ABSTRACT
Extensive research has highlighted the role of infection-induced epigenetic events in the development of cancer. More recently, attention has focused on the ability of non-carcinogenic infections, as well as vaccines, to modify the human epigenome and modulate the immune response. This review explores this rapidly evolving area of investigation and outlines the many and varied ways in which vaccination and natural infection can influence the human epigenome from modulation of the innate and adaptive immune response, to biological ageing and modification of disease risk. The implications of these epigenetic changes on immune regulation and their potential application to the diagnosis and treatment of chronic infection and vaccine development are also discussed.
Introduction
Recognition of the factors that influence the human epigenome has expanded in parallel with understanding of the molecular mechanisms of epigenetic regulation. Many intrinsic and extrinsic influences can mark the epigenome, including age, sex, smoking and diet [Citation1–Citation3]. Maternal factors, including stress and famine, have persistent effects on the epigenome of subsequent generations supporting the notion that epigenetic modifications are heritable [Citation3].
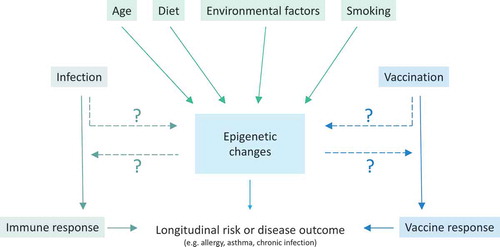
There is growing evidence that vaccination and natural infection alter the epigenome, modulating both the initial immune response and longitudinal disease risk. () Much of the current understanding of the impact of infection on the human epigenome comes from studies of carcinogenic viruses and bacteria, including human papilloma virus (HPV), Epstein Barr virus (EBV), hepatitis C virus (HCV), and Helicobacter pylori [Citation4]. Induction of epigenetic modifications, particularly aberrant DNA methylation, is considered the primary mechanism by which viral and bacterial infections lead to cancer development.
Despite significant research into the role of infection-induced epigenetic changes in the development of cancer, less is known about the non-carcinogenic effects of infections on the human epigenome. Even less is known about the impact of vaccinations on the epigenetic landscape. One area in which epigenetics may play a role is in the increasingly recognized heterologous (‘non-specific’) effects of vaccines. Heterologous effects are additional effects exerted by the vaccine beyond the specific protection afforded against the targeted disease [Citation5]. The immunological mechanisms underlying these heterologous effects are only beginning to be understood but there is emerging evidence that epigenetic mechanisms are involved.
This systematic review focuses on vaccine and infection-induced epigenetic modifications that do not directly influence the development of cancer.
Methods
Search strategy and selection criteria
Original studies investigating the non-carcinogenic effects of (i) vaccinations and (ii) bacterial, viral, fungal and parasitic pathogens or their constituents on the human epigenome were identified using two independent search strategies.
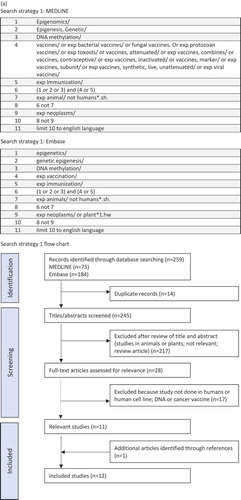
In August 2019, MEDLINE (1946 to present) and Embase (1947 to present) were searched using the Ovid interface. Additional publications were identified through hand-searching of reference lists of relevant retrieved articles. Results were limited to English language and studies in humans. The search thesaurus terms and keywords together with the histories are listed in Figure 2(,). Studies were excluded if: the experiments were done in animals or plants; the study pertained directly to carcinogenesis; the epigenetic modification occurred in the genome of the infecting pathogen; the study assessed only modifying enzymes such as DNA methyltransferases (DNMT) or histone deacetylases (HDAC); or the identified epigenetic change preceded infection.
Systematic review results
The first search, identifying studies of vaccine-induced epigenetic changes, identified 259 studies, of which 28 full-text articles were assessed for relevance and 11 were included in the review (). One additional reference was identified during the second search resulting in a total of 12 studies. These investigated five different vaccines: Bacillus Calmette-Guérin (BCG) vaccine, yellow fever virus (YFV) vaccine, influenza A virus (IAV) vaccine, hepatitis B virus (HBV) vaccine, tetanus toxoid vaccine and modified Vaccinia Ankara 85A vaccine (MVA 85A). () These studies all investigated the epigenetic effects of in vivo vaccination of human subjects, except two studies that exposed human monocytes to BCG vaccine or MVA 85A vaccine in vitro. Three studies investigating the epigenetic effects of BCG vaccine used chromatin immunoprecipitation (ChIP) to detect histone modifications in monocytes and the fourth used the Illumina Infinium HumanMethylation450 BeadChip array to measure genome-wide methylation in CD4 + T cells. The study of MVA 85A also used ChIP to assess histone modifications in monocytes. The remaining seven studies investigated DNA methylation in whole blood, peripheral blood mononuclear cells (PBMC) or CD8 + T cells using either the Illumina Infinium HumanMethylation450 BeadChip array to measure genome-wide methylation or bisulphite sequencing to detect site-specific CpG methylation in regulatory regions. One study examining the epigenetic effects of YFV vaccine in CD8 + T cells also used Assay for Transposase-Accessible Chromatin with high-throughput sequencing (ATAC-seq) to assess chromatin accessibility across the genome. ()
Table 1. Host epigenetic modifications of infectious pathogens and vaccines.
Table 2. Analysis technique by cell type.
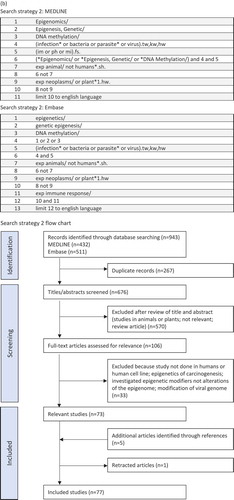
The second search, identifying studies of pathogen-induced epigenetic changes, identified 943 articles, of which 106 full-text articles were assessed and 73 were included in the review (). Five additional relevant articles were identified through hand searching of reference lists and one article was retracted, resulting in a total of 77 studies.
The articles were grouped according to whether they investigated epigenetic effects in human subjects (38 studies) or in human-derived cells or cell lines (41 studies), with some articles investigating both. Of the studies done in human subjects, eight focused on bacteria with six assessing pathogenic bacteria, including Mycobacterium tuberculosis and Helicobacter pylori, and two assessing commensal bacteria in the gut and placental microbiota. There were 21 studies involving viral pathogens, including human immunodeficiency virus-1 (HIV), human T-cell lymphotropic virus-1 (HTLV), cytomegalovirus (CMV), EBV, hepatitis B virus (HBV), HCV, respiratory syncytial virus (RSV) and dengue virus. Five studies looked at parasites including Leishmania braziliensis, Schistosoma haematobium, Ascaris lumbricoides, Plasmodium falciparum and Trypanosoma cruzi. The remaining five articles considered syndromes of clinical infection, including neonatal sepsis, periodontitis and chorioamnionitis. ()
Of the 41 studies done in human cells or cell lines, 16 assessed bacteria with 12 investigating pathogenic bacteria, including M. tuberculosis, Burkholderia pseudomallei, Escherichia coli, Anaplasma phagocytophilum, Listeria monocytogenes and Chlamydia trachomatis, and four investigating specific bacteria of the oral, airway and gut microbiota. One article investigated the effects of the parasite Leishmania donovani and 19 investigated viruses including HIV, IAV, dengue virus, EBV, human rhinovirus (HRV), RSV and human herpesvirus-6B (HHV-6B). Six articles investigated pathogen constituents, including β-glucan derived from the cell wall of Candida albicans, lipopolysaccharide (LPS) from Porphyromonas gingivalis, E. coli and Fusobacterium nucleatum, Bacillus anthracis suppressor-of-variegation, enhancer-of-zeste, trithorax protein (BaSET) and 2-aminoacetophenone (2-AA) from Pseudomonas aeruginosa. ()
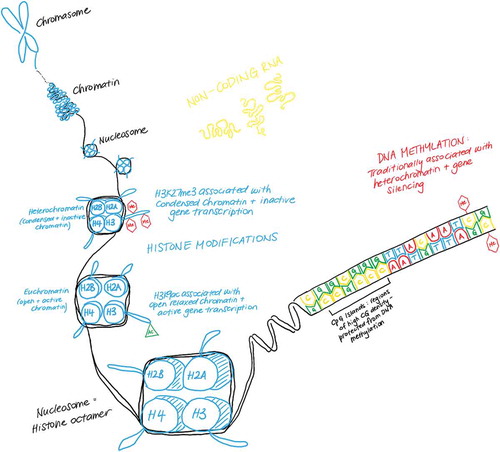
Most of the studies measured either DNA methylation or histone modifications, with only three investigating non-coding RNA. () Genome-wide DNA methylation was analysed in 19 articles, site-specific DNA methylation was analysed in 45 articles and histone modifications were analysed in 21 articles. The studies included in the review were heterogeneous in design with a broad range of human cells, tissues and human-derived cell lines investigated using a variety of laboratory techniques (). Some studies analysed epigenetic changes in more than one cell type using more than one analysis technique.
Results and discussion
The results of the systematic review are summarized in –. Despite the variation between studies in design, methodology and outcome, several themes emerge that highlight the many and diverse ways in which vaccines and infectious pathogens modify and regulate the human epigenome.
Table 3. Epigenetic effects of vaccines.
Table 4. Epigenetic effects of infectious pathogens: studies conducted in human cells or cell lines.
Table 5. Epigenetic effects of infectious pathogens: studies conducted in humans.
The influence of vaccination on the epigenome
Immune modulation
Neonatal BCG vaccination confers a significant survival benefit in high-mortality settings above and beyond its protective effect against tuberculosis. This has been attributed to heterologous protection against mortality from neonatal sepsis, pneumonia, and diarrhoeal disease [Citation41]. These heterologous effects of BCG vaccine are proposed to be the result of epigenetic reprogramming of innate immune cells and the development of innate immune memory, termed ‘trained immunity’ [Citation42].
In vitro ‘training’ of human monocytes with live or gamma-irradiated BCG vaccine invokes a strongly potentiated pro-inflammatory cytokine response upon re-stimulation with LPS [Citation43]. This is accompanied by the upregulation of the activating histone mark H3K4me3 at the level of the TNF-α and IL-6 promoters. These in vitro findings are consistent with human studies in which monocytes isolated from healthy adults 3 months after BCG vaccination demonstrate increased production of IFN-γ, TNF-α and IL-1β following exposure to mycobacterial and non-mycobacterial stimuli [Citation44]. ChIP analysis and sequencing also showed genome wide changes in H3K27ac and enrichment of H3K4me3 at the TNF-α and IL-6 promoters in monocytes following BCG vaccination [Citation44,Citation45]. In a controlled human infection model, administration of BCG vaccination one month before vaccination with the live attenuated YFV vaccine resulted in lower levels of YFV viraemia in BCG-vaccinated subjects [Citation45]. This demonstrates a correlation between the induction of trained immunity and protection against an unrelated viral infection.
MVA85A is a recombinant strain of modified Vaccinia Ankara expressing the immunodominant MTB protein 85A [Citation46]. It is being investigated as a booster vaccination for BCG vaccine to protect against tuberculosis [Citation46]. In vitro priming of human monocytes with Vaccinia virus induces trained immunity similar to the effect observed with BCG priming. In contrast, monocytes primed with MVA85A show decreased heterologous IL-6 and TNF-α responses suggestive of induced innate immune tolerance. While MVA85A did not induce any changes in H3K4me3 in the IL-6 or TNF-α promoters in monocytes, the tolerising effect of MVA85A was reversed by the addition of a histone methyltransferase inhibitor. This suggests the induction of innate immune tolerance may also be epigenetically mediated [Citation47].
There is emerging evidence that BCG vaccine may also protect against the progression of certain autoimmune diseases including diabetes and multiple sclerosis [Citation48,Citation49]. In females with type 1 diabetes mellitus administration of BCG vaccination results in improved glycaemic control and demethylation of the methylation control sites in six central T-regulatory genes [Citation50].
Vaccine response
Predicting the degree to which an individual will mount an adequate immune response to vaccination and develop sustained immunity poses an ongoing challenge to clinicians and researchers [Citation51]. In the elderly, a poor humoral immune response to influenza vaccination is attributed to immunosenescence [Citation52]. In a study in healthy 50–74 year olds, although the global DNA methylation profile underwent minimal changes, a specific group of CpG sites, when co-ordinately hypomethylated, was associated with lower humoral immune response to influenza vaccination [Citation53]. A study comparing influenza vaccine responses in older and younger populations showed larger epigenetic remodelling in vaccine responders aged over 50 years [Citation54]. The differentially methylated probes mapped to genes involved in immunosenescence. Similarly, hypomethylation of RNF39 (Ring Finger Protein 39), a transcription factor in the major histocompatibility complex (MHC) class I region, is associated poor HBV vaccine responses in infants [Citation55].
Disease risk
The role of epigenetics in the ability of vaccines, including BCG and pertussis, to modulate allergic disease risk is poorly understood [Citation56,Citation57]. In a whole population birth cohort on the Isle of Wight in 1989, tetanus vaccination was associated with differential DNA methylation and a reduced risk of asthma in adolescence [Citation58].
The influence of infection on the epigenome
Innate immune system modulation
Similar to the studies of BCG vaccine-induced trained innate immunity, exposure of human monocytes to β-glucan, the main cell wall constituent of C. albicans, results in a genome-wide increase in H3K4me3 and increased production of TNF-α and IL-6 upon re-stimulation with C. albicans and unrelated innate immune stimuli [Citation59,Citation60]. β-glucan ‘training’ also results in dynamic genome-wide changes in H3K27ac in monocytes [Citation61]. The Fulani and Mossi, sympatric ethnic groups in Burkina Faso, are known to have different susceptibility to malaria. The Fulani group have lower rates of malaria infection and demonstrate a ‘high alert’ immune state similar to that of trained immunity. Monocytes from Plasmodium falciparum infected and uninfected Fulani and Mossi males showed stable DNA methylation profiles between disease states and ethnic groups but significantly different transcriptional responses in the malaria infected Fulani with increased expression of chromatin factors involved in H3K4me [Citation62].
CMV has also been shown to contribute to innate immune memory. CMV infection induces subsets of ‘adaptive’ natural killer (NK) cells that lack expression of B cell and myeloid cell-related signalling proteins and display epigenetic diversification compared to naïve NK cells. These subpopulations of NK cells possess a global methylation signature similar to cytotoxic T lymphocytes and undergo expansion in response to CMV–infected cells in an antibody-dependent manner [Citation63–Citation65]. Study of these adaptive NK cells in chronic viral hepatitis shows that these cells preferentially expand in CMV seropositive chronically HBV–infected patients and that their distinct methylation pattern of the FCER1 G and IFNG promoter regions is conserved [Citation66].
At the other end of the spectrum of innate immune memory, tolerance results in dampening of host inflammatory responses and facilitates pathogen persistence [Citation67]. Pre-treatment of human macrophages with 2-AA, a quorum-sensing molecule excreted by Pseudomonas aeruginosa, reduces H3K18ac at the TNF promoter on re-exposure to 2-AA and results in attenuated transcription of pro-inflammatory cytokines [Citation68].
Adaptive immune system modulation
Studies have also focused on the epigenetic modulation of the adaptive immune system in response to infection, in particular T cell dysregulation in the setting of acute and chronic viral infection. Regulatory T cells (Tregs) are required for peripheral immune tolerance and Foxp3 is the major transcription factor essential for maintaining the suppressive function of Tregs. Demethylation of a CpG island in the FOXP3 locus, the Treg-specific demethylated region (TSDR), is associated with stable Foxp3 expression during Treg development in the thymus [Citation69]. In vitro HIV infection modifies the phenotype and function of Tregs isolated from healthy donors through altered methylation of the FOXP3 gene. Increased methylation of the FOXP3 locus is associated with loss of Treg suppressive capacity and an altered cytokine profile that may contribute to heightened immune activation in HIV infection [Citation70]. Increased methylation of the FOXP3 TSDR and decreased functional suppression was also found in Tregs isolated from patients with HTLV associated myelopathy/tropical spastic paraparesis (HAM/TSP) [Citation71]. In contrast, the FOXP3 promoter is significantly demethylated in colonic mucosa and PBMC from HIV–infected patients [Citation72].
Chronic viral infection is associated with persistent antigen presentation and progressive T cell dysfunction [Citation73]. Sustained expression of the inhibitory programmed death-1 (PD-1) receptor on exhausted CD8 + T cells is associated with lack of viral control and correlates with disease progression in HIV [Citation74]. HIV-specific CD8 + T cells isolated from donors with acute HIV infection and viraemia demonstrate demethylation of the PD-1 transcriptional regulatory region compared to donor matched naïve CD8 + T cells. The PD-1 transcriptional regulatory region remains demethylated in the chronic stages of HIV infection, even following spontaneous or pharmacological reduction in viral load [Citation75]. Persistent demethylation of the PD-1 regulatory region is also observed in other chronic viral infections including CMV and EBV [Citation76]. In a model of acute viraemia, YFV-specific CD8 + T cells isolated from donors 14 days after YFV vaccination show transient expression of PD-1 and near complete demethylation of the PD-1 regulatory region. After contraction of the YFV-specific CD8 + T cells 90 days post-vaccination, a functional population of memory CD8 + T cells emerges with re-methylation of the PD-1 regulatory region [Citation76]. Using a similar controlled human infection model, deuterium labelling of CD8 + T cells that proliferate in response to YFV vaccine shows that long-lived memory CD8 + T cells retain epigenetic marks of their effector history and are distinct from naïve cells [Citation77].
Host evasion and latent infection
Invading pathogens can also use epigenetic modification as a strategy to promote viral latency and host evasion. A genome wide analysis of histone modifications in an in vitro model of latent HIV infection revealed minimal overall changes in histone modifications, but several regions within gene promoters showed changes in H3K4me3 and H3K9ac signal. These genes include the cell cycle regulatory genes CDKN1 and CCND2, which may play a role in the maintenance of HIV latency [Citation78]. In vitro HIV infection also increases global DNA methylation in the cellular genome [Citation79]. HHV-6B infection induces hypomethylation in vitro in regions close to telomeres that may facilitate viral integration and promote latency [Citation80].
Epigenetic manipulation of the host immune system may also play a role in chronic infection with HBV. Increased methylation of a CpG island in a promoter of the CIITA gene, which regulates expression of major histocompatibility complex (MHC) class II, is associated with persistent HBV infection [Citation81].
A. phagocytophilum infection of THP-1 cells results in epigenetic silencing of host defence genes through histone modifications that promote intracellular survival of the bacterium [Citation82]. M. tuberculosis infection of THP-1 cells is also associated with histone modifications through the recruitment of HDAC1 to the IL-12B promoter and histone deacetylation. This results in downregulation of IL-12B gene expression, which is known to play a role in Th1 responses, and may contribute to the mechanisms by which M. tuberculosis subverts the host immune system [Citation83]. Genome-wide DNA methylation profiling following infection with L. donovani identified a group of over 400 CpG sites with altered methylation corresponding to genes involved in host immune defence [Citation84]. In vitro L. monocytogenes infection induces a dramatic dephosphorylation of histone 3 and deacetylation of histone 4 that is mediated by the toxin listeriolysin O. These histone modifications are associated with reduced transcriptional activity of a subset of host immunity genes. Cholesterol-dependent cytolysins (CDC) from other bacterial pathogens also induce a similar degree of histone dephosphorylation, suggesting that these bacterial toxins regulate the host immune response via epigenetic manipulation [Citation85]. Comparison of the epigenetic changes induced by infection with B. pseudomallei and M. tuberculosis reveal a subset of over 100 genes containing differentially methylated regions (DMR) common to both pathogens. This demonstrates that there are both pathogen-specific and pathogen-common epigenetic changes that occur in response to microbial infection [Citation86].
Disease pathogenesis and progression
Periodontitis, a chronic inflammatory disease predominantly driven by P. gingivalis, is associated with blunted Toll-like receptor (TLR) expression and signalling. The TLR2 promoter is hypermethylated in gingival epithelial cells isolated from periodontitis-affected tissue [Citation87,Citation88]. Consistent with this finding, in vitro infection with P. gingivalis induces hypermethylation of several gene promoters, including TLR2 [Citation89]. C. trachomatis infection of human conjunctival epithelial cells increases methylation of the CDH1 promoter and downregulates expression of E-Cadherin, which is hypothesized to contribute to the conjunctival fibrosis that causes blindness from trachoma [Citation90]. Global DNA methylation analysis of myocardium from patients with chronic Chagas cardiomyopathy shows differential methylation of more than 7000 CpG sites [Citation90]. These sites correlate with the differential expression of pathogenically relevant cardiovascular and immune system genes. The cytokine storm of severe dengue virus infection is characterized by the overexpression of proinflammatory cytokines, including TNF-α and IFN-γ. Individuals with dengue virus infection have reduced methylation of the TNF-α promoter and increased TNF-α messenger RNA (mRNA) in whole blood compared with uninfected controls but no difference in methylation of the IFN-γ promoter [Citation91].
In a cohort of patients with chronic HBV, those with acute on chronic HBV liver failure had higher methylation of the GSTM3 gene promoter compared with those without liver failure. The GSTM3 gene codes for glutathione-s-transferase M3 that mediates oxidative stress in the liver. Hypermethylation of the GSTM3 promoter may correlate with disease severity and progression in chronic HBV [Citation92]. In a cohort of tuberculosis (TB) exposed children in Swaziland, helminth co-infection with S. haemotobium was associated with increased global DNA methylation that persisted six months after successful eradication therapy [Citation93]. These DNA methylation changes were associated with a decrease in the proliferation of TB-specific CD4 + T cells, supporting the hypothesis that helminth co-infection perturbs the immune response to M. tuberculosis and increases the risk of tuberculosis progression.
The modifying effect of long-term antiviral therapy on the epigenome has also been assessed. Acute untreated HIV-1 infection is associated with demethylation of the CCR5 intron and increased expression of CCR5 [Citation94]. Suppression of viral replication, either spontaneously or pharmacologically, is correlated with increased methylation of the CCR5 intron [Citation94]. An inverse correlation between DNA methylation and active viral replication is also seen in the setting of long-term antiretroviral therapy for HIV [Citation95].
Disease biomarker
The use of DNA methylation signatures as biomarkers for latent and occult infection has been explored for several pathogens and clinical infection syndromes. The DNA methylation profiles of granulocytes and monocytes isolated from patients with active TB and latent TB show multiple DMR enriched in several immune regulatory pathways [Citation96]. Macrophages from patients with active TB and latent TB also show different methylation profiles that could potentially be used as diagnostic biomarkers [Citation97,Citation98]. Additionally, in vitro infection of THP-1 cells with M. tuberculosis strains isolated from patients with pulmonary or disseminated TB show distinct methylation signatures [Citation97,Citation98].
The differential methylation of more than 500 genes in umbilical cord blood from infants born to mothers with active HBV infection suggests a possible role for DNA methylation status at birth as a biomarker of prenatal HBV exposure [Citation99]. Similarly, differential DNA methylation was found in placental tissue from preterm infants born in the setting of chorioamnionitis compared with preterm infants without significant pathology. The placentas with chorioamnionitis showed altered DNA methylation signatures that were not observed in the absence of inflammation [Citation100]. These changes were hypothesized to reflect both increased number and altered function of innate immune cells in the placenta. The composition and diversity of the placental microbiome also influences DNA methylation patterns in placental tissue. A study examining 16 bacterial species in the placentas of 84 preterm infants found methylation signatures unique to the particular bacterial species present [Citation101]. The differentially methylated genes are involved in immune and inflammatory responses and are enriched in the NF-κB signalling pathway that is critical for foetal development.
In preterm infants, altered DNA methylation patterns within the CALCA gene promoter, that codes for calcitonin, are associated with early and late onset neonatal sepsis [Citation102]. A clinical diagnosis of neonatal sepsis is also associated with increased methylated genomic DNA [Citation103]. Uropathogenic E. coli infection results in increased methylation of CDKN2A exon 1, that may have clinical utility as a biomarker for urinary tract infection susceptibility and recurrence [Citation104].
Biological ageing
The ‘epigenetic clock’ is a validated biomarker of ageing that comprises a linear combination of 353 CpG sites. It is applicable to most human cell and tissue types and is strongly correlated with chronological age throughout life [Citation1]. Advanced ‘epigenetic age’ is associated with reduced physical and mental fitness and is prognostic of all-cause mortality in later life [Citation105,Citation106]. Chronic viral infection has been shown to induce biological age acceleration, as measured by the epigenetic clock. Chronic treated HIV infection is associated with global dysregulation of the methylome in blood and an average biological age advancement of approximately 5 years [Citation107]. In brain specimens from HIV subjects, this advancement is more than 7 years [Citation108]. Similarly, CMV infection is associated with accelerated epigenetic age [Citation109]. In chronic HCV infection age acceleration is only seen in the presence of fibrosis or co-infection with HIV [Citation110]. Using a peripheral blood immunophenotyping model to infer epigenetic age, slowing of age acceleration was observed in HBV or HCV mono-infection with sustained virological response on therapy [Citation110]. In the study examining the influence of chronological age on influenza vaccine responses there was no difference in the epigenetic age of vaccine responders and non-responders [Citation54].
Disease risk
The modulation of long-term disease risk by infection or bacterial colonization may be epigenetically mediated in several disease processes. Whole genome methylation analysis of pregnant women with different gut microbiota profiles found an association between predominant gut bacterial phyla and methylation patterns. A predominance of Firmicutes was associated with differential methylation of gene promoters linked to cardiovascular disease and metabolic syndrome [Citation111].
Epigenetic variations may also play a role in susceptibility to malaria and efficacy of anti-malarial drugs. Analysis of whole blood from healthy controls and P. falciparum infected subjects with varying severities of clinical disease found lower global DNA methylation is associated with untreated disease, complicated malaria and higher parasitaemia. Malaria affected subjects also had specific demethylation of the ABCB1 promoter which codes for P-glycoprotein, an efflux protein that is proposed to modulate the effect of antimalarial drugs on intracellular parasites.
Early life viral respiratory infections, including RSV and HRV, are implicated in the development of childhood asthma [Citation112]. Children with a history of severe RSV bronchiolitis in infancy have decreased methylation of CpG sites within the proximal enhancer of the PRF1 gene. This gene codes for perforin, which is involved in the cellular immune response to viral infection. Decreased methylation of these CpG sites is also independently associated with persistent wheeze and maternal asthma. This methylation signature may be a potential biomarker for the development of complications of severe RSV infection, including persistent wheeze and asthma. In vitro HRV infection of nasal epithelial cells from children with and without asthma showed genome-wide differences in DNA methylation. These differences were associated with altered transcription of genes involved in the host immune response to viral pathogens and asthma pathogenesis [Citation113]. These findings support the theory that HRV infection may contribute to the persistence and progression of asthma via epigenetic mechanisms.
Conclusion
Our understanding of the complex interaction between vaccines and infectious pathogens, and modification of the human epigenome is rapidly evolving. Until recently, epigenetic research has focused predominantly on epigenomic profiling with functional inference made through correlation with modifying enzymes and gene transcription. New technological advances enable the manipulation and ‘editing’ of epigenomic features that allow causality and function to be directly established [Citation114]. Refined methods of epigenomic profiling will more clearly define the role of vaccines and pathogens in the dynamic regulation of the epigenetic landscape. A more comprehensive understanding of this interplay will identify potential targets to modulate the immune system and novel therapies to treat chronic infection. This is particularly important in vaccine development where the potential heterologous effects of vaccines may have significant implications on immune regulation. Future research needs to focus on the epigenetic impact of vaccines in early childhood, when the epigenome is particularly susceptible to modification and the consequences on immune maturation and development are most significant. This will both inform the development of new vaccines and guide global vaccine policy.
Authors’ Contributions
SB formulated the research question, performed the literature search, conducted data analysis and drafted and edited the manuscript. NM contributed to data analysis and interpretation and revision of the manuscript. BN contributed to drafting and revision of the manuscript. NC formulated the research question and contributed to drafting and editing of the final manuscript. All authors read and approved the final manuscript.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14(10):R115.
- Lee KW, Pausova Z. Cigarette smoking and DNA methylation. Front Genet. 2013;4:132.
- Heijmans BT, Tobi EW, Stein AD, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 2008;105(44):17046–17049.
- Hattori N, Ushijima T. Epigenetic impact of infection on carcinogenesis: mechanisms and applications. Genome Med. 2016;8(1):10.
- Pollard AJ, Finn A, Curtis N. Non-specific effects of vaccines: plausible and potentially important, but implications uncertain. Arch Dis Child. 2017;102:1077–1081.
- Wei M, Wang L, Wu T, et al. NLRP3 activation was regulated by DNA methylation modification during mycobacterium tuberculosis infection. Biomed Res Int. 2016;2016:4323281.
- Cizmeci D, Dempster EL, Champion OL, et al. Mapping epigenetic changes to the host cell genome induced by Burkholderia pseudomallei reveals pathogen-specific and pathogen-generic signatures of infection. Sci Rep. 2016;6:30861.
- Schmeck B, Beermann W, van Laak V, et al. Intracellular bacteria differentially regulated endothelial cytokine release by MAPK-dependent histone modification. J Immunol. 2005;175(5):2843–2850.
- Eskandarian HA, Impens F, Nahori MA, et al. A role for SIRT2-dependent histone H3K18 deacetylation in bacterial infection. Science. 2013;341(6145):1238858.
- Ghadimi D, Helwig U, Schrezenmeir J, et al. Epigenetic imprinting by commensal probiotics inhibits the IL-23/IL-17 axis in an in vitro model of the intestinal mucosal immune system. J Leukoc Biol. 2012;92(4):895–911.
- Wang L, Wu G, Qin X, et al. Expression of nodal on bronchial epithelial cells influenced by lung microbes through DNA methylation modulates the differentiation of T-helper cells. Cell Physiol Biochem. 2015;37(5):2012–2022.
- Wang Y, Zhong H, Xie X, et al. Long noncoding RNA derived from CD244 signaling epigenetically controls CD8+ T-cell immune responses in tuberculosis infection. Proc Natl Acad Sci U S A. 2015;112(29):E3883–92.
- Shin SH, Park SY, Ko JS, et al. Aberrant CpG island hypermethylation in pediatric gastric mucosa in association with Helicobacter pylori infection. Arch Pathol Lab Med. 2011;135(6):759–765.
- Hong SJ, Lee HJ, Oh JH, et al. Age-related methylation patterning of housekeeping genes and tissue-specific genes is distinct between the stomach antrum and body. Epigenomics. 2013;5(3):283–299.
- Kuhtreiber W, Plager S, Kim T, et al. Permanent epigenetic changes in Treg signature genes of type 1 diabetic subjects after in vivo BCG vaccinations. Diabetes. 2017;66(Supplement 1):A483.
- Shimura M, Toyoda Y, Iijima K, et al. Epigenetic displacement of HP1 from heterochromatin by HIV-1 Vpr causes premature sister chromatid separation. J Cell Biol. 2011;194(5):721–735.
- Chandel N, Ayasolla KS, Lan X, et al. Epigenetic modulation of human podocyte vitamin D receptor in HIV milieu. J Mol Biol. 2015;427(20):3201–3215.
- Mikovits JA, Young HA, Vertino P, et al. Infection with human immunodeficiency virus type 1 upregulates DNA methyltransferase, resulting in de novo methylation of the gamma interferon (IFN-gamma) promoter and subsequent downregulation of IFN-gamma production. Mol Cell Biol. 1998;18(9):5166–5177.
- Britton LM, Sova P, Belisle S, et al. A proteomic glimpse into the initial global epigenetic changes during HIV infection. Proteomics. 2014;14(19):2226–2230.
- Nunes JM, Furtado MN, de Morais Nunes ER, et al. Modulation of epigenetic factors during the early stages of HIV-1 infection in CD4+ T cells in vitro. Virology. 2018;523:41–51.
- Fang J, Hao Q, Liu L, et al. Epigenetic changes mediated by microRNA miR29 activate cyclooxygenase 2 and lambda-1 interferon production during viral infection. J Virol. 2012;86(2):1010–1020.
- Mukherjee S, Vipat VC, Chakrabarti AK. Infection with influenza A viruses causes changes in promoter DNA methylation of inflammatory genes. Influenza Other Respi Viruses. 2013;7(6):979–986.
- Li W, Sun W, Liu L, et al. IL-32: a host proinflammatory factor against influenza viral replication is upregulated by aberrant epigenetic modifications during influenza A virus infection. J Immunol. 2010;185(9):5056–5065.
- Tang B, Zhao R, Sun Y, et al. Interleukin-6 expression was regulated by epigenetic mechanisms in response to influenza virus infection or dsRNA treatment. Mol Immunol. 2011;48(8):1001–1008.
- Marcos-Villar L, Diaz-Colunga J, Sandoval J, et al. Epigenetic control of influenza virus: role of H3K79 methylation in interferon-induced antiviral response. Sci Rep. 2018;8(1):1230.
- Hernando H, Shannon-Lowe C, Islam AB, et al. The B cell transcription program mediates hypomethylation and overexpression of key genes in Epstein-Barr virus-associated proliferative conversion. Genome Biol. 2013;14(1):R3.
- McErlean P, Favoreto S Jr., Costa FF, et al. Human rhinovirus infection causes different DNA methylation changes in nasal epithelial cells from healthy and asthmatic subjects. BMC Med Genomics. 2014;7:37.
- Qi Y, Li Y, Zhang L, et al. MicroRNA expression profiling and bioinformatic analysis of dengue virus-infected peripheral blood mononuclear cells. Mol Med Rep. 2013;7(3):791–798.
- Zhang Y, Li SK, Yi Yang K, et al. Whole genome methylation array reveals the down-regulation of IGFBP6 and SATB2 by HIV-1. Sci Rep. 2015;5:10806.
- Gindin Y, Gaggar A, Kottilil S, et al. DNA methylation and immune cell markers demonstrate evidence of accelerated aging in patients with chronic HBV infection, with improvement during treatment. J Hepatol. 2018;68(Supplement 1):S63–S4.
- Elgizouli M, Logan C, Grychtol R, et al. Reduced PRF1 enhancer methylation in children with a history of severe RSV bronchiolitis in infancy: an association study. BMC Pediatr. 2017;17(1):(no pagination)(65).
- Kennedy RB, Ovsyannikova IG, Haralambieva IH, et al. Immunosenescence-related transcriptomic and immunologic changes in older individuals following influenza vaccination. Front Immunol. 2016;7:450.
- Almeida L, Silva JA, Andrade VM, et al. Analysis of expression of FLI1 and MMP1 in American cutaneous leishmaniasis caused by Leishmania braziliensis infection. Infect Genet Evol. 2017;49:212–220.
- Gupta AP, Bozdech Z. Epigenetic landscapes underlining global patterns of gene expression in the human malaria parasite, Plasmodium falciparum. Int J Parasitol. 2017;47(7):399–407.
- Laugier L, Frade AF, Ferreira FM, et al. Whole-genome cardiac DNA methylation fingerprint and gene expression analysis provide new insights in the pathogenesis of chronic chagas disease cardiomyopathy. Clin Infect Dis. 2017;65(7):1103–1111.
- Quintin J, Cheng SC, van der Meer JW, et al. Innate immune memory: towards a better understanding of host defense mechanisms. Curr Opin Immunol. 2014;29:1–7.
- Martins MD, Jiao Y, Larsson L, et al. Epigenetic modifications of histones in periodontal disease. J Dent Res. 2016;95(2):215–222.
- Mujtaba S, Winer BY, Jaganathan A, et al. Anthrax SET protein: a potential virulence determinant that epigenetically represses NF-kappaB activation in infected macrophages. J Biol Chem. 2013;288(32):23458–23472.
- Ganesan M, Zhang J, Bronich T, et al. Acetaldehyde accelerates HCV-induced impairment of innate immunity by suppressing methylation reactions in liver cells. Am J Physiol. 2015;309(7):G566–77.
- Tolg C, Sabha N, Cortese R, et al. Uropathogenic E. coli infection provokes epigenetic downregulation of CDKN2A (p16INK4A) in uroepithelial cells. Lab Invest. 2011;91(6):825–836.
- Roth A, Garly ML, Jensen H, et al. Bacillus calmette-guerin vaccination and infant mortality. Expert Rev Vaccines. 2006;5(2):277–293.
- Netea MG, Quintin J. van der Meer JW. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011;9(5):355–361.
- Arts RJ, Blok BA, Aaby P, et al. Long-term in vitro and in vivo effects of gamma-irradiated BCG on innate and adaptive immunity. J Leukoc Biol. 2015;98(6):995–1001.
- Kleinnijenhuis J, Quintin J, Preijers F, et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A. 2012;109(43):17537–17542.
- Arts RJW, Moorlag S, Novakovic B, et al. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe. 2018;23(1):89–100.e5.
- McShane H, Pathan AA, Sander CR, et al. Recombinant modified vaccinia virus ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat Med. 2004;10(11):1240–1244.
- Blok BA, Jensen KJ, Aaby P, et al. Opposite effects of vaccinia and modified vaccinia ankara on trained immunity. Eur J Clin Microbiol Infect Dis. 2019;38(3):449–456.
- Faustman DL, Wang L, Okubo Y, et al. Proof-of-concept, randomized, controlled clinical trial of bacillus-calmette-guerin for treatment of long-term type 1 diabetes. PLoS One. 2012;7(8):e41756.
- Ristori G, Romano S, Cannoni S, et al. Effects of bacille calmette-guerin after the first demyelinating event in the CNS. Neurology. 2014;82(1):41–48.
- Kuhtreiber WM, Tran L, Kim T, et al. Long-term reduction in hyperglycemia in advanced type 1 diabetes: the value of induced aerobic glycolysis with BCG vaccinations. Npj Vaccines. 2018;3(1):(no pagination)(23).
- Furman D, Davis MM. New approaches to understanding the immune response to vaccination and infection. Vaccine. 2015;33(40):5271–5281.
- Haq K, McElhaney JE. Immunosenescence: influenza vaccination and the elderly. Curr Opin Immunol. 2014;29:38–42.
- Zimmermann MT, Oberg AL, Grill DE, et al. System-wide associations between DNA-methylation, gene expression, and humoral immune response to influenza vaccination. PLoS One. 2016;11(3):e0152034.
- Gensous N, Franceschi C, Blomberg BB, et al. Responders and non-responders to influenza vaccination: A DNA methylation approach on blood cells. Exp Gerontol. 2018;105:94–100.
- Lu Y, Cheng Y, Yan W, et al. Exploring the molecular causes of hepatitis B virus vaccination response: an approach with epigenomic and transcriptomic data. BMC Med Genomics. 2014;7:12.
- Linehan MF, Nurmatov U, Frank TL, et al. Does BCG vaccination protect against childhood asthma? Final results from the manchester community asthma study retrospective cohort study and updated systematic review and meta-analysis. J Allergy Clin Immunol. 2014;133(3):688–95 e14.
- Nilsson L, Kjellman NI, Bjorksten B. Allergic disease at the age of 7 years after pertussis vaccination in infancy: results from the follow-up of a randomized controlled trial of 3 vaccines. Arch Pediatr Adolesc Med. 2003;157(12):1184–1189.
- Janjanam VD, Mukherjee N, Lockett GA, et al. Tetanus vaccination is associated with differential DNA-methylation: reduces the risk of asthma in adolescence. Vaccine. 2016;34(51):6493–6501.
- Cheng SC, Quintin J, Cramer RA, et al. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science (New York, NY). 2014;345(6204):1250684.
- Quintin J, Saeed S, Martens JH, et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe. 2012;12(2):223–232.
- Arts RJ, Novakovic B, Ter Horst R, et al. Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab. 2016;24(6):807–819.
- Quin JE, Bujila I, Cherif M, et al. Major transcriptional changes observed in the Fulani, an ethnic group less susceptible to malaria. elife. 2017;6:e29156.
- Schlums H, Cichocki F, Tesi B, et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity. 2015;42(3):443–456.
- Lee J, Zhang T, Hwang I, et al. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity. 2015;42(3):431–442.
- Luetke-Eversloh M, Hammer Q, Durek P, et al. Human cytomegalovirus drives epigenetic imprinting of the IFNG locus in NKG2Chi natural killer cells. PLoS Pathog. 2014;10(10):e1004441.
- Zecher B, Schuch A, Correia MP, et al. Metabolic properties of epigenetically regulated adaptive versus conventional NK cells are conserved in chronic viral hepatitis. J Hepatol. 2017;66(1 Supplement 1):S541.
- Novakovic B, Habibi E, Wang SY, et al. beta-glucan reverses the epigenetic state of LPS-induced immunological tolerance. Cell. 2016;167(5):1354–68 e14.
- Bandyopadhaya A, Tsurumi A, Maura D, et al. A quorum-sensing signal promotes host tolerance training through HDAC1-mediated epigenetic reprogramming. Nat. 2016;1:16174.
- Toker A, Huehn J. To be or not to be a treg cell: lineage decisions controlled by epigenetic mechanisms. Sci Signal. 2011;4(158):pe4.
- Pion M, Jaramillo-Ruiz D, Martinez A, et al. HIV infection of human regulatory T cells downregulates Foxp3 expression by increasing DNMT3b levels and DNA methylation in the FOXP3 gene. AIDS. 2013;27(13):2019–2029.
- Anderson MR, Enose-Akahata Y, Massoud R, et al. Epigenetic modification of the FoxP3 TSDR in HAM/TSP decreases the functional suppression of tregs. J Neuroimmune Pharmacol. 2014;9(4):522–532.
- Abdel-Hameed EA, Ji H, Sherman KE, et al. Epigenetic modification of FOXP3 in patients with chronic HIV infection. J Acquired Immune Deficiency Syndromes. 2014;65(1):19–26.
- Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–687.
- Zhang JY, Zhang Z, Wang X, et al. PD-1 up-regulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors but not in long-term nonprogressors. Blood. 2007;109(11):4671–4678.
- Youngblood B, Noto A, Porichis F, et al. Cutting edge: prolonged exposure to HIV reinforces a poised epigenetic program for PD-1 expression in virus-specific CD8 T cells. J Immunol. 2013;191(2):540–544.
- Youngblood B, Oestreich KJ, Ha SJ, et al. Chronic virus infection enforces demethylation of the locus that encodes PD-1 in antigen-specific CD8(+) T cells. Immunity. 2011;35(3):400–412.
- Akondy RS, Fitch M, Edupuganti S, et al. Origin and differentiation of human memory CD8 T cells after vaccination. Nature. 2017;552(7685):362–367.
- Park J, Lim CH, Ham S, et al. Genome-wide analysis of histone modifications in latently HIV-1 infected T cells. AIDS. 2014;28(12):1719–1728.
- Maricato JT, Furtado MN, Takenaka MC, et al. Epigenetic modulations in activated cells early after HIV-1 infection and their possible functional consequences. PLoS One. 2015;10(4):e0119234.
- Engdahl E, Dunn N, Niehusmann P, et al. Human herpesvirus 6B induces hypomethylation on chromosome 17p13.3, correlating with increased gene expression and virus integration. J Virol. 2017;91(11):01.
- He Y, Zhao Y, Zhang S, et al. Not polymorphism but methylation of class II transactivator gene promoter IV associated with persistent HBV infection [Erratum appears in J Clin Virol. 2010 Mar;47(3):300]. J Clin Virol. 2006;37(4):282–286.
- Garcia-Garcia JC, Barat NC, Trembley SJ, et al. Epigenetic silencing of host cell defense genes enhances intracellular survival of the rickettsial pathogen Anaplasma phagocytophilum. PLoS Pathog. 2009;5(6):e1000488.
- Chandran A, Antony C, Jose L, et al. Mycobacterium tuberculosis infection induces HDAC1-mediated suppression of IL-12B gene expression in macrophages. Front Cell Infect Microbiol. 2015;5(DEC):90.
- Marr AK, MacIsaac JL, Jiang R, et al. Leishmania donovani infection causes distinct epigenetic DNA methylation changes in host macrophages. PLoS Pathog. 2014;10(10):e1004419.
- Hamon MA, Batsche E, Regnault B, et al. Histone modifications induced by a family of bacterial toxins. Proc Natl Acad Sci U S A. 2007;104(33):13467–13472.
- Pacis A, Tailleux L, Morin AM, et al. Bacterial infection remodels the DNA methylation landscape of human dendritic cells. Genome Res. 2015;25(12):1801–1811.
- Benakanakere M, Abdolhosseini M, Hosur K, et al. TLR2 promoter hypermethylation creates innate immune dysbiosis. J Dent Res. 2015;94(1):183–191.
- de Faria Amormino SA, Arao TC, Saraiva AM, et al. Hypermethylation and low transcription of TLR2 gene in chronic periodontitis. Hum Immunol. 2013;74(9):1231–1236.
- Yin L, Chung WO. Epigenetic regulation of human beta-defensin 2 and CC chemokine ligand 20 expression in gingival epithelial cells in response to oral bacteria. Mucosal Immunol. 2011;4(4):409–419.
- Rajic J, Inic-Kanada A, Stein E, et al. Chlamydia trachomatis infection is associated with E-cadherin promoter methylation, downregulation of E-cadherin expression, and increased expression of fibronectin and alpha-SMA-implications for epithelial-mesenchymal transition. Front. 2017;7:253.
- Gomes AV, de Souza Morais SM, Menezes-Filho SL, et al. Demethylation profile of the TNF-alpha promoter gene is associated with high expression of this cytokine in Dengue virus patients. J Med Virol. 2016;88(8):1297–1302.
- Qi L, ZQ Z, Wang LY, et al. Methylation of the glutathione-S-transferase M3 gene promoter is associated with oxidative stress in acute-on-chronic hepatitis B liver failure. Tohoku J Exp Med. 2012;228(1):43–51.
- DiNardo AR, Nishiguchi T, Mace EM, et al. Schistosomiasis induces persistent DNA methylation and tuberculosis-specific immune changes. J Immunol. 2018;201(1):124–133.
- Gornalusse GG, Mummidi S, Gaitan AA, et al. Epigenetic mechanisms, T-cell activation, and CCR5 genetics interact to regulate T-cell expression of CCR5, the major HIV-1 coreceptor. Proc Natl Acad Sci U S A. 2015;112(34):E4762–71.
- Rosca A, Anton G, Ene L, et al. Immunoassay and molecular methods to investigate DNA methylation changes in peripheral blood mononuclear cells in HIV infected patients on cART. J Immunoassay Immunochem. 2017;38(3):299–307.
- Esterhuyse MM, Weiner J, Caron E, et al. Epigenetics and proteomics join transcriptomics in the quest for tuberculosis biomarkers. mBio. 2015;6(5):e01187–15.
- Ip M, Zheng L, Leung ET, et al. Human epigenetic alterations in Mycobacterium tuberculosis infection: a novel platform to eavesdrop interactions between M tuberculosis and host immunity. Hong Kong Med. 2015;21(Suppl 7):S31–5.
- Zheng L, Leung ETY, Wong HK, et al. Unraveling methylation changes of host macrophages in Mycobacterium tuberculosis infection. Tuberculosis. 2016;98:139–148.
- Cheng Q, Zhao B, Huang Z, et al. Epigenome-wide study for the offspring exposed to maternal HBV infection during pregnancy, a pilot study. Gene. 2018;658:76–85.
- Konwar C, Price EM, Robinson WP. Using DNA methylation signatures in placental tissue and cells to gain insight into chorioamnionitis. Placenta. 2016;45:113.
- Tomlinson MS, Bommarito PA, Martin EM, et al. Microorganisms in the human placenta are associated with altered CpG methylation of immune and inflammation-related genes. PLoS ONE. 2017;12(12):e0188664.
- Tendl KA, Schulz SM, Mechtler TP, et al. DNA methylation pattern of CALCA in preterm neonates with bacterial sepsis as a putative epigenetic biomarker. Epigenetics. 2013;8(12):1261–1267.
- Dhas BB, Antony HA, Bhat V, et al. Global DNA methylation in neonatal sepsis. Indian J Pediatr. 2015;82(4):340–344.
- Tolg C, Kapila A, Cortese R, et al. Uropathogenic E. coli infection provokes epigenetic downregulation of CDKN2A (P16INK4A) in uroepithelial cells. J Urol. 2011;1:e546.
- Marioni RE, Shah S, McRae AF, et al. The epigenetic clock is correlated with physical and cognitive fitness in the Lothian Birth Cohort 1936. Int J Epidemiol. 2015;44(4):1388–1396.
- Marioni RE, Shah S, McRae AF, et al. DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol. 2015;16:25.
- Gross AM, Jaeger PA, Kreisberg JF, et al. Methylome-wide analysis of chronic HIV infection reveals five-year increase in biological age and epigenetic targeting of HLA. Mol Cell. 2016;62(2):157–168.
- Horvath S, Levine AJ. HIV-1 infection accelerates age according to the epigenetic clock. J Infect Dis. 2015;212(10):1563–1573.
- Kananen L, Nevalainen T, Jylhava J, et al. Cytomegalovirus infection accelerates epigenetic aging. Exp Gerontol. 2015;72:227–229.
- Kottilil S, Gindin Y, Emmanuel B, et al. DNA methylation and immune cell markers demonstrate associations of accelerated aging with chronic HCV/HIV co-infection and liver fibrosis. J Hepatol. 2018;68(Supplement 1):S309–S10.
- Kumar H, Lund R, Laiho A, et al. Gut microbiota as an epigenetic regulator: pilot study based on whole-genome methylation analysis. mBio. 2014;5(6):16.
- Kusel M, Holt PG. Do early-life viral infections cause asthma? J Allergy Clin Immunol. 2010;125(6):1202–1205.
- Pech M, Weckmann M, Konig IR, et al. Rhinovirus infections change DNA methylation and mRNA expression in children with asthma. PLoS One. 2018;13(11):e0205275.
- Stricker SH, Koferle A, Beck S. From profiles to function in epigenomics. Nat Rev Genet. 2017;18(1):51–66.