the internal control. PCR data were calculated by the Delta-Delta Ct (ddCt) method. Means of mRNA levels are expressed relative to control subjects.
Western blot analysis
Total protein from hippocampus, extracted using RIPA lysis buffer and quantified by Enhanced BCA Protein Assay Kit (Beyotime P0010S), was separated by SDS-PAGE and transferred to PVDF membrane. After being blocked in TBS buffer containing 0.05% Tween-20 and 5% skim milk, the membranes were incubated overnight at 4°C with the following primary antibodies: anti-DNMT1 (Imagenex; 1:1000) and anti-BDNF (Santa Cruz Biotechnology; 1:500). After incubation with the corresponding secondary antibody, the immunoreactive signals were visualized by ECL Plus Western Blotting Detection System and quantitated using Quantity One software. The levels of these proteins were normalized by β-actin protein levels.
Methylated DNA immunoprecipitation
Genomic DNA was isolated from
Total genomic DNA was isolated from hippocampus. DNA methylation (enrichment of 5mC) on Bdnf promoters was measured using Methylated DNA Immunoprecipitation [MeDIP Kit (Diagenode, Denville, New Jersey)]. Genomic DNA isolated was sonicated to 200 bp and subjected to immunoprecipitation using specific 5mC antibody followed by qPCR. Primers for PCR are listed in Table 1. Input genomic DNA that is not subjected to the methylation enrichment procedure was used as a control. The procedures for sample treatment and immunoprecipitation are described in the kit instruction manuals. The percentage of methylated versus unmethylated promoter was calculated using the following equation:
Statistical analysis
Results are expressed as mean ± SEM. Experimental differences were assessed by 2-way ANOVA followed by Bonferroni post-hoc comparisons using Predictive Analytics Software v.18 (SPSS, Inc., Chicago, Illinois). The criterion for significance was P < 0.05, two-tailed.
Disclosure of Potential Conflicts of Interest
The authors report no biomedical financial interests or potential conflicts of interest.
Tab1_supplemental_information.doc
Download MS Word (37.5 KB)Acknowledgments
This work is supported by National Natural Science Foundation of China. Grant Number: NSFC 81271264.
Additional information
Funding
References
- Otte C, Gold SM, Penninx BW, et al. Major depressive disorder. Nat Rev Dis Primers. 2016;2:16065. doi:10.1038/nrdp.2016.65. PMID:27629598
- Lépine JP, Briley M. The increasing burden of depression. Neuropsychiatr Dis Treat. 2011;(Suppl 1):3–7. doi:10.2147/NDT.S19617. PMID:21750622
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455(7215):894–902. doi:10.1038/nature07455. PMID:18923511
- Harmer CJ, Goodwin GM, Cowen PJ. Why do antidepressants take so long to work? A cognitive neuropsychological model of antidepressant drug action. Br J Psychiatry. 2009;195(2):102–108. doi:10.1192/bjp.bp.108.051193. PMID:19648538
- Cipriani A, Geddes JR, Furukawa TA, et al. Metareview on short term effectiveness and safety of antidepressants for depression: an evidence-based approach to inform clinical practice. Can J Psychiatry. 2007;52(9):553–562. doi:10.1177/070674370705200903
- Ruhé HG, Huyser J, Swinkels JA, et al. Switching antidepressants after a first selective serotonin reuptake inhibitor in major depressive disorder: a systematic review. J Clin Psychiatry. 2006;67(12):1836–1855. doi:10.4088/JCP.v67n1203. PMID:17194261
- Montejo AL, Montejo L, Navarro-Cremades F. Sexual side effects of antidepressant and antipsychotic drugs. Curr Opin Psychiatry. 2015;28(6):418–423. doi:10.1097/YCO.0000000000000198. PMID:26382168
- Charil A, Laplante DP, Vaillancourt C, et al. Prenatal stress and brain development. Brain Res Rev. 2010;65:56–79. doi:10.1016/j.brainresrev.2010.06.002. PMID:20550950
- Fine R, Zhang J, Stevens HE. Prenatal stress and inhibitory neuron systems: implications for neuropsychiatric disorders. Mol Psychiatry. 2014;19:641–651. doi:10.1038/mp.2014.35. PMID:24751963
- Markham JA, Koenig JI. Prenatal stress: Role in psychotic and depressive diseases. Psychopharmacology. 2011;214:89. doi:10.1007/s00213-010-2035-0. PMID:20949351
- Mulder EJ, Robles de Medina PG, Huizink AC, et al. Prenatal maternal stress: effects on pregnancy and the (unborn) child. Early Hum Dev. 2002;70(1-2):3–14. doi:10.1016/S0378-3782(02)00075-0. PMID:12441200
- Weinstock M. The long-term behavioural consequences of prenatal stress. Neurosci Biobehav Rev. 2008;32(6):1073–1086. doi:10.1016/j.neubiorev.2008.03.002. PMID:18423592
- Szyf M. DNA methylation, behavior and early life adversity. J Genet Genomics. 2017;40:331–338. doi:10.1016/j.jgg.2013.06.004
- Dong E, Ruzicka WB, Grayson DR, et al. DNA-methyltransferase1 (DNMT1) binding to CpG rich GABAergic and BDNF promoters is increased in the brain of schizophrenia and bipolar disorder patients. Schizophr Res. 2015;167(1-3):35–41. doi:10.1016/j.schres.2014.10.030. PMID:25476119
- Roth TL, Lubin FD, Funk AJ, et al. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry. 2009;65:760–769.
- Murgatroyd C, Spengler D. Epigenetics of early child development. Front Psychiatry. 2011;18:2–16.
- Tsankova N, Renthal W, Kumar A, et al. Epigenetic regulation in psychiatric disorders. Nature Rev Neurosci. 2007;8(5):355–367. doi:10.1038/nrn2132
- Nestler EJ. Epigenetic mechanisms of depression. JAMA Psychiatry. 2014;71(4):454–456. doi:10.1001/jamapsychiatry.2013.4291. PMID:24499927
- Fumagalli F, Molteni R, Racagni G, et al. Stress during development: impact on neuroplasticity and relevance to psychopathology. Prog Neurobiol. 2007;81:197–217. doi:10.1016/j.pneurobio.2007.01.002. PMID:17350153
- Mehler MF. Epigenetic principles and mechanisms underlying nervous system functions in health and disease. Prog Neurobiol. 2008;86:305–341. doi:10.1016/j.pneurobio.2008.10.001. PMID:18940229
- Feng J, Fan G. The role of DNA methylation in the central nervous system and neuropsychiatric disorders. Int Rev Neurobiol. 2009;89:67–84. doi:10.1016/S0074-7742(09)89004-1. PMID:19900616
- Day JJ, Kennedy AJ, Sweatt JD. DNA methylation and its implications and accessibility for neuropsychiatric therapeutics. Annu Rev Pharmacol Toxicol. 2015;55:591–611. doi:10.1146/annurev-pharmtox-010814-124527. PMID:25340930
- Roy B, Shelton RC, Dwivedi Y. DNA methylation and expression of stress related genes in PBMC of MDD patients with and without serious suicidal ideation. J Psychiatr Res. 2017;89:115–124. doi:10.1016/j.jpsychires.2017.02.005. PMID:28246044
- Dwivedi Y. Brain-derived neurotrophic factor: role in depression and suicide. Neuropsychiatr Dis Treat. 2009;5:433–449. doi:10.2147/NDT.S5700. PMID:19721723
- Robertson KD. DNA methylation and human disease. Nat Rev Genet. 2005;6:597–610. doi:10.1038/nrg1655. PMID:16136652
- Tian JS, Cui YL, Hu LM, et al. Antidepressant-like effect of genipin in mice. Neurosci Lett. 2010;479(3):236–239. doi:10.1016/j.neulet.2010.05.069
- Cai L, Li R, Tang WJ, et al. Antidepressantlike effect of geniposide on chronic unpredictable mild stress-induced depressiverats by regulating the hypothalamus-pituitary-adrenal axis. Eur Neuropsychopharmacol. 2015;25(8):1332–1341. doi:10.1016/j.euroneuro.2015.04.009
- Tian JS, Shi BY, Xiang H, et al. 1H-NMR-based metabonomic studies on the anti-depressant effect of genipin in the chronicunpredictable mild stress rat model. PLoS One. 2013;8(9):e75721. doi:10.1371/journal.pone.0075721. PMID:24058700
- Wang J, Duan P, Cui Y, et al. Geniposide alleviates depression-like behavior via enhancing BDNF expression in hippocampusof streptozotocin-evoked mice. Metab Brain Dis. 2016;31(5):1113–1122. doi:10.1007/s11011-016-9856-4
- Dong E, Dzitoyeva S, Matrisciano F, et al. BDNF epigenetic modifications associated with schizophrenia-like phenotype induced by prenatal stress in mice. Biol Psych. 2014;77(6):589–596. doi:10.1016/j.biopsych.2014.08.012
- Zheng Y, Fan W, Zhang X, et al. Gestational stress induces depression-like and anxiety-like phenotypes through epigenetic regulation of BDNFexpression in offspring hippocampus. Epigenetics. 2016;11(2):150–162. doi:10.1080/15592294.2016.1146850. PMID:26890656
- Dong E, Tueting P, Matrisciano F, et al. Behavioral and molecular neuroepigenetic alterations in prenatally stressed mice: relevance for the study of chromatin remodeling properties of antipsychotic drugs. Transl Psychiatry. 2016;6:e711. doi:10.1038/tp.2015.191
- Akao T, Kobashi K, Aburada M. Enzymic studies on the animal and intestinal bacterial metabolism of geniposide. Biol Pharm Bull. 1994;17(12):1573–1576. doi:10.1248/bpb.17.1573. PMID:7735197
- Hou YC, Tsai SY, Lai PY, et al. Metabolism and pharmacokinetics of genipin and geniposide in rats. Food Chem Toxicol. 2008;46(8):2764–2769. doi:10.1016/j.fct.2008.04.033
- Koo HJ, Lim KH, Jung HJ, et al. Anti-inflammatory evaluation of gardenia extract, geniposide and genipin. J Ethnopharmacol. 2006;103(3):496–500. doi:10.1016/j.jep.2005.08.011. PMID:16169698
- Park EH, Joo MH, Kim SH, et al. Antiangiogenic activity of Gardenia jasminoides fruit. Phytother Res. 2003;17(8):961–962. doi:10.1002/ptr.1259
- Zhang CY, Parton LE, Ye CP, et al. Genipin inhibits UCP2-mediated proton leak and acutely reverses obesity- and high glucose-induced beta cell dysfunction in isolated pancreatic islets. Cell Metab. 2006;3(6):417–427. doi:10.1016/j.cmet.2006.04.010
- Koriyama Y, Chiba K, Yamazaki M, et al. Long-acting genipin derivative protects retinal ganglion cells from oxidative stress models in vitro and in vivo through the Nrf2/antioxidant response element signaling pathway. J Neurochem. 2010;115(1):79–91. doi:10.1111/j.1471-4159.2010.06903.x. PMID:20681953
- Yamazaki M, Chiba K. Genipin exhibits neurotrophic effects through a common signaling pathway in nitric oxidesynthase-expressing cells. Eur J Pharmacol. 2008;581(3):255–261. doi:10.1016/j.ejphar.2007.12.001. PMID:18178184
- Ayyasamy V, Owens KM, Desouki MM, et al. Cellular model of Warburg effect identifies tumor promoting function of UCP2 in breast cancerand its suppression by genipin. PLoS One. 2011;6(9):e24792. doi:10.1371/journal.pone.0024792. PMID:21935467
- Tan HY, Wang N, Tsao SW, et al. IRE1α inhibition by natural compound genipin on tumour associated macrophages reduces growth of hepatocellular carcinoma. Oncotarget. 2016;7(28):43792–43804. doi:10.18632/oncotarget.9696. PMID:27270308
- Becker G., Kowall M. Crucial role of the postnatal maternal environment in the expression of prenatal stress effects in the male rats. J Comp Physiol Psychol. 1977;91:1432–1446. doi:10.1037/h0077401. PMID:563871
- Chapman R.H., Stern J.M. Failure of severe maternal stress or ACTH during pregnancy to affect emotionality of male rat offspring: implications of litter effects for prenatal studies. Dev Psychobiol. 1979;12:255–267. doi:10.1002/dev.420120309. PMID:220123
- Yankelevitch-Yahav R, Franko M, Huly A, et al. The forced swim test as a model of depressive-like behavior. J Vis Exp. 2015;(97):52587. doi:10.3791/52587. PMID:25867960
