ABSTRACT
Children conceived by Assisted Reproductive Technologies (ART) are at moderately increased risk for a number of undesirable outcomes, including low birth weight. Whether the additional risk is associated with specific procedures used in ART or biological factors that are intrinsic to infertility has been the subject of much debate, as has the mechanism by which ART or infertility might influence this risk. The potential effect of ART clinical and laboratory procedures on the gamete and embryo epigenomes heads the list of mechanistic candidates that might explain the association between ART and undesirable clinical outcomes. The reason for this focus is that the developmental time points at which ART clinical and laboratory procedures are implemented are precisely the time points at which large-scale reorganization of the epigenome takes place during normal development. In this manuscript, we review the many human studies comparing the epigenomes of ART children with children conceived in vivo, as well as assess the potential of individual ART clinical and laboratory procedures to alter the epigenome.
KEYWORDS:
More than five million children have been born with the aid of assisted reproductive technologies (ART). Though the great majority of these infants are born healthy, epidemiological studies suggest that infants born following ART are at increased risk for adverse perinatal outcomes, including fetal growth restriction, preeclampsia, and preterm birth [Citation1–Citation5]. These observations are not related solely to multiple pregnancy as adverse effects are seen even in singleton conceptions [Citation1]. It has been suggested that the underlying infertility may be responsible for the adverse outcomes seen following IVF [Citation6]. However, animal studies have also demonstrated phenotypic and molecular changes in placenta and offspring following ART in the absence of any underlying infertility. These data suggest that procedures utilized during IVF lead to changes in the fetus and/or placenta that increase the risk of adverse outcomes.
Assisted reproductive technologies, and specifically in vitro fertilization (IVF), involve multiple exposures including superovulation, embryo manipulation, embryo culture and embryo transfer, exposing gametes and embryos to an altered hormonal environment and changes in temperature, pH and oxygen tension. These exposures occur at critical times of development, which coincide with global reprogramming of the epigenome and the establishment of epigenetic changes that persist into adulthood [Citation7]. The most well studied epigenetic modification associated with ART is DNA methylation, due to its discrete nature and the availability of robust tools to interrogate localized as well as global changes. Both animal and human studies suggest that ART are associated with epigenetic changes in embryonic and extra-embryonic tissues [Citation8–Citation13]. It is therefore likely that epigenetic modifications that occur due to the exposures required for IVF could be central to the pathogenesis of at least some of the observed adverse outcomes seen after ART. In this review, we summarize existing data examining the link between procedures involved in IVF, changes in DNA methylation, and the development of short- and long-term adverse outcomes.
What adverse outcomes are commonly associated with IVF?
Initial studies examining the effects of IVF found an increased risk of adverse outcomes in pregnancies conceived by IVF when compared to pregnancies conceived through natural conception. Initially, these complications were attributed to the increased risk of multiple gestation following ART [Citation14–Citation16]. These findings led to changes in guidelines and the implementation of strategies to reduce multiple pregnancies including blastocyst transfer and elective single embryo transfer [Citation17]. However, further studies demonstrated that the increase in adverse outcomes persisted even in singleton pregnancies. Multiple meta-analyses and retrospective cohort studies identified an increased risk of preeclampsia, preterm delivery, placenta previa, placental abruption, intrauterine growth restriction and low birth weight (< 2500g) in singleton pregnancies following IVF [Citation2,Citation18–Citation22]. Many of these outcomes have been associated with disordered placentation. The establishment of the human placenta occurs early in gestation and involves appropriate and regulated invasion of placental trophoblast cells into the maternal decidua. Recent studies have linked changes in chromatin structure, DNA methylation and miRNA expression to changes in trophoblast migration and invasion, suggesting that epigenetic regulation during early pregnancy is critical for proper placentation, and perturbation of this regulation by ART could lead to the development of the adverse outcomes seen [Citation23–Citation25].
Studies have also demonstrated an increased risk of birth defects in infants conceived by IVF. In addition to single congenital defects, ART has been linked to a number of rare imprinting disorders [Citation27,Citation26,Citation28,Citation29]. A recent meta-analysis of 23 studies reported positive associations between ART and four imprinting disorders: Beckwith-Wiedemann, Angelman, Prader-Willi and Silver-Russell syndromes [Citation29]. Imprinted genes have parental-specific expression, through silencing of one allele by epigenetic modifications such as DNA methylation. In disorders of imprinting, allele-specific DNA methylation is perturbed, leading to disruption of mono-allelic gene expression [Citation26]. The increased prevalence of these disorders after IVF supports the role of abnormal epigenetic regulation in the development of adverse outcomes after ART.
The effect of ART on post-natal life has been more difficult to elucidate, as the majority of cases of IVF births have occurred in the past three decades. Studies have, however, observed alterations in growth and metabolism in children born following IVF; children conceived by ART have been found to have increased fat mass, impaired insulin sensitivity and elevated fasting glucose levels when compared with children from unassisted conception [Citation30–Citation32]. Children born following IVF have also been observed to have elevated systolic and diastolic blood pressures as well as elevated triglyceride levels compared to controls [Citation33]. These findings persist when controlling for birth weight and are even more pronounced in children born after intrauterine growth restriction [Citation33]. It is well known that low birth weight (LBW) infants are at increased risk of adverse neonatal outcomes such as a four-fold increased risk of neonatal death [Citation34] and they are at increased risk of metabolic abnormalities later in life including obesity, coronary heart disease and diabetes [Citation35–Citation38]. Growth restriction is also associated with premature puberty, cardiovascular, renal and neurological disease [Citation39]. Prior studies that have followed children born after IVF have been hindered by small sample size, and potential confounders arising from adverse gestational outcomes also associated with IVF (refs) [Citation30,Citation40,Citation41]. It is clear that well-controlled, long-term follow-up studies are necessary to validate and understand these findings.
Why are pregnancies conceived by ART susceptible to changes in DNA methylation?
The many procedures required for IVF occur during critical periods of epigenetic reprograming, which involves the erasure of existing epigenetic modifications followed by replacement with new modifications, in both the gamete and the somatic tissues of the embryo. The exact dynamics of epigenetic reprograming during reproduction have been well described in murine models, demonstrating two main windows for epigenetic reprogramming: 1) gametogenesis, when oocytes undergo DNA demethylation and then gradual remethylation of the genome, including at imprinted genes and transposon regions; and 2) after fertilization during early embryo development where there is again global demethylation (with the exception of imprinted genes) and remethylation to allow for lineage establishment [Citation42,Citation43]. There is less known about the sequence of events in humans, though recent work suggests several global similarities: during gametogenesis, demethylation during germ cell specification is seen even at imprinted regions albeit with some timing differences, [Citation44] followed by global demethylation after fertilization, though genes apart from imprinted genes are also protected in the maternal genome, suggesting a lower level of demethylation [Citation45–Citation48].
During these periods of epigenetic reprograming, the genome is particularly susceptible to environmentally-induced epigenetic defects. Superovulation, or controlled ovarian hyperstimulation, is used ubiquitously during IVF, and has been shown to impact epigenetic programing during oogenesis [Citation49–Citation54]. Embryo culture, embryo transfer and preimplantation genetic testing are additional ART procedures that coincide with epigenetic reprograming during embryo development. Because environmental exposures are known to lead to stable epigenetic changes [Citation55], it is likely that the environmental perturbations associated with ART could lead to changes in DNA methylation dynamics during gametogenesis and early embryo development.
What are the existing data regarding the effect of assisted reproductive technologies on the epigenome?
The association between ART, adverse pregnancy outcomes, and imprinting disorders, and understanding of the timing of epigenetic reprogramming, led investigators to examine the role of epigenetic reprograming in the adverse outcomes associated with IVF. Over the past 15 years multiple studies have examined DNA methylation in fetal and/or placental tissues from pregnancies conceived by IVF. There is great heterogeneity in the sample size, methodology and approach of these studies which has led to the inconsistency in the results of these studies.
Examination of epigenetic change in candidate genes
The initial studies on epigenetic changes following ART were restricted to candidate gene approaches, focused primarily on imprinted genes and imprinting control regions (ICR) [Citation11,Citation56–Citation81]. (Supplemental Table 1). These studies were carried out in cord blood, peripheral blood, buccal samples and early/late gestation placental tissues. Many of these studies, though not all, reported significant differences between ART and naturally conceived children [Citation10,Citation11,Citation56,Citation57,Citation59,Citation69,Citation74–Citation77,Citation79,Citation81,Citation82]. Of those that reported differences, the KvDMR1 gene [Citation11,Citation69,Citation79], and the H19/IGF2 genes and imprinting control region were found perturbed in several studies [Citation56,Citation61,Citation76,Citation81–Citation83]. There was little consistency in both the genes tested and the epigenetic change found in other genes. The discordance in these results may be attributed to limited sample sizes in many of these studies.
Skewing of X-chromosome inactivation (XCI) has also been studied in female newborns conceived by IVF, though the data does not support a disruption in XCI following IVF. In one study, XCI patterns in cord blood samples of 30 IVF and 44 naturally conceived female infants were analyzed but XCI skewing was not found to be significantly different in the two groups [Citation84]. In another larger study with 60 ICSI, 73 IVF and 52 naturally conceived female newborns, XCI skewing was not found to be associated with the mode of conception [Citation85].
Examination of genome-wide epigenetic change: cord blood/placenta
With the advent of genome-wide approaches, more studies have focused on interrogating global DNA methylation differences in children conceived by ART and unassisted conception providing a more unbiased look at epigenome changes with ART. () Our group was one of the first to examine ART-associated DNA methylation differences at multiple areas of the human genome. In our initial studies we utilized a custom Illumina GoldenGate assay to examine DNA methylation of 1536 CpG sites, largely in the promoters of 736 genes selected for importance in development and cancer, in placenta and cord blood samples from pregnancies conceived either through IVF or through natural conception [Citation10]. We reported hypermethylation in placental and hypomethylation in cord blood samples of in vitro conceived children. In addition, we were also able to demonstrate gene expression differences in a subset of the differentially methylated genes. Interestingly, methylation and expression differences were observed in both imprinted and non-imprinted genes. Camprubi et al [Citation73] also used a custom Illumina GoldenGate methylation array to determine differences between ART and non-ART populations in cord blood and placenta, selecting 25 imprinted differentially methylated regions (DMRs). These authors also interrogated repeat sequences (Alu Yb8, LINE-1 and α- satellites) using pyrosequencing. However, in their study, there were no significant methylation differences at the imprinted domains in the ART and non-ART groups.
Table 1. Studies using genome-wide approaches to investigate epigenetic differences due to ART.
The emergence of Illumina Infinium Human Methylation Arrays, capable of interrogating 27,000 CpGs (27K array) and 450,000 CpGs (450K array), respectively, allowed researchers to interrogate larger numbers of sites in a single assay. Melamed et al. [Citation86] analyzed methylation differences in cord blood samples of ten ART and eight control samples using the Illumina Infinium HumanMethylation 27K array. They observed significant differences in 2.7% of the assayed sites with hypomethylation in the ART group. The ART group also had increased variance in methylation at the interrogated CpG sites. These findings were corroborated in cord blood by two studies using the Illumina Infinium Human Methylation 450K BeadChip assay, where differential methylation was noted in the ART group, with the most significant differences noted in samples that underwent ICSI [Citation87,Citation88]. Genome-wide studies using the Illumina 450K BeadChip assay have also been carried out in the placenta by two groups, though no statistically significant methylation differences were noted [Citation89,Citation90].
One study used genome-wide methylated DNA immunoprecipitation followed by deep sequencing (MeDIP-seq) to analyze differential methylation in IVF and non IVF twins [Citation91]. In addition to the examination of either individual genes or specific CpGs throughout the genome, other studies have examined DNA methylation at repeated sequences as a surrogate measure of global DNA methylation. Because repeated sequences make up a large portion of the genome, methylation changes found in these regions is more likely to be representative of the whole genome. We observed global hypermethylation at CCGG sites (~2.3 million such sites in the human genome and hypomethylation at LINE1 repeat elements in placenta from pregnancies conceived by ART when compared to control placenta [Citation92,Citation93]. However, while another group failed to find significant differences in methylation at LINE1 elements in placentas from IVF pregnancies, the study did identify an ART ‘outlier’ group with global hypomethylation, based on methylation levels at sites assayed on the Illumina 450K array [Citation90]. It is noteworthy that we first proposed the ‘outlier’ concept in an earlier study [Citation94], observing that ‘epigenetic outliers’ may be responsible for inconsistent results regarding methylation differences between ART and natural conceptions.
Examination of genome-wide epigenetic change: early pregnancy tissues
The majority of studies examining epigenetic changes are conducted in samples resulting from live births at the time of delivery. One of the limitations of these analyses is that we are examining tissue 9 months following the initial exposure. Few studies have examined first trimester tissue. In a study by Xu et al, they examined chorionic villus samples from first trimester pregnancies. The authors did not observe any significant methylation differences between the IVF and controls in the CVS samples [Citation95]. However, the study identified 34 differentially methylated loci between IVF and non-IVF fertility treatments (NIFT) groups. The authors were also able to demonstrate expression differences in five of the 11 differentially methylated genes in a validation cohort. Several other studies have examined epigenetic changes in specimens following spontaneous abortions, though these data must be interpreted with caution as the samples may be from ‘abnormal’ pregnancies [Citation56,Citation61,Citation64,Citation66,Citation78,Citation96]. Results from these studies vary significantly. In one of these studies, the authors examined the methylation status of seven autosomal imprinted genes, the XIST gene and repetitive sequences (LINE1 and Alu elements) in 78 ART and 38 non-ART aborted samples [Citation56]. The authors found aberrant paternal and maternal imprints in ART samples. A study of seven DMRs of imprinted genes and two non-imprinted gene promoters in CVS samples from pregnancy losses after ART and spontaneous conceptions revealed significant methylation differences between ART and non-ART CVS for LIT1 and H19 [Citation61]. Another group, however, was unable to demonstrate methylation differences in CVS tissue in the imprinted PEG1 gene based on the mode of conception, and observed similar methylation patterns in H19, LIT1 and SNRPN genes in ART and control groups [Citation64,Citation66]. Similarly, a follow up study from this group interrogated GRB10, IGF2 and PEG3 genes in CVS tissue and reported insignificant methylation differences between the two groups [Citation78]. Lou et al., [Citation96] used multifetal reduction samples to study methylation levels of genes involved in cholesterol metabolism in 3 groups – IVF, ICSI and COH (control group). Differential methylation and expression of INSIG1 was observed for ICSI conceived fetuses. They also observed differential methylation levels for INSIG1 and SREBF1 genes in the ICSI placental samples, where placenta from natural conceptions served as controls. Similarly, in a recent study conducted by the same group, the authors were able to show differential methylation in DMRs of H19, IGF2 and SNRPN in IVF-conceived fetuses compared to COH [Citation83].
In all studies performed so far it is clear that there are remarkable inconsistencies in whether or not there are epigenetic changes following ART.
In those studies that provided a list of genes perturbed by ART after using a genome-wide approach (), we attempted to identify any overlap between gene lists [Citation10,Citation86,Citation87,Citation91,Citation97]. We found only four genes that appeared in more than one gene list – GNAS, PEG10, PRCP2 and RUNX3, of which GNAS was the only gene that appeared in 3 studies further corroborating the inconsistent nature of genes affected.
Table 2. Genes epigenetically perturbed by ART identified by studies using whole genome approaches.
A recent meta-analysis of 18 studies using candidate gene approaches to examine epigenetic differences in ART samples attempted to reconcile some of these differences [Citation98]. The meta-analysis confirmed the increased incidence of imprinting disorders in IVF and ICSI conceived children. However, methylation levels in the genes studied (H19, PEG1-MEST, GRB0, IGF2, SNRPN, KvDMR1/KCNQ10T1 and PEG3) were not significantly different between the groups. The authors attributed the results to heterogeneity in various aspects of the studies included (such as fertility treatment, tissue, measurement methods, imprinted regions) [Citation98]. In addition, we speculate that the absence of technical as well as biological validation and insufficient sample sizes in most of the studies are responsible for the conflicting reports. Divergent results may be also be due to the stochastic nature of the DNA methylation disturbances, the chosen samples, and/or the interrogated CpG sites.
An alternative to the hypothesis that IVF leads to epigenetic changes is that a subfertility diagnosis may be responsible for epigenetic differences seen in patients undergoing ART. However, we have investigated a subset of patients who used donor oocytes for diminished ovarian reserve, in the absence of any other infertility diagnosis (therefore removing infertility diagnosis as a confounder), and found significant methylation differences between placentas from pregnancies using donor egg and naturally conceived controls [Citation99]. These data suggest that, in fact, the procedures used in IVF are at least partially responsible for differences in DNA methylation. However, these results are not replicated in every study. For example, one study found significant methylation and expression differences between a subfertile group not using ART and a control group, but no differences between the IVF and control groups [Citation89]. However, the role of IVF in epigenetic changes is further confirmed by animal data, from our lab and many others, demonstrating that in the absence of infertility, IVF can lead to changes in DNA methylation in the placenta and offspring [Citation9,Citation13,Citation49,Citation50,Citation100–Citation106].
Are there specific interventions utilized in IVF that may be responsible for the epigenetic changes?
As discussed above, both experiments with donor oocyte and animal studies suggest that it is ART and not (only) the underlying infertility that is responsible for epigenetic differences seen after IVF. In addition, animal models also allow for the separation of individual procedures, to examine the effect of each one in isolation. IVF involves multiple manipulations to the gamete and early embryo. These include hormonal stimulation of the ovaries (superovulation), retrieval of oocytes, in vitro fertilization by sperm or injection of a single sperm into the oocyte, culture of fertilized embryos and transfer of fresh or cryopreserved embryos into the uterus. () Apart from the manipulations themselves, gametes and embryos are also subjected to ex-vivo exposures such as changes in temperature, light, oxygen levels, pH and culture media. Each of these factors could potentially affect either the establishment or maintenance of epigenetic marks and affect placentation.
Figure 1. In vitro fertilization involved multiple exposures and manipulations to the gamete and embryo including superovulation, the surgical retrieval of oocytes, in vitro fertilization by sperm or injection of a single sperm into the oocyte (Intracytoplasmic sperm injection), the culture of fertilized embryos for 3–5 days, and the transfer of fresh embryos and/or embryo cryopreservation.
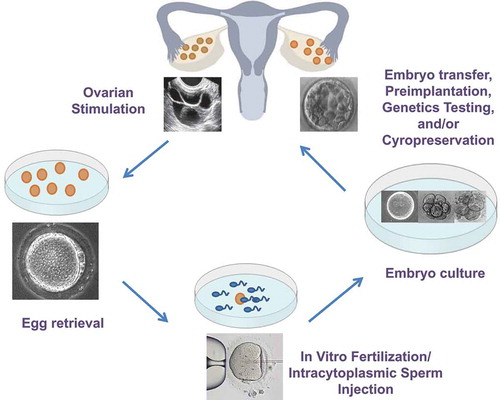
Superovulation, or controlled ovarian stimulation, involves the administration of exogenous gonadotropins to stimulate oocyte growth and is ubiquitous in IVF. Epigenetic establishment in oocytes occurs in a growth and maturation dependent manner, and superovulation has been hypothesized to interfere with this process by altering the timeline of development, recruitment of low quality oocytes or affecting enzymes required for appropriate epigenetic programming [Citation107,Citation108]. It is therefore unsurprising that superovulation has shown an effect on epigenetic regulation in both murine and human studies. Mouse blastocysts exposed to low and high doses of gonadotropin hormones used during superovulation were found to have perturbations in imprint acquisition and maintenance, at maternal and paternal alleles [Citation49]. These perturbations were present in both the embryo and placenta [Citation49,Citation109] as well as in somatic tissues of offspring [Citation50] and within the germline, leading to transgenerational effects [Citation110]. Using a mouse model that specifically isolated the effects of superovulation, we have additionally found changes in global somatic gene expression in offspring after superovulation. Genes affected include several cytochrome P450 genes which are regulated by glucocorticoids and could affect blood pressure regulation [Citation51]. In human oocytes, selected imprinted genes including H19, PEG1 and KCNQ1OT1 similarly show abnormalities in methylation following superovulation [Citation52–Citation54]. Most studies examining epigenetic effects of superovulation have, however, focused on imprinted genes, and global examination of epigenetic perturbations on larger sample sizes, especially in humans, is necessary.
Apart from superovulation, studies examining the effect of ART procedures on epigenetic modification have predominantly focused on in vitro culture of embryos, which has been shown to have a detrimental effect on the regulation of DNA methylation. Readers are directed to a recent review from our group for a detailed discussion of the effect of embryo culture, culture medium and oxygen tension on epigenetic changes after ART. Overall, global epigenetic changes due to embryo culture and oxygen tension have been found in animal models, though, as with superovulation, the majority of studies have focused on imprinted genes. DNA methylation changes have been seen in a tissue-specific manner, with placental tissue showing increased susceptibility to changes in methylation due to embryo culture. Studies have also found that epigenetic changes due to culture are stochastic in nature with not all embryos or loci are affected equally. Evidence for epigenetic disturbances on a whole genome level have also been found, though to a large extent these studies use immunofluorescence. The application of microarrays and next-generation sequencing technology to examine the effects of embryo culture on epigenetic regulation will allow for more locus/gene-specific changes to be identified.
The cryopreservation of oocytes and embryos through vitrification has emerged as an important technique associated with ART, allowing for the preservation of fertility over time and embryo transfer into a more physiologic hormonal environment. Studies suggest frozen embryo transfers may result in improved implantation rates and decreased risk of adverse outcomes following ART [Citation111–Citation116]. While a number of studies have explored the effect of oocyte vitrification on DNA methylation during oogenesis and embryogenesis [Citation117,Citation118], there are fewer studies that examine how embryo vitrification affects epigenetic regulation. Our group has shown LINE1 methylation levels in placentas from frozen embryo transfers resembled levels in controls, while LINE1 methylation levels of placentas after fresh embryo transfers was significantly different [Citation92]. This observation supports a study comparing gene expression in frozen compared to fresh human preimplantation embryos, which showed that frozen embryos displayed more consistent expression of genes required for developmental competence, while fresh embryos were highly variable in terms of expression [Citation119].
The process of embryo transfer itself, which is minimally invasive, has been shown to affect DNA methylation at imprinted genes. This suggests that exposure of the embryo to minimal environmental changes in temperature; humidity or pH can in itself lead to aberrant epigenetic regulation [Citation103].
It is likely that no one procedure is solely responsible for changes in DNA methylation after ART. In fact, we have shown that the morphological effects of individual ART procedures are cumulative in a mouse model, suggesting that epigenetic effects might follow a similar pattern [Citation13]. The above human studies, and similar findings from murine studies, suggest that multiple aspects of embryo manipulation affect epigenetic modifications in the embryo. It should be noted that extensive work in other animals, including rabbit, bovine and ovine models are outside the scope of this review, but additionally support these observations [Citation120–Citation124].
Conclusion
Pregnancy success rates with IVF are improving constantly and we can now offer patients additional technologies for embryo and gamete cryopreservation and selection which may prove to even further advance the field. Minimizing multiple gestation pregnancies has been at the forefront of our clinical goals and single embryo transfer is becoming more routine in our practices. However, little progress has been made to improve the safety of our procedures and investigate the mechanisms responsible for the adverse outcomes associated with ART. It is critical that we identify modifiable elements in our protocols to minimize the risks to our patients and their offspring. As discussed in this review, a growing body of literature suggests that techniques utilized in ART affect epigenetic marks in the fetus and placenta that may be responsible for the increased risk of adverse outcomes associated with IVF. It is still unclear whether these epigenetic changes and adverse outcomes will have long-term effects on health and disease. It is imperative that we validate our findings and further investigate the effects of our interventions. It is suspected that specific individuals or genes are more ‘at risk’ and new global profiling methods on small numbers of cells can be performed to further investigate these questions.
Another underutilized avenue of investigation is isolating the ART population for research furthering our understanding of adverse perinatal outcomes such as preterm birth and preeclampsia. Knowing that IVF increases the risk of these conditions, this population may represent an at risk population with a potential common mechanism responsible for the adverse outcome. We recently utilized this strategy, using an IVF population to study the etiology of preterm birth, a likely multifactorial disease with multiple potential etiologies. We found that IVF patients who delivered preterm had a high incidence of abnormal DNA methylation in the ADAMTS gene family. The ADAMTS genes play a role in extracellular matrix regulation and cell invasion and are expressed in the developing placenta [Citation125]. Further research into the role of these genes in early placentation is now underway. By targeting a population at high risk for PTB, we identified a role for disordered epigenetic regulation of a family of genes in a disorder of placentation and susceptibility to pre-term birth. We suspect further investigation into IVF pregnancies with adverse outcomes may be able to contribute significantly to our knowledge of early implantation and placentation.
Establishing the link between aberrant epigenetic marks and adverse perinatal outcomes in humans is difficult. Therefore, in addition to validating the current studies it critical that we also utilize animal models to establish the link between abnormal epigenetic marks, gene expression and disease. In addition, animal studies can be helpful in examining potential long-term effects of epigenetic changes, as these findings in humans could take decades to reveal. Further investigations in both human and animal models will not only provide insight into the role of epigenetic changes in adverse outcomes associated with ART, but into the mechanisms by which early exposure to the embryo lead to adverse perinatal and long-term health outcomes.
Supplemental Material
Download MS Word (41.4 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Jackson RA, Gibson KA, Wu YW, et al. Perinatal outcomes in singletons following in vitro fertilization: a meta-analysis. Obstet Gynecol. 2004;103(3):551–563.
- Schieve LA, Meikle SF, Ferre C, et al. Low and very low birth weight in infants conceived with use of assisted reproductive technology. N Engl J Med. 2002;346(10):731–737.
- Chen X-K, Wen SW, Bottomley J, et al. In vitro fertilization is associated with an increased risk for preeclampsia. Hypertens Pregnancy. 2009;28(1):1–12.
- LB R, PR R, Sunde A, et al. Increased risk of placenta previa in pregnancies following IVF/ICSI; a comparison of ART and non-ART pregnancies in the same mother. Hum Reprod. 2006;21(9):2353–2358.
- McDonald SD, Han Z, Mulla S, et al. Preterm birth and low birth weight among in vitro fertilization singletons: a systematic review and meta-analyses. Eur J Obstet Gynecol Reprod Biol. 2009;146(2):138–148.
- Romundstad LB, Romundstad PR, Sunde A, et al. Effects of technology or maternal factors on perinatal outcome after assisted fertilisation: a population-based cohort study. Lancet. 2008;372(9640):737–743.
- Gosden R, Trasler J, Lucifero D, et al. Rare congenital disorders, imprinted genes, and assisted reproductive technology. Lancet. 2003;361(9373):1975–1977.
- El Hajj N, Haaf T. Epigenetic disturbances in in vitro cultured gametes and embryos: implications for human assisted reproduction. Fertil Steril. 2013;99(3):632–641.
- de Waal E, Mak W, Calhoun S, et al. In vitro culture increases the frequency of stochastic epigenetic errors at imprinted genes in placental tissues from mouse concepti produced through assisted reproductive technologies. Biol Reprod. 2014;90(2):22.
- Katari S, Turan N, Bibikova M, et al. DNA methylation and gene expression differences in children conceived in vitro or in vivo. Hum Mol Genet. 2009;18(20):3769–3778.
- Gomes MV, Huber J, Ferriani RA, et al. Abnormal methylation at the KvDMR1 imprinting control region in clinically normal children conceived by assisted reproductive technologies. Mol Hum Reprod. 2009;15(8):471–477.
- Lazaraviciute G, Kauser M, Bhattacharya S, et al. A systematic review and meta-analysis of DNA methylation levels and imprinting disorders in children conceived by IVF/ICSI compared with children conceived spontaneously. Hum Reprod Update. 2014;20(6):840–852.
- de Waal E, Vrooman LA, Fischer E, et al. The cumulative effect of assisted reproduction procedures on placental development and epigenetic perturbations in a mouse model. Hum Mol Genet. 2015;24(24):6975–6985.
- Sunderam S, Kissin DM, Crawford SB, et al. Assisted reproductive technology surveillance - United States, 2015. MMWR Surveill Summ. 2018;67(3):1–28.
- Practice Committee of the American Society for Reproductive M. Multiple pregnancy associated with infertility therapy. Fertil Steril. 2006;86(5 Suppl 1):S106–110.
- Santana DS, Cecatti JG, Surita FG, et al. Twin pregnancy and severe maternal outcomes: the World Health Organization multicountry survey on maternal and newborn health. Obstet Gynecol. 2016;127(4):631–641.
- Practice Committee of the American Society for Reproductive Medicine. Electronic address aao, practice committee of the society for assisted reproductive T. Guidance on the limits to the number of embryos to transfer: a committee opinion. Fertil Steril. 2017;107(4):901–903.
- Kalra SK, Barnhart KT. In vitro fertilization and adverse childhood outcomes: what we know, where we are going, and how we will get there. A glimpse into what lies behind and beckons ahead. Fertil Steril. 2011;95(6):1887–1889.
- Helmerhorst FM, Perquin DA, Donker D, et al. Perinatal outcome of singletons and twins after assisted conception: a systematic review of controlled studies. BMJ. 2004;328(7434):261.
- Pandey S, Shetty A, Hamilton M, et al. Obstetric and perinatal outcomes in singleton pregnancies resulting from IVF/ICSI: a systematic review and meta-analysis. Hum Reprod Update. 2012;18(5):485–503.
- Zhu L, Zhang Y, Liu Y, et al. Maternal and live-birth outcomes of pregnancies following assisted reproductive technology: a retrospective cohort study. Sci Rep. 2016;6:35141.
- Halliday J. Outcomes of IVF conceptions: are they different? Best Pract Res Clin Obstet Gynaecol. 2007;21(1):67–81.
- Maccani MA, Marsit CJ. Epigenetics in the placenta. Am J Reprod Immunol. 2009;62(2):78–89.
- Nelissen EC, van Montfoort AP, Dumoulin JC, et al. Epigenetics and the placenta. Hum Reprod Update. 2011;17(3):397–417.
- Kohan-Ghadr HR, Kadam L, Jain C, et al. Potential role of epigenetic mechanisms in regulation of trophoblast differentiation, migration, and invasion in the human placenta. Cell Adh Migr. 2016;10(1–2):126–135.
- Odom LN, Segars J. Imprinting disorders and assisted reproductive technology. Curr Opin Endocrinol Diabetes Obes. 2010;17(6):517–522.
- Gosden R, Trasler J, Lucifero D, et al. Rare congenital disorders, imprinted genes, and assisted reproductive technology. Lancet. 2003;361(9373):1975–1977.
- Le Bouc Y, Rossignol S, Azzi S, et al. Epigenetics, genomic imprinting and assisted reproductive technology. Ann Endocrinol. 2010;71(3):237–238.
- Cortessis VK, Azadian M, Buxbaum J, et al. Comprehensive meta-analysis reveals association between multiple imprinting disorders and conception by assisted reproductive technology. J Assist Reprod Genet. 2018;35(6):943–952.
- Ceelen M, van Weissenbruch MM, Prein J, et al. Growth during infancy and early childhood in relation to blood pressure and body fat measures at age 8-18 years of IVF children and spontaneously conceived controls born to subfertile parents. Hum Reprod. 2009;24(11):2788–2795.
- Ceelen M, van Weissenbruch MM, Vermeiden JP, et al. Cardiometabolic differences in children born after in vitro fertilization: follow-up study. J Clin Endocrinol Metab. 2008;93(5):1682–1688.
- Chen M, Wu L, Zhao J, et al. Altered glucose metabolism in mouse and humans conceived by in-vitro fertilization (IVF). Diabetes. 2014.
- Sakka SD, Loutradis D, Kanaka-Gantenbein C, et al. Absence of insulin resistance and low-grade inflammation despite early metabolic syndrome manifestations in children born after in vitro fertilization. Fertil Steril. 2010;94(5):1693–1699.
- Ashworth A. Effects of intrauterine growth retardation on mortality and morbidity in infants and young children. Eur J Clin Nutr. 1998;52(Suppl 1):S34–41. discussion S41-32
- Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295(7):349–353.
- Arends NJ, Boonstra VH, Duivenvoorden HJ, et al. Reduced insulin sensitivity and the presence of cardiovascular risk factors in short prepubertal children born small for gestational age (SGA). Clin Endocrinol (Oxf). 2005;62(1):44–50.
- Jaquet D, Gaboriau A, Czernichow P, et al. Insulin resistance early in adulthood in subjects born with intrauterine growth retardation. J Clin Endocrinol Metab. 2000;85(4):1401–1406.
- Valdez R, Athens MA, Thompson GH, et al. Birthweight and adult health outcomes in a biethnic population in the USA. Diabetologia. 1994;37(6):624–631.
- Hart R, Norman RJ. The longer-term health outcomes for children born as a result of IVF treatment: part I–general health outcomes. Hum Reprod Update. 2013;19(3):232–243.
- Ceelen M, van Weissenbruch MM, Roos JC, et al. Body composition in children and adolescents born after in vitro fertilization or spontaneous conception. J Clin Endocrinol Metab. 2007;92(9):3417–3423.
- Knoester M, Helmerhorst FM, van der Westerlaken LA, et al. Matched follow-up study of 5 8-year-old ICSI singletons: child behaviour, parenting stress and child (health-related) quality of life. Hum Reprod. 2007;22(12):3098–3107.
- Morgan HD, Santos F, Green K, et al. Epigenetic reprogramming in mammals. Hum Mol Genet. 2005;14: R47–58. Spec No 1.
- Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293(5532):1089–1093.
- von Meyenn F, Reik W. Forget the Parents: epigenetic Reprogramming in Human Germ Cells. Cell. 2015;161(6):1248–1251.
- Smith ZD, Chan MM, Humm KC, et al. DNA methylation dynamics of the human preimplantation embryo. Nature. 2014;511(7511):611–615.
- Reik W, Epigenetics: KG. Cellular memory erased in human embryos. Nature. 2014;511(7511):540–541.
- Okae H, Chiba H, Hiura H, et al. Genome-wide analysis of DNA methylation dynamics during early human development. PLoS Genet. 2014;10(12):e1004868.
- Guo H, Zhu P, Yan L, et al. The DNA methylation landscape of human early embryos. Nature. 2014;511(7511):606–610.
- Market-Velker BA, Zhang L, Magri LS, et al. Dual effects of superovulation: loss of maternal and paternal imprinted methylation in a dose-dependent manner. Hum Mol Genet. 2010;19(1):36–51.
- de Waal E, Yamazaki Y, Ingale P, et al. Gonadotropin stimulation contributes to an increased incidence of epimutations in ICSI-derived mice. Hum Mol Genet. 2012;21(20):4460–4472.
- Weinerman R, Ord T, Bartolomei MS, et al. The superovulated environment, independent of embryo vitrification, results in low birthweight in a mouse model. Biol Reprod. 2017;97(1):133–142.
- Sato C, Shimada M, Mori T, et al. Assessment of human oocyte developmental competence by cumulus cell morphology and circulating hormone profile. Reprod Biomed Online. 2007;14(1):49–56.
- Khoueiry R, Mery L, Mery L, et al. Dynamic CpG methylation of the KCNQ1OT1 gene during maturation of human oocytes. J Med Genet. 2008;45(9):583–588.
- Geuns E, Hilven P, Van Steirteghem A, et al. Methylation analysis of KvDMR1 in human oocytes. J Med Genet. 2007;44(2):144–147.
- Xin F, Susiarjo M, Bartolomei MS. Multigenerational and transgenerational effects of endocrine disrupting chemicals: A role for altered epigenetic regulation?. Semin Cell Dev Biol. 2015;43:66–75.
- Kobayashi H, Hiura H, John RM, et al. DNA methylation errors at imprinted loci after assisted conception originate in the parental sperm. Eur J Hum Genet. 2009;17(12):1582–1591.
- Hiura H, Hattori H, Kobayashi N, et al. Genome-wide microRNA expression profiling in placentae from frozen-thawed blastocyst transfer. Clin Epigenetics. 2017;9:79.
- Tierling S, Souren NY, Gries J, et al. Assisted reproductive technologies do not enhance the variability of DNA methylation imprints in human. J Med Genet. 2010;47(6):371–376.
- Kanber D, Buiting K, Zeschnigk M, et al. Low frequency of imprinting defects in ICSI children born small for gestational age. Eur J Hum Genet. 2009;17(1):22–29.
- Li L, Wang L, Le F, et al. Evaluation of DNA methylation status at differentially methylated regions in IVF-conceived newborn twins. Fertil Steril. 2011;95(6):1975–1979.
- Zechner U, Pliushch G, Schneider E, et al. Quantitative methylation analysis of developmentally important genes in human pregnancy losses after ART and spontaneous conception. Mol Hum Reprod. 2010;16(9):704–713.
- Feng C, Tian S, Zhang Y, et al. General imprinting status is stable in assisted reproduction-conceived offspring. Fertil Steril. 2011;96(6):1417–1423.e1419.
- Shi X, Ni Y, Zheng H, et al. Abnormal methylation patterns at the IGF2/H19 imprinting control region in phenotypically normal babies conceived by assisted reproductive technologies. Eur J Obstet Gynecol Reprod Biol. 2011;158(1):52–55.
- Zheng HY, Shi XY, Wu FR, et al. Assisted reproductive technologies do not increase risk of abnormal methylation of PEG1/MEST in human early pregnancy loss. Fertil Steril. 2011;96(1):84–89.e82.
- Zheng HY, Shi XY, Wang LL, et al. Study of DNA methylation patterns of imprinted genes in children born after assisted reproductive technologies reveals no imprinting errors: A pilot study. Exp Ther Med. 2011;2(4):751–755.
- Zheng HY, Tang Y, Niu J, et al. Aberrant DNA methylation of imprinted loci in human spontaneous abortions after assisted reproduction techniques and natural conception. Hum Reprod. 2013;28(1):265–273.
- Oliver VF, Miles HL, Cutfield WS, et al. Defects in imprinting and genome-wide DNA methylation are not common in the in vitro fertilization population. Fertil Steril. 2012;97(1):147–153.e147.
- Sakian S, Louie K, Wong EC, et al. Altered gene expression of H19 and IGF2 in placentas from ART pregnancies. Placenta. 2015;36(10):1100–1105.
- Vincent RN, Gooding LD, Louie K, et al. Altered DNA methylation and expression of PLAGL1 in cord blood from assisted reproductive technology pregnancies compared with natural conceptions. Fertil Steril. 2016;106(3):739–748.e733.
- Wong EC, Hatakeyama C, Robinson WP, et al. DNA methylation at H19/IGF2 ICR1 in the placenta of pregnancies conceived by in vitro fertilization and intracytoplasmic sperm injection. Fertil Steril. 2011;95(8):2524–2526.e2521-2523.
- Rancourt RC, Harris HR, Michels KB. Methylation levels at imprinting control regions are not altered with ovulation induction or in vitro fertilization in a birth cohort. Hum Reprod. 2012;27(7):2208–2216.
- Puumala SE, Nelson HH, Ross JA, et al. Similar DNA methylation levels in specific imprinting control regions in children conceived with and without assisted reproductive technology: a cross-sectional study. BMC Pediatr. 2012;12:33.
- Camprubí C, Iglesias-Platas I, Martin-Trujillo A, et al. Stability of genomic imprinting and gestational-age dynamic methylation in complicated pregnancies conceived following assisted reproductive technologies. Biol Reprod. 2013;89(3):50.
- Loke YJ, Galati JC, Saffery R, et al. Association of in vitro fertilization with global and IGF2/H19 methylation variation in newborn twins. J Dev Orig Health Dis. 2015;6(2):115–124.
- Whitelaw N, Bhattacharya S, Hoad G, et al. Epigenetic status in the offspring of spontaneous and assisted conception. Hum Reprod. 2014;29(7):1452–1458.
- Choux C, Binquet C, Carmignac V, et al. The epigenetic control of transposable elements and imprinted genes in newborns is affected by the mode of conception: ART versus spontaneous conception without underlying infertility. Hum Reprod. 2018;33(2):331–340.
- Dimitriadou E, Noutsopoulos D, Markopoulos G, et al. Abnormal DLK1/MEG3 imprinting correlates with decreased HERV-K methylation after assisted reproduction and preimplantation genetic diagnosis. Stress. 2013;16(6):689–697.
- Liu Y, Tang Y, Ye D, et al. Impact of abnormal DNA methylation of imprinted loci on human spontaneous abortion. Reprod Sci. 2018;25(1):131–139.
- Chen XJ, Chen F, Lv PP, et al. Maternal high estradiol exposure alters CDKN1C and IGF2 expression in human placenta. Placenta. 2018;61:72–79.
- Tang L, Liu Z, Zhang R, et al. Imprinting alterations in sperm may not significantly influence ART outcomes and imprinting patterns in the cord blood of offspring. PLoS One. 2017;12(11):e0187869.
- Turan N, Katari S, Gerson LF, et al. Inter- and intra-individual variation in allele-specific DNA methylation and gene expression in children conceived using assisted reproductive technology. PLoS Genet. 2010;6(7):e1001033.
- Nelissen EC, Dumoulin JC, Daunay A, et al. Placentas from pregnancies conceived by IVF/ICSI have a reduced DNA methylation level at the H19 and MEST differentially methylated regions. Hum Reprod. 2013;28(4):1117–1126.
- Lou H, Le F, Hu M, et al. Aberrant DNA methylation of IGF2-H19 locus in human fetus and in spermatozoa from assisted reproductive technologies. Reprod Sci. 2019;26(7):997–1004.
- JL K, Yang B, AE S, et al. Skewed X inactivation and IVF-conceived infants. Reprod Biomed Online. 2010;20(5):660–663.
- Wu EX, Stanar P, Ma S. X-chromosome inactivation in female newborns conceived by assisted reproductive technologies. Fertil Steril. 2014;101(6):1718–1723.
- Melamed N, Choufani S, Wilkins-Haug LE, et al. Comparison of genome-wide and gene-specific DNA methylation between ART and naturally conceived pregnancies. Epigenetics. 2015;10(6):474–483.
- Estill MS, Bolnick JM, Waterland RA, et al. Assisted reproductive technology alters deoxyribonucleic acid methylation profiles in bloodspots of newborn infants. Fertil Steril. 2016;106(3):629–639.e610.
- El Hajj N, Haertle L, Dittrich M, et al. DNA methylation signatures in cord blood of ICSI children. Hum Reprod. 2017;32(8):1761–1769.
- Litzky JF, Deyssenroth MA, Everson TM, et al. Placental imprinting variation associated with assisted reproductive technologies and subfertility. Epigenetics. 2017;12(8):653–661.
- Choufani S, Turinsky AL, Melamed N, et al. Impact of assisted reproduction, infertility, sex, and paternal factors on the placental DNA methylome. Hum Mol Genet. 2018;28(3):372–385.
- Castillo-Fernandez JE, Loke YJ, Bass-Stringer S, et al. DNA methylation changes at infertility genes in newborn twins conceived by in vitro fertilisation. Genome Med. 2017;9(1):28.
- Ghosh J, Coutifaris C, Sapienza C, et al. Global DNA methylation levels are altered by modifiable clinical manipulations in assisted reproductive technologies. Clin Epigenetics. 2017;9:14.
- Ball MP, Li JB, Gao Y, et al. Targeted and genome-scale strategies reveal gene-body methylation signatures in human cells. Nat Biotechnol. 2009;27(4):361–368.
- Ghosh J, Mainigi M, Coutifaris C, et al. Outlier DNA methylation levels as an indicator of environmental exposure and risk of undesirable birth outcome. Hum Mol Genet. 2016;25(1):123–129.
- Xu N, Barlow GM, Cui J, et al. Comparison of genome-wide and gene-specific DNA methylation profiling in first-trimester chorionic villi from pregnancies conceived with infertility treatments. Reprod Sci. 2017;24(7):996–1004.
- Lou H, Le F, Zheng Y, et al. Assisted reproductive technologies impair the expression and methylation of insulin-induced gene 1 and sterol regulatory element-binding factor 1 in the fetus and placenta. Fertil Steril. 2014;101(4):974–980.e972.
- El Hajj N, Haaf T. Epigenetic disturbances in in vitro cultured gametes and embryos: implications for human assisted reproduction. Fertil Steril. 2013;99(3):632–641.
- Lazaraviciute G, Kauser M, Bhattacharya S, et al. A systematic review and meta-analysis of DNA methylation levels and imprinting disorders in children conceived by IVF/ICSI compared with children conceived spontaneously. Hum Reprod Update. 2014;20(6):840–852.
- Song S, Ghosh J, Mainigi M, et al. DNA methylation differences between in vitro- and in vivo-conceived children are associated with ART procedures rather than infertility. Clin Epigenetics. 2015;7(1):41.
- Mainigi M, Rosenzweig JM, Lei J, et al. Peri-implantation hormonal milieu: elucidating mechanisms of adverse neurodevelopmental outcomes. Reprod Sci. 2016;23(6):785–794.
- Mainigi MA, Weinerman R, Ord T, et al. Low birthweight during fresh IVF cycles is due to altered placental vasculogenesis: evidence from a mouse model. Paper presented at: American Society of Reproductive Medicine2016; 2016.
- Doherty AS, Mann MR, Tremblay KD, et al. Differential effects of culture on imprinted H19 expression in the preimplantation mouse embryo. Biol Reprod. 2000;62(6):1526–1535.
- Rivera RM, Stein P, Weaver JR, et al. Manipulations of mouse embryos prior to implantation result in aberrant expression of imprinted genes on day 9.5 of development. Hum Mol Genet. 2008;17(1):1–14.
- Lucifero D, Mann MRW, Bartolomei MS, et al. Gene-specific timing and epigenetic memory in oocyte imprinting. Hum Mol Genet. 2004;13(8):839–849.
- Mann MRW, Lee SS, Doherty AS, et al. Selective loss of imprinting in the placenta following preimplantation development in culture. Development. 2004;131(15):3727–3735.
- Rivera R, Stein P, Weaver J, et al. Manipulations of mouse embryos prior to implantation result in aberrant expression of imprinted genes on day 9.5 of development. Hum Mol Genet. 2008;17(1):1–14.
- Huntriss J, Balen AH, Sinclair KD, et al., Royal College of Obstetricians G. Epigenetics and Reproductive Medicine: scientific Impact Paper No. 57. BJOG. 2018.
- Tomizawa S, Nowacka-Woszuk J, Kelsey G. DNA methylation establishment during oocyte growth: mechanisms and significance. Int J Dev Biol. 2012;56(10–12):867–875.
- Fortier AL, Lopes FL, Darricarrère N, et al. Superovulation alters the expression of imprinted genes in the midgestation mouse placenta. Hum Mol Genet. 2008;17(11):1653–1665.
- Stouder C, Deutsch S, Paoloni-Giacobino A. Superovulation in mice alters the methylation pattern of imprinted genes in the sperm of the offspring. Reprod Toxicol. 2009;28(4):536–541.
- Weinerman R, Mainigi M. Why we should transfer frozen instead of fresh embryos: the translational rationale. Fertil Steril. 2014;102(1):10–18.
- Pelkonen S, Koivunen R, Gissler M, et al. Perinatal outcome of children born after frozen and fresh embryo transfer: the Finnish cohort study 1995-2006. Hum Reprod. 2010;25(4):914–923.
- Ozgur K, Berkkanoglu M, Bulut H, et al. Perinatal outcomes after fresh versus vitrified-warmed blastocyst transfer: retrospective analysis. Fertil Steril. 2015;104(4):899–907.e893.
- Shapiro BS, Daneshmand ST, Restrepo H, et al. Matched-cohort comparison of single-embryo transfers in fresh and frozen-thawed embryo transfer cycles. Fertil Steril. 2013;99(2):389–392.
- Zhang W, Xiao X, Zhang J, et al. Clinical outcomes of frozen embryo versus fresh embryo transfer following in vitro fertilization: a meta-analysis of randomized controlled trials. Arch Gynecol Obstet. 2018.
- Maheshwari A, Pandey S, Amalraj Raja E, et al. Is frozen embryo transfer better for mothers and babies? Can cumulative meta-analysis provide a definitive answer? Hum Reprod Update. 2018;24(1):35–58.
- Denomme MM, Mann MR. Genomic imprints as a model for the analysis of epigenetic stability during assisted reproductive technologies. Reproduction. 2012;144(4):393–409.
- Moussa M, Shu J, Zhang X, et al. Cryopreservation of mammalian oocytes and embryos: current problems and future perspectives. Sci China Life Sci. 2014;57(9):903–914.
- Shaw L, Sneddon SF, Brison DR, et al. Comparison of gene expression in fresh and frozen-thawed human preimplantation embryos. Reproduction. 2012;144(5):569–582.
- Young LE, Fernandes K, McEvoy TG, et al. Epigenetic change in IGF2R is associated with fetal overgrowth after sheep embryo culture. Nat Genet. 2001;27(2):153–154.
- Zaitseva I, Zaitsev S, Alenina N, et al. Dynamics of DNA-demethylation in early mouse and rat embryos developed in vivo and in vitro. Mol Reprod Dev. 2007;74(10):1255–1261.
- Salilew-Wondim D, Fournier E, Hoelker M, et al. Genome-wide DNA methylation patterns of bovine blastocysts developed in vivo from embryos completed different stages of development in vitro. PLoS One. 2015;10(11):e0140467.
- Salvaing J, Peynot N, Bedhane MN, et al. Assessment of ‘one-step’ versus ‘sequential’ embryo culture conditions through embryonic genome methylation and hydroxymethylation changes. Hum Reprod. 2016;31(11):2471–2483.
- Urrego R, Rodriguez-Osorio N, Niemann H. Epigenetic disorders and altered gene expression after use of Assisted Reproductive Technologies in domestic cattle. Epigenetics. 2014;9(6):803–815.
- Mani S, Ghosh J, Lan Y, et al. Epigenetic changes in preterm birth placenta suggest a role for ADAMTS genes in spontaneous preterm birth. Hum Mol Genet. 2018.
