ABSTRACT
Infants born preterm are at increased risk of multiple morbidities and mortality. Why some women deliver preterm remains poorly understood. Prior studies have shown that cervical microRNA expression and DNA methylation are associated with the length of gestation. However, no study has examined the role of long noncoding RNAs (lncRNAs) in the cervix during pregnancy. To determine whether expression of lncRNAs is associated with length of gestation at delivery, we analyzed RNA from cervical swabs obtained from 78 women during pregnancy (mean 15.5, SD 5.0, weeks of gestation) who were participating in the Spontaneous Prematurity and Epigenetics of the Cervix (SPEC) Study in Boston, MA, USA. We used a PCR-based platform and found that 9 lncRNAs were expressed in at least 50% of the participants. Of these, a doubling of the expression of TUG1, TINCR, and FALEC was associated with shorter lengths of gestation at delivery [2.8 (95% CI: 0.31, 5.2); 3.3 (0.22, 6.3); and 4.5 (7.3, 1.6) days shorter respectively]. Of the lncRNAs analyzed, none was statistically associated with preterm birth, but expression of FALEC was 2.6-fold higher in women who delivered preterm vs. term (P = 0.051). These findings demonstrate that lncRNAs can be measured in cervical samples obtained during pregnancy and are associated with subsequent length of gestation at delivery. Further, this study supports future work to replicate these findings in other cohorts and perform mechanistic studies to determine the role of lncRNAs in the cervix during pregnancy.
Introduction
Infants born preterm are at higher risk than full-term infants for multiple morbidities [Citation1]. While many epidemiologic studies have identified risk factors for preterm birth, including prior spontaneous preterm birth, short mid-pregnancy cervical length, black race (in the United States), bacterial vaginosis, and smoking [Citation2], the molecular mechanisms underlying preterm birth are incompletely understood. Inflammation has long been implicated in the pathophysiology of preterm birth [Citation3], but how it results in premature rupture of membranes, preterm labor, and cervical remodeling, all of which can lead to spontaneous preterm birth, remains unknown [Citation4].
Cervical samples obtained in pregnancy can be analyzed for epigenetic biomarkers such as DNA methylation and microRNA (miRNA) [Citation5–Citation7]. Specifically, we found that DNA methylation [Citation7] and miRNA expression [Citation6] mid-pregnancy are associated with the subsequent length of gestation at delivery. Others also have shown that miRNA expression is associated with the risk of spontaneous preterm birth [Citation8] and that miRNAs affect cervical remodeling [Citation9]. However, we are unaware of other studies that have analyzed expression of long noncoding RNA (lncRNA) obtained from the cervix during pregnancy in association with the length of gestation.
Less than 2% of the human genome is transcribed and translated into proteins [Citation10]. Much of the transcribed, but untranslated, DNA results in RNAs that do not encode for proteins but have critical cellular regulatory functions, including control of gene expression [Citation11–Citation16]. LncRNAs are > 200 nucleotides long and have more diverse functions than miRNAs [Citation17]. In the nucleus, they typically negatively regulate gene expression by recruiting DNA methyltransferase 3 and histone modifying complexes, such as the Polycomb Repressive Complex [Citation18]. In the cytoplasm, lncRNAs can enhance or repress translation by affecting messenger RNA (mRNA) stability (both positively and negatively) and by base pairing with mRNA [Citation18]. LncRNAs also can act as competing endogenous ‘miRNA sponges’ by binding to specific miRNAs to prevent them from inhibiting translation [Citation11]. LncRNAs are differentially expressed in the setting of inflammation [Citation19] and thus may play a role in the length of gestation or preterm birth.
As lncRNA expression varies by tissue type, it is critical to collect samples from the tissues relevant to the physiologic processes of interest. Cervical change is a necessary step for parturition. For this reason, to determine whether expression of lncRNAs is associated with length of gestation at delivery, we analyzed RNA from cervical swabs obtained before 28 weeks of gestation from pregnant participants in the Spontaneous Prematurity and Epigenetics of the Cervix (SPEC) Study in Boston, MA, USA.
Results
The majority of participants (55.8%) were white and well-educated, with 60.8% of participants having obtained at least a college degree. Characteristics of the 78 participants are presented in . Of the 87 lncRNAs analyzed on the PCR array, 9 were expressed in over 50% of participants’ samples and retained for downstream analysis (). The number of participants’ samples in which each lncRNA was detected ranged from 40 to 78. Correlations among each of the lncRNAs ranged from 0 to 0.8 (Supplemental ). Expression is denoted as difference in the cycle threshold for PCR amplification of each lncRNA compared to the housekeeping genes (ΔCt), where a higher ΔCt means lower expression and a lower ΔCt means higher expression. Raw Ct values for all assays are provided in the supplemental material. Bivariate analyses showed that UCA1 expression was negatively associated with maternal age and negatively associated with pre-pregnancy body mass index (BMI) (Supplemental ). TINCR expression was associated with race/ethnicity (P = 0.016). Specifically, expression was lower among non-Hispanic black women compared with white women (ΔCt β = 1.3, P = 0.02). ZFAS1 and SRA1 expression were both lower when swabs were collected later in pregnancy; per week increment of gestation ΔCt was 0.05 (P = 0.04) and 0.08 (P = 0.01) higher, respectively.
Table 1. Study participant characteristics (n = 78*).
Table 2. Long noncoding RNAs (lncRNAs) detected in cervical samples from 78 pregnant women.
Unadjusted linear regression models revealed that expression of TUG1 and FALEC was significantly associated with the length of gestation, although using a conservative Bonferroni adjustment for multiple comparisons (P < 0.0056), just FALEC remained significant. Introduction of the following covariates did not appreciably change the effect estimates: maternal age, income, education, parity, marital status, multiple gestations, and gestational age at which swabs were collected. Maternal race/ethnicity did substantially affect the effect estimates and was retained in final models. In race/ethnicity-adjusted models, 3 of the 9 lncRNAs were associated with the length of gestation: TUG1, TINCR, and FALEC (). For each of these lncRNAs, higher expression levels were associated with shorter gestations. Per doubling of expression (-ΔCt), the lengths of gestation were 2.8 (95%CI: 0.31, 5.2), 3.3 (95%CI: 0.22, 6.3), and 4.5 (95%I CI: 1.6 7.3) days shorter, respectively (). The lengths of gestation were similar among women with and without expression of each of these lncRNAs. For TUG1, gestations were 0.2 days shorter among the 70 women with expression compared to the 8 women without (P = 0.96). For TINCR, gestations were 1.1 days shorter among the 48 women with expression compared to the 30 without (P = 0.69). For FALEC, gestations were 1.2 days shorter among the 40 women with expression compared to the 38 without (P = 0.63). Scatter plots of the remaining 6 lncRNAs and gestational age at delivery are presented in Supplemental .
Table 3. Associations of long noncoding RNAs (lncRNAs) from cervical samples obtained prior to 28 weeks of gestation and subsequent length of gestation at delivery.
Figure 1. Scatter plots of long noncoding RNA expression (-ΔCt values on x-axis; less negative values indicate higher expression) and gestational age at delivery.
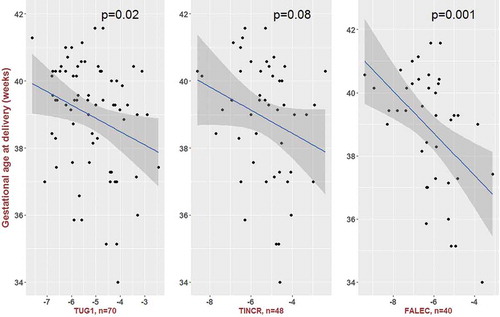
The coefficient of variance (CV) among the duplicate samples of the 9 lncRNAs analyzed was low, ranging from < 0.1% to 3.2%. The duplicate samples associated with the length of gestation also had high correlation coefficients (TUG1 0.95, TINCR 0.73, FALEC 0.77), demonstrating technical validity. Further, for most duplicates, there was agreement with respect to detection/non-detection (70% for TUG1, 88% for TINCR, 84% for FALEC). When we excluded the discordant samples, effect estimates for the association of lncRNA expression and length of gestation were similar to the models presented in . Per doubling of expression (-ΔCt value), gestations were 3.1 (95% CI: 0.72, 5.43), 2.3 (−0.4, 5.0), and 5.2 (2.1, 8.3) days shorter for TUG1, TINCR, and FALEC, respectively. There were 7 preterm deliveries among the 78 participants; however, the range of preterm births for which there was lncRNA expression varied from 4/43 for MALAT1 to 7/78 for NEAT1(). In unadjusted analyses, just FALEC expression was associated with preterm birth. Specifically, those who delivered preterm had 2.5-fold higher expression than women who delivered at term (P = 0.03). Adjustment for race/ethnicity did not change the effect estimate (2.6-fold higher expression) but the effect estimate was no longer statistically significant (P = 0.051).
Of the 73 histopathologic slides available for staining, 29 (39.7%) participants had a low leukocyte burden. Leukocyte burden was associated with expression of 6 of the 9 lncRNAs (Supplemental ). In a sensitivity analysis among the women for whom we had leukocyte burden data, associations of lncRNA expression and gestational age were similar to the whole study sample and further adjustment for leukocyte burden did not alter the estimates (Supplemental ).
Discussion
We found that the expression of 3 lncRNAs measured in cervical samples obtained mid-pregnancy was associated with the subsequent length of gestation at delivery. Specifically, higher expression of TUG1, TINCR, FALEC was associated with shorter gestations. Further, expression of FALEC was higher among women who went on to deliver preterm in the unadjusted, but not the adjusted, analysis.
While historically thought of as ‘junk’ sequences, noncoding RNAs recently have gained attention as critical regulators of cancer biology and epigenetic processes involved in human development, including X chromosome inactivation and growth [Citation20]. Increasingly, apart from cancer, lncRNA expression has been found to be associated with inflammatory diseases [Citation21] such as systemic lupus erythematosus [Citation20,Citation21], renal inflammation and fibrosis [Citation21], and perhaps most relevant, early spontaneous abortion [Citation22] and premature rupture of membranes [Citation22]. Wang and colleagues compared embryonic tissues from spontaneous and induced abortions and analyzed lncRNAs using a microarray platform that measures expression of 33,045 lncRNAs. Of the lncRNAs measured, they found that over 1,700 were differentially expressed between the two groups [Citation22]. Using the same microarray as Wang et al. [Citation22], Luo et al. studied the placentas from 20 term deliveries, with and without premature rupture of membranes, and 20 preterm deliveries, with and without premature rupture of membranes [Citation23]. They found that 449 to 3,607 lncRNAs differed among the groups and that lncRNAs involved in infection and inflammation pathways differed among the four groups. While studies of cervical lncRNA expression are emerging in the cervical cancer literature [Citation9,Citation22,Citation24,Citation25], we are not aware of other published studies evaluating cervical samples for lncRNA expression in pregnancy.
While little is known about the role the three lncRNAs (TUG1, TINCR, and FALEC) play in the cervix, they all have been studied with respect to cancer. The gene for TUG1 is located on chromosome 22. Li et al. reported that TUG1 expression was inhibited in glioma tissues and that upregulation could activate caspase-3 and −9, inhibiting expression of Bcl-2 and resulting in apoptosis [Citation26]. They also reported that downregulation of TUG1 was associated with poor prognosis in patients with glioma. However, in other studies, TUG1 has been reported to function as an oncogene [Citation27–Citation32]. With respect to the cervix, upregulation of TUG1 has been shown to promote cervical cancer cell proliferation and migration in a study of 60 patients treated for cervical cancer [Citation33]. The TINCR gene is located on chromosome 19 and its expression has been shown to be associated with the progression of gastric cancer [Citation34], a poor prognosis in patients with hepatocellular carcinoma [Citation34], and invasion of esophageal squamous cell carcinoma cells [Citation35]; conversely, loss of its expression may promote proliferation and metastasis in colorectal carcinoma [Citation36]. The FALEC gene is located on chromosome 1. Hu et al. have shown that expression of FALEC is much higher in epithelial tumors than other types of tumors and associated with progression in ovarian cancer [Citation37]. Zhao et al. also found that FALEC expression was higher in prostate cancer tissues compared to adjacent normal prostate cells [Citation38].
While our study is on the forefront of studying cervical lncRNAs with respect to birth outcomes in a tissue relevant to preterm birth, it has limitations. First, there were just 7 preterm births in the cohort, limiting the power to detect this clinically important outcome. Yet, gestational age is a continuous variable and even among term infants, health outcomes are worse at 37 and 38 weeks than at 39 weeks [Citation39]. Thus, gestational age matters even in the term population. Second, RNA obtained from cervical cells can be degraded and contaminated; therefore, our PCR platform may have underestimated the presence of other lncRNAs considered ‘undetected’ in our sample. Nonetheless, for the PCR reaction to occur, intact sequences must be detected by the primers, suggesting lncRNAs that we did detect likely are truly present. We ran 25 samples in duplicate with good agreement of detection and non-detection. In cases where there was one expressed replicate and one non-expressed replicate, the expression levels were quite low. When these samples were excluded from the analysis, the associations were strengthened, suggesting potential bias toward the null by including them. PCR platforms differ from sequencing tools that can detect novel sequences and microarrays that can detect tens of thousands of lncRNAs. The platform we used can measure only the 87 lncRNAs for which there were primers, but it measures lncRNAs that are known to play biologic roles in cellular differentiation and organism development and thus were likely candidates for expression during pregnancy. However, lncRNA expression is cell-type dependent and the assay was not designed specifically for cervical samples and thus we may have missed biologically relevant lncRNAs [Citation40]. One benefit of the platform is that it is PCR-based and does not require additional validation as sequencing or affinity-based microarrays would. Nonetheless, our findings require replication in a separate cohort and could be broadened to study other lncRNAs and cervical function in pregnancy.
In conclusion, our study demonstrated that lncRNAs can be measured in cervical samples and that higher TUG1, TINCR, and FALEC expression may be associated with shorter lengths of gestation, a finding that requires replication. Further work to delineate the determinants of lncRNA expression and their mechanistic roles in pregnancy is warranted.
Methods
SPEC cohort enrollment and participant selection
We enrolled women receiving routine prenatal care at a single medical center in Boston, Massachusetts, USA. We obtained written informed consent from all participants. Women were eligible if they were at least 18 years of age and less than 28 weeks of gestation. Women self-reported race/ethnicity, pre-pregnancy height and weight, and history of smoking. Samples from the first 99 women enrolled in the cohort were sent for lncRNA analysis; 8 samples had insufficient yields and concentrations for lncRNA analysis and 10 samples did not pass quality control. Of the remaining 81 samples, birth outcomes were available for 78 women. Birth outcomes were obtained from the medical record for all but 6 women who delivered elsewhere. We contacted those women by phone to obtain self-reported birth outcomes. Preterm birth was defined as delivery prior to 37 completed weeks of gestation. This study was approved by the Institutional Review Board at Beth Israel Deaconess Medical Center (Protocol 2013P-000125).
Cervical lncRNA ascertainment
After cervical mucus was wiped away, we collected cervical samples using dacron swabs during a speculum examination. Each swab was twirled 5 times within the endocervix. One swab was used for RNA and another for histopathologic slide analysis. Mean gestational age at the time of cervical swab collection was 15.5 weeks (SD 5.0 weeks). Samples were stored in RNAlater (Qiagen, Germantown, MD) at 4°C. Within 7 days, RNA was extracted using the Maxwell 16 Cell LEV Total RNA Purification Kit (Promega, Madison, WI) and then frozen at −80°C. The RNA was analyzed using a custom 384-well lncRNA PCR Array (Qiagen, Cat. no. CLAH00013), which simultaneously measures the expression of 87 lncRNAs. The array was based on the Human Cell Development & Differentiation RT2 lncRNA PCR Array (Qiagen, Cat. no. LAHS-003Z) that profiles the expression of 84 lncRNAs expressed during cellular differentiation and organism development, as well as three additional lncRNAs: THRIL, PINT, and SRA1 [Citation41,Citation42]. The array uses laboratory-verified SYBR® Green qPCR assays. We included controls in each assay to assess the presence of genomic DNA contamination, reverse transcription performance, and PCR performance. The protocol includes a genomic DNA elimination step prior to reverse transcription, which was done using the RT2 First Strand Kit (Qiagen) according to the manufacturer’s protocol. For the cDNA synthesis (using the RT2 First Strand Kit), we used 25 ng of RNA in 8 ul. The synthesized cDNA was mixed with RT2 SYBR Green Mastermix, and aliquoted into the PCR plate using a Microlab STARlet (Hamilton) Liquid Handling Workstation. Real-time qRT-PCR was performed on a Bio-Rad CFX384 instrument with an initial HotStartTaq DNA polymerase activation step at 95°C for 10 min, followed by 40 cycles of 15 s at 95°C and 1 min at 60°C. We ran 25 samples in duplicate and randomly, blindly positioned them across plates and batches. Additionally, the laboratory personnel were blinded with respect to the outcome. In agreement with the manufacturer’s recommendations, we considered a lncRNA as unexpressed if more than 35 cycles were required for amplification and fluorescence signaling (Ct > 35). To assess RNA quality, we analyzed 9 samples from this cohort that were not included in this analysis using the Agilent RNA 6000 Nano Kit following manufacturer’s protocol (Agilent 2100 Bioanalyzer, Agilent Technologies, Foster City, CA).
Histopathologic slide assessment for leukocyte burden
Given cell type can affect expression of lncRNAs, we stained the contents of the cervical swab using a modified hematoxylin and eosin staining (H&E) protocol and then quantified each sample as high or low leukocyte burden. We defined leukocyte burden as high when leukocytes were overlapping, and thus obscuring, epithelial cells throughout the slide or if there were at least 3 fields at 100X magnification with greater than 2 clusters of leukocytes, which were predominantly neutrophils. A cluster was defined as > 15 cells. Samples not meeting these criteria were included in the low leukocyte burden category.
Statistical analysis
We retained lncRNAs that were expressed in more than half of the samples for downstream analyses. Subsequently, to normalize lncRNA expression and calculate the ΔCt value, we subtracted the geometric mean of Ct values for 4 housekeeping genes including in the PCR array (ACTB, B2M, RPLP0, and RN7SK) from each lncRNA Ct value. To explore bivariate associations of known predictors of birth outcomes and lncRNAs, we used unadjusted linear regression models with the ΔCt as the dependent variable. To determine if cervical lncRNAs were associated with the length of gestation, we used multivariable linear regression models with – ΔCt (doubling of expression) as the independent variable, adjusting for potential confounders. We retained covariates in models if they affected the effect estimates by 10 percent or more. We considered estimates to be statistically significant if the P value was < 0.05, but also explored whether estimates would have remained significant after adjustment for multiple comparisons using a Bonferroni correction. We used a complete case approach; participants who did not have a lncRNA expressed were not included in models. To determine if lncRNA expression differed among women who delivered preterm versus term, we used unadjusted and adjusted linear regression models with the lncRNAs as the dependent variable and calculated fold-changes for each lncRNA in the two groups of women using the formula 2^(-ΔΔCt) [Citation43].
We performed three additional analyses. First, to understand whether technical factors in the PCR reaction contributed to our findings, we analyzed 25 sets of duplicates and calculated Pearson correlations in samples that had lncRNAs detected in both samples and evaluated whether there was agreement with respect to detection/non-detection in each replicate. We compared our primary analysis (in which we used the samples with the most lncRNAs detected) to one in which discordant pairs of samples were dropped. Second, we compared the length of gestation for the three significantly associated lncRNAs among participants with and without expression of these lncRNAs using unpaired t-tests. Last, to understand the potential contribution of cell proportions, we repeated the primary analysis in the subset of participants for whom slide data were available and then further adjusted for leukocyte burden in multivariable models. Analyses were performed using SAS 9.4 (SAS Institute, Cary, NC) and R 3.4.2 (2017–09–28).
Supplemental Material
Download Zip (151.3 KB)Acknowledgments
We are thankful to Melissa Ada, Allison O’Neill, Emily Nuss, Erin Kennedy, and Allyson Redhunt who have all served as research assistants for the SPEC study. We acknowledge the clinical staff that made this study possible at the Center for Maternal Fetal Medicine and outpatient prenatal clinics at Beth Israel Deaconess Medical Center as well as the Bowdoin Street clinic. We also thank the staff at the Clinical Research Center at Beth Israel Deaconess Medical Center. We thank Oksar Karlsson for his assistance in setting up the lncRNA assay. We also appreciated the support and feedback from members of the NeoWIP. We are also grateful to the SPEC study participants.
Disclosure Statement
Conflicts of interest/financial disclosures relevant to this work: None
Supplementary Material
Supplemental data for this article can be accessed here
Additional information
Funding
References
- Behrman RE, Butler AS, editors. Preterm birth: causes, consequences, and prevention. Washington (DC): The National Academies Collection: Reports funded by National Institutes of Health; 2007.
- Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84.
- Trivedi S, Joachim M, McElrath T, et al. Fetal-placental inflammation, but not adrenal activation, is associated with extreme preterm delivery. Am J Obstet Gynecol. 2012;206(3):236e1–8.
- Moutquin JM. Classification and heterogeneity of preterm birth. BJOG. 2003;110(Suppl 20):30–33.
- Sanders AP, Burris HH, Just AC, et al. Altered miRNA expression in the cervix during pregnancy associated with lead and mercury exposure. Epigenomics. 2015;7(6):885–896.
- Sanders AP, Burris HH, Just AC, et al. microRNA expression in the cervix during pregnancy is associated with length of gestation. Epigenetics. 2015;10(3):221–228.
- Burris HH, Baccarelli AA, Motta V, et al. Association between length of gestation and cervical DNA methylation of PTGER2 and LINE 1-HS. Epigenetics. 2014;9(8):1083–1091.
- Elovitz MA, Brown AG, Anton L, et al. Distinct cervical microRNA profiles are present in women destined to have a preterm birth. Am J Obstet Gynecol. 2014;210(3):221e1–11.
- Anton L, DeVine A, Sierra LJ, et al. miR-143 and miR-145 disrupt the cervical epithelial barrier through dysregulation of cell adhesion, apoptosis and proliferation. Sci Rep. 2017;7(1):3020.
- Alexander RP, Fang G, Rozowsky J, et al. Annotating non-coding regions of the genome. Nat Reviews Genet. 2010;11(8):559–571.
- Esteller M. Non-coding RNAs in human disease. Nat Reviews Genet. 2011;12(12):861–874.
- Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43(6):904–914.
- Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Reviews Genet. 2009;10(3):155–159.
- Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21(6):354–361.
- Whitehead J, Pandey GK, Kanduri C. Regulation of the mammalian epigenome by long noncoding RNAs. Biochim Biophys Acta. 2009;1790(9):936–947.
- Ransohoff JD, Wei Y, Khavari PA. The functions and unique features of long intergenic non-coding RNA. Nat Rev Mol Cell Biol. 2018;19(3):143–157.
- Volders PJ, Helsens K, Wang X, et al. LNCipedia: a database for annotated human lncRNA transcript sequences and structures. Nucleic Acids Research. 2013;41(Databaseissue):D246–51.
- Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat Reviews Genet. 2014;15(1):7–21.
- Marques-Rocha JL, Samblas M, Milagro FI, et al. Noncoding RNAs, cytokines, and inflammation-related diseases. FASEB J. 2015;29(9):3595–3611.
- Leviton A, Fichorova R, Yamamoto Y, et al. Inflammation-related proteins in the blood of extremely low gestational age newborns. The contribution of inflammation to the appearance of developmental regulation. Cytokine. 2011;53(1):66–73.
- Yang H, Liang N, Wang M, et al. Long noncoding RNA MALAT-1 is a novel inflammatory regulator in human systemic lupus erythematosus. Oncotarget. 2017;8(44):77400–77406.
- Wang H, Cao Q, Ge J, et al. LncRNA-regulated infection and inflammation pathways associated with pregnancy loss: genome wide differential expression of lncRNAs in early spontaneous abortion. Am J Reprod Immunol. 2014;72(4):359–375.
- Luo X, Shi Q, Gu Y, et al. LncRNA pathway involved in premature preterm rupture of membrane (PPROM): an epigenomic approach to study the pathogenesis of reproductive disorders. PLoS One. 2013;8(11):e79897.
- Jiang B, Sun R, Fang S, et al. Lnc-CC3 increases metastasis in cervical cancer by increasing Slug expression. Oncotarget. 2016;7(27):41650–41661.
- Liu Q, Guo X, Que S, et al. LncRNA RSU1P2 contributes to tumorigenesis by acting as a ceRNA against let-7a in cervical cancer cells. Oncotarget. 2017;8(27):43768–43781.
- Li J, Zhang M, An G, et al. LncRNA TUG1 acts as a tumor suppressor in human glioma by promoting cell apoptosis. Exp Biol Med (Maywood). 2016;241(6):644–649.
- Xu Y, Wang J, Qiu M, et al. Upregulation of the long noncoding RNA TUG1 promotes proliferation and migration of esophageal squamous cell carcinoma. Tumour Biol. 2015;36(3):1643–1651.
- Zhang EB, Yin DD, Sun M, et al. P53-regulated long non-coding RNA TUG1 affects cell proliferation in human non-small cell lung cancer, partly through epigenetically regulating HOXB7 expression. Cell Death Dis. 2014;5:e1243.
- Zhang Q, Geng PL, Yin P, et al. Down-regulation of long non-coding RNA TUG1 inhibits osteosarcoma cell proliferation and promotes apoptosis. Asian Pac J Cancer Prev. 2013;14(4):2311–2315.
- Ma B, Li M, Zhang L, et al. Upregulation of long non-coding RNA TUG1 correlates with poor prognosis and disease status in osteosarcoma. Tumour Biol. 2016;37(4):4445–4455.
- Huang MD, Chen WM, Qi FZ, et al. Long non-coding RNA TUG1 is up-regulated in hepatocellular carcinoma and promotes cell growth and apoptosis by epigenetically silencing of KLF2. Mol Cancer. 2015;14:165.
- Wang L, Zhao Z, Feng W, et al. Long non-coding RNA TUG1 promotes colorectal cancer metastasis via EMT pathway. Oncotarget. 2016;7(32):51713–51719.
- Hu Y, Sun X, Mao C, et al. Upregulation of long noncoding RNA TUG1 promotes cervical cancer cell proliferation and migration. Cancer Med. 2017;6(2):471–482.
- Xu TP, Wang YF, Xiong WL, et al. E2F1 induces TINCR transcriptional activity and accelerates gastric cancer progression via activation of TINCR/STAU1/CDKN2B signaling axis. Cell Death Dis. 2017;8(6):e2837.
- Xu Y, Qiu M, Chen Y, et al. Long noncoding RNA, tissue differentiation-inducing nonprotein coding RNA is upregulated and promotes development of esophageal squamous cell carcinoma. Dis Esophagus. 2016;29(8):950–958.
- Zhang ZY, Lu YX, Zhang ZY, et al. Loss of TINCR expression promotes proliferation, metastasis through activating EpCAM cleavage in colorectal cancer. Oncotarget. 2016;7(16):22639–22649.
- Hu X, Feng Y, Zhang D, et al. A functional genomic approach identifies FAL1 as an oncogenic long noncoding RNA that associates with BMI1 and represses p21 expression in cancer. Cancer Cell. 2014;26(3):344–357.
- Zhao R, Sun F, Bei X, et al. Upregulation of the long non-coding RNA FALEC promotes proliferation and migration of prostate cancer cell lines and predicts prognosis of PCa patients. Prostate. 2017;77(10):1107–1117.
- American College of O, Gynecologists. ACOG committee opinion no. 560: medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2013;121(4):908–910.
- Goff LA, Groff AF, Sauvageau M, et al. Spatiotemporal expression and transcriptional perturbations by long noncoding RNAs in the mouse brain. Proc Natl Acad Sci U S A. 2015;112(22):6855–6862.
- Rodosthenous RS, Burris HH, Sanders AP, et al. Second trimester extracellular microRNAs in maternal blood and fetal growth: an exploratory study. Epigenetics. 2017;12(9):804–810.
- Karlsson O, Rodosthenous RS, Jara C, et al. Detection of long non-coding RNAs in human breastmilk extracellular vesicles: implications for early child development. Epigenetics. 2016;11(10):721–729.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408.
