ABSTRACT
The outbreak of vaping-related severe lung injuries and deaths and the epidemic of teen vaping in the U.S. underscore the urgent need for determining the biological consequences of electronic cigarette (e-cig) use. We have investigated the association between vaping and epigenetic changes by quantifying DNA methylation levels in Long Interspersed Nucleotide Element 1 (LINE-1) and global DNA hydroxymethylation (5-hmC) levels and measuring the expression level of enzymes catalysing the respective processes in peripheral blood of exclusive vapers, smokers, and controls, matched for age, gender, and race (n = 45). Both vapers and smokers showed significant loss of methylation in LINE-1 repeat elements in comparison to controls (P = 0.00854 and P = 0.03078, respectively). Similarly, vapers and smokers had significant reductions in 5-hmC levels relative to controls (P = 0.04884 and P = 0.0035, respectively). Neither the LINE-1 methylation levels nor the global 5-hmC levels were different between vapers and smokers. There was a direct correlation between methylation levels in the LINE-1 elements and global 5-hmC levels in the study subjects (r = 0.31696, P = 0.03389). Inverse and statistically significant correlations were found between both the LINE-1 methylation levels and the global 5-hmC levels and various vaping/smoking metrics in the study subjects. There were modest but not statistically significant changes in transcription of DNA methyltransferases and ten-eleven translocation enzymes in both vapers and smokers relative to controls. Our findings support follow-up genome-wide investigations into the epigenetic effects of vaping, which may further clarify the health consequences of e-cig use.
Abbreviations
5-mC: 5-methylcytosine; 5-hmC: 5-hydroxymethylcytosine; 8-OHdG: 8-hydroxy-2ʹ-deoxyguanosine; ACTIN: actin beta; ANOVA: Analysis of Variance; BER: base excision repair; BMI: body mass index; CO: carbon monoxide; COHb: carboxyhaemoglobin; COBRA: combined bisulphite restriction analysis; COPD: chronic obstructive pulmonary disease; DNMT1: DNA methyltransferase 1; DNMT3A: DNA methyltransferase 3A; DNMT3B: DNA methyltransferase 3B; e-cigs: electronic cigarettes; ELISA: enzyme-linked immunosorbent assay; ENDS: electronic nicotine delivery systems; FDA: Food and Drug Administration; GAPDH; glyceraldehyde-3-phosphate dehydrogenase; HPLC: high-performance liquid chromatography; LINE-1: Long Interspersed Nucleotide Element 1; PBS: phosphate-buffered saline; RFU: relative fluorescence units; RT-qPCR: quantitative reverse-transcription polymerase chain reaction; ROS: reactive oxygen species; SAM, S-adenosylmethionine; SE: standard error; TET1: ten-eleven translocation 1; TET2: ten-eleven translocation 2; TET3: ten-eleven translocation 3.
Introduction
Electronic cigarettes (e-cigs) – otherwise known as vapes, vape pens, pod mods, JUUL, or electronic nicotine delivery systems (ENDS) – are handheld battery-powered vaporizing devices that simulate tobacco smoking [Citation1]. E-cigs heat a liquid to produce an aerosol (vapour) that users inhale into their lungs [Citation2]. The liquid, also referred to as ‘e-liquid/e-juice’, contains a mixture of propylene glycol, glycerine, flavours, and other additives, and nicotine at variable concentrations (incl. zero) [Citation3]. E-cig use is commonly referred to as ‘vaping’, and e-cig users are interchangeably called ‘vapers’ [Citation4]. Vaping replicates some of the behavioural aspects of cigarette smoking, including the hand-to-mouth action, but without burning tobacco, which is proven to produce a myriad of toxicants and carcinogens [Citation1–Citation3]. Because vapour generation by e-cigs does not involve combustion of tobacco, vaping is claimed to be, at best, a safe, and at worst, a less-harmful alternative to smoking [Citation3,Citation4]. However, chemical analyses of e-cig liquid and vapour have shown the presence of many of the same toxicants and carcinogens as those found in tobacco smoke, albeit in generally lower concentrations [Citation5]. Currently, investigating the biological consequences of exposure to e-cig-derived toxicants and carcinogens is a high priority research area [Citation4].
Recently, we have demonstrated that vapers, similarly to smokers, exhibit differential expression of genes in the oral epithelium, a major target organ for smoking-associated cancer [Citation6]. The cancer-causing effects of many carcinogens present in both e-cig vapour and cigarette smoke are ascribed to their ability to induce genetic and/or epigenetic alterations [Citation7,Citation8]. Global loss of DNA methylation involving reactivation of latent retrotransposons leading to genomic instability is an epigenetic hallmark of human cancer [Citation9,Citation10]. More recently, DNA hydroxymethylation has also emerged as an important mechanism of gene deregulation in human carcinogenesis [Citation11,Citation12]. Building on our recent findings [Citation6], we have now investigated the association between vaping and these key epigenetic effects in a well-defined population of exclusive e-cig users, smokers only, and control non-vapers non-smokers, matched for age, gender, and approximately race (n = 15, each group). We have quantified DNA methylation levels in Long Interspersed Nucleotide Element 1 (LINE-1) repeats, as an indicator of global 5-methylcytosine (5-mC) content of the DNA [Citation10,Citation13], and measured global levels of 5-hydroxymethycytosine (5-hmC), which is the oxidation product of 5-mC [Citation11,Citation12], in leucocytes DNA from the study population, using enzyme-linked immunosorbent assays (ELISA). We have also quantitated the expression level of enzymes catalysing DNA methylation, including DNA methyltransferases (DNMT1, DNMT3A, and DNMT3B), and hydroxymethylation, including the ten-eleven translocation (TET) family of methylcytosine dioxygenases (TET1, TET2, and TET3) [Citation14], in total RNA isolated from the respective samples, using reverse-transcription quantitative polymerase chain reaction (RT-qPCR) analysis. Furthermore, we have verified the vaping/smoking status of the study population by measuring the concentrations of plasma cotinine, a prime metabolite of nicotine [Citation15], by ELISA, and the exhaled carbon monoxide (CO) and carboxyhaemoglobin (COHb) levels by a breath CO monitor.
Materials and methods
Subject recruitment and enrolment
The study was approved by the Health Sciences Institutional Review Board of the University of Southern California (HSIRB-USC), under the protocol number HS-16-00175. The study was advertised in online forums, including Craigslist, Reddit, and myUSC (http://my.usc.edu), and through social media (Twitter, Instagram, and Facebook). Also, flyers and leaflets were used to advertise the study in local colleges, universities, and vape shops. Moreover, an online survey was developed, validated, and subsequently employed to solicit and query potential participants (http://geteo.usc.edu). Individuals who appeared to have met the study criteria were contacted by phone to complete a screening questionnaire. Based on the information obtained during the phone screen, those who were deemed potentially eligible were scheduled for an in-person visit to our laboratory. During the visit, an expanded version of the phone screen was administered to reconfirm eligibility, and informed consent was obtained, afterwards (see, below).
Personal interview
Upon reconfirmation of the eligibility and obtaining informed consent, all participants underwent a personal interview to provide detailed information about demographics, socio-economic status, use of e-cigs, cigarettes, or other tobacco products, dietary habits, lifestyle, specifically, use of recreational or illicit drugs, alcohol, and prescription- or over-the-counter medicine, occupational and residential history, and family history of disease.
Study population
Eligible candidates for the study included healthy adults – both males and females of diverse ages, races, and ethnicities – who could read and write in English and understand and give informed consent. The catchment area for this study was the Greater Los Angeles Area. The study population consisted of 45 subjects divided equally into three groups (i.e., 15 subjects per group), including Group 1: exclusive vapers; Group 2: cigarette smokers only; and Group 3: control non-smokers non-vapers. All groups were matched for age, gender, and approximately race (age range: Vapers: 22–43; Smokers: 23–46; Controls: 22–47, see, ). Detailed characteristics of the study population are listed in . Dual users of both e-cigs and conventional cigarettes or poly users of e-cigs, cigarettes, or other tobacco products were excluded from the study. Criteria for classification of the study subjects, as vapers, cigarette smokers, or controls, were as follows: vapers were those who reported current use of e-cigs for at least 3 times a week for a minimum of 6 months, and no use of conventional cigarettes or any other tobacco products in the past 6 months. Smokers were those who reported current smoking of tobacco cigarettes at least 3 times per week for a minimum of 1 year, and no use of any other tobacco products, including e-cigs, in the past 6 months. Controls were those who reported no use of any tobacco product (e-cigs or combustible) more than 5 times in their life (lifetime consumption: fewer than 100 cigarettes or less than 5 vaping sessions), with no use in the past 6 months. Unlike combustible cigarettes that have been in the market for many years, e-cigs are a relatively new tobacco products. Therefore, we set the minimum use criteria for vapers and smokers to 6 months and 1 year, respectively, to be able to recruit a sufficient number of participants for this study.
Table 1. Characteristics of the study population.
Health indicators for exclusion from the study consisted of respiratory diseases (e.g., asthma or chronic obstructive pulmonary disease (COPD)), immune system disorders, diabetes, kidney diseases, body mass index <18 kg/m2 or >40 kg/m2, oral infection or inflammation, gum disease, dental decay, or any medical disorder/medication that could affect subject’s safety or study results. Any unstable or significant medical condition in the past 12 months, including but not limited to symptomatic heart conditions, stroke, severe angina, and hypertension was ground for exclusion. Being pregnant or having a baby in the past 12 months was also exclusionary. Other exclusion criteria included uncontrolled mental illness or substance abuse or inpatient treatment for those conditions in the past 12 months, use of recreational or illicit drugs (e.g., marijuana, heroin, etc.) in the past 6 months, and use of any medication known to induce/inhibit CYP450 2A6 enzyme. Physical examination and health assessment of all the study subjects were performed by highly trained staff during the personal visits and interviews.
Sampling and processing of peripheral blood
Peripheral blood (30 ml) was drawn from the study subjects by venipuncture. Plasma fraction was first collected by centrifugation and afterwards leukocytes and erythrocytes were separated using the LeucosepTM tubes according to the manufacturer’s instructions (Greiner Bio-One Inc., Monroe, NC). The collected plasma, leukocytes, and erythrocytes fractions were aliquoted into multiple microtubes (Eppendorf, Inc., San Diego, CA), snap-frozen, and preserved at −80°C until further analysis. An aliquot of the thawed leukocyte samples from each subject was used for genomic DNA isolation using a standard protocol published previously [Citation16]. Another aliquot was used for total RNA isolation using the RNeasy Mini Kit (Qiagen, Valencia, CA).
Quantification of global DNA methylation by ELISA
Global 5-methylcytosine (5-mC) content of leukocyte DNA was measured using the Global DNA Methylation LINE-1 kit according to the manufacturer’s instructions (Active Motif, Carlsbad, CA). Briefly, 1 µg of genomic DNA from each sample and standards (containing known quantities of 5-mC) was incubated with MseI at 37°C for 4 h, followed by heat inactivation at 65°C for 20 min. The MseI-digested DNA was hybridized to a LINE-1 Probe by incubating in a thermal cycler at 98°C for 10 min, at 68°C for 1 h, and a quick ramp down to 25°C. Following hybridization, 20 ng of samples and 100 ng of standard controls were loaded in triplicate onto a 96-well streptavidin-coated plate, and incubation was performed at room temperature for 1 h on a shaker set at mild agitation (~100 rpm). The unbound DNA was then removed from the wells by multiple washes with the provided 1X Wash Buffer (200 µl per wash). Subsequently, a blocking buffer was added to each well (200 µl), and incubation was performed at room temperature for 30 min on the shaker. After removing the blocking buffer by tapping the inverted plate on absorbent paper towels, a primary antibody for 5-mC (1:100 dilution) was added (100 µl per well) with 1-h incubation at room temperature on the shaker. The primary antibody has no or negligible cross-reactivity to unmethylated cytosine or hydroxymethylated DNA (5-hmC) (Technical Note #55017, Active Motif). The plate was rinsed three times with the 1X Wash Buffer and afterwards a horseradish peroxidase-conjugated secondary antibody (1:25 dilution) was added (100 µl per well) and incubation was done for 1 h at room temperature on the shaker. After washing the wells three times with the 1X Wash Buffer, a colorimetric reaction was initiated by adding the Developing Solution provided (100 µl per well). The reaction was terminated by adding a stop solution (100 µl per well) when the colour of the highest concentration DNA standards turned into a medium blue. Absorbance was read at 450 nm, with a reference wavelength of 655 nm, using a SpectraMax® i3x Multi-Mode Microplate Reader (Molecular Devices, San Jose, CA). Results are expressed as % 5-mC measured relative to total cytosine content of the LINE-1 elements.
Quantitation of global DNA hydroxymethylation by ELISA
Global 5-hmC content of genomic DNA isolated from leukocytes was determined using the MethylFlash Hydroxymethylated DNA Quantification Kit (Fluorometric) according to the instructions of the manufacturer (Epigentek, Farmingdale, NY). Briefly, 500 ng of samples and standards (containing known amounts of 5-hmC) were loaded in duplicate onto a multi-well plate with 8-well assay strips, pre-filled with a binding solution (80 µl per well). The strips were then incubated at 37°C for 90 min on a shaker set at mild agitation (~100 rpm). Unbound DNA was washed off by rinsing wells three times with the provided 1X Wash Buffer (150 µl per well). A primary antibody recognizing 5-hmC (1:1,000 dilution) was added (50 µl per well) and the plate was incubated at room temperature for 1 h on the shaker. The primary antibody has no or negligible cross-reactivity to unmethylated cytosine or methylcytosine (5-mC); it detects hydroxymethylated DNA (5-hmC) with high specificity (Technical Note #P-1037, Epigentek). The wells were then rinsed three times with 1X Wash Buffer. Subsequently, a secondary antibody (1:2,000 dilution) was added (50 µl per well), and the plate was incubated at room temperature for 30 min on the shaker. Following four rinses of all the wells with 1X Wash Buffer, an enhancer solution was added (50 µl per well), and the plate was incubated at room temperature for 30 min on the shaker. After removing the enhancer solution from the wells, the plate was rinsed five times with 1X Wash Buffer (150 µl per well) and once with 1X phosphate-buffered saline (PBS) (150 µl per well). Finally, a fluorescent development solution was added (50 µl per well) and incubation was performed for 4 min at room temperature, after which the relative fluorescence units (RFU) were read using a SpectraMax® i3x Multi-Mode Microplate Reader (Molecular Devices) at 530EX/590EM nm. Results are expressed as % 5-hmC measured in total DNA.
Expression analysis of DNMT and TET enzymes by RT-qPCR
Total RNA (250 ng) isolated from leukocytes was reverse transcribed into cDNA using the iScript™ Reverse-Transcription Supermix for RT-qPCR (iScript RT Supermix) (Bio-Rad laboratories, Inc., Hercules, CA). The synthesized cDNA was diluted 2:5 with low TE buffer (10 mmol/L Tris-HCl, 0.1 mmol/L EDTA, pH 8.0), of which 2 µl was used per reaction in a mastermix containing gene-specific primers and SsoAdvanced™ Universal SYBR® Green Supermix (Bio-Rad laboratories, Inc.). The human actin beta (ACTIN) gene and the human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene were used as references. All PCR assays were performed using the CFX96 Touch™ Real-Time PCR detection system (Bio-Rad Laboratories, Inc.). The cycling conditions included a pre-incubation at 95°C for 2 min, followed by 40 cycles at 95°C for 5 s, and 58°C for 30 s. Fifteen samples per biological group (i.e., vapers, smokers, and controls) were run in triplicate for a total of 135 reactions for each gene of interest. Transcript levels within each sample were calculated using the Bio-Rad CFX Maestro™ software (BioRad Laboratories, Inc.). The primer sets used for RT-qPCR are listed in .
Table 2. Primers used for RT-qPCR gene expression analysis.
Plasma cotinine measurement by ELISA
Plasma cotinine was measured by a solid-phase competitive ELISA kit according to the instructions of the manufacturer (Abnova Corp., Walnut, CA). Briefly, aliquots of standard controls and plasma samples from the study subjects were loaded in triplicate (10 µl each) onto a 96-microwell plate pre-coated with a polyclonal antibody raised against cotinine. After adding a cotinine horseradish peroxidase enzyme (100 µl per well), the microplate was incubated for 1 h at room temperature in the dark. Unbound cotinine and cotinine enzyme-conjugate were washed off by rinsing the wells six times with distilled water (300 µl each wash). A chromogenic substrate (3,3ʹ,5,5ʹ-Tetramethylbenzidine) was added (100 µl per well), and the plate was incubated for 30 min at room temperature. The reaction was terminated by adding a stop solution (100 µl per well), and absorbance was read at 450 nm using an iMarkTM Mircroplate Absorbance Reader (BioRad Laboratories, Inc.). Results are expressed as nanograms (ng) of cotinine measured per millilitre of plasma.
Measurement of exhaled CO and COHb by breath CO monitor
Exhaled CO levels and %COHb were measured using the Bedfont Micro+TM Smokerlyzer® according to the manufacturer’s instructions (Bedfont Scientific Ltd., Harrietsham, UK). Briefly, study participants were instructed to inhale and hold their breath for 15 s. Following the completion of the 15-s countdown, participants blew slowly into the device mouthpiece aiming to empty their lungs completely. The CO levels (ppm) and equivalent %COHb were recorded by the device and shown on the touchscreen display.
Statistical analysis
Normal distribution of data was examined both visually and by the Shapiro–Wilk test. Given the small sample size and non-normal distribution of the data, non-parametric tests were used throughout. In all figures presented and all analyses performed, data distribution was visually displayed by a combination of scatter plots (to show individual values) and box and whisker plots (to highlight the minimum, first quartile, median, third quartile, and maximum values as well as the means and outlier(s) (if any)). Results are expressed as mean ± SE in the text. Comparisons of all variables between two groups were performed by the Wilcoxon Rank-Sum test. Relationships between different variables were examined by the Spearman Rank correlation analysis. All statistical tests were two-sided. P values <0.05 were considered statistically significant. All statistical analyses were performed using the R environment for statistical computing, available at RStudio (https://rstudio.com/), which is a free and open source software.
Results
Quantification of global DNA methylation and hydroxymethylation
We quantified DNA methylation levels in LINE-1 repeat elements, as a measure of global 5-methylcytosine (5-mC) content of the DNA, in peripheral blood leukocytes from vapers and smokers as compared to control non-vapers non-smokers . As shown in ), both vapers and smokers showed significant loss of methylation in LINE-1 repeat elements in comparison to controls (P = 0.00854 and P = 0.03078, respectively). Specifically, the methylation levels of LINE-1 elements in vapers and smokers were decreased ~18% and 13%, respectively, relative to controls. The methylation levels of LINE-1 repeats were not significantly different between vapers and smokers (P = 0.80258).
Figure 1. Quantification of global DNA methylation and hydroxymethylation. DNA methylation levels in LINE-1 repeat elements, as a measure of global 5-mC, and global DNA hydroxymethylation (5-hmC) levels were determined in vapers, smokers, and controls by ELISA, as described in the text. All samples were assayed in triplicate (for 5-mC) and in duplicate (for 5-hmC). Distribution of data within each group is shown by a combination of scatter plots (to display individual values) and box and whisker plots (to highlight the minimum, first quartile, median, third quartile, and maximum values as well as the means and outlier(s) (if any)). In the scatter plots, identical values are overlaid and presented as a single circle (‘°’). In the box and whisker plots, the ‘lower’ and ‘upper’ edges of boxes represent the 1st and 3rd quartiles, respectively (25 and 75 percentiles, resp.). Horizontal lines within the boxes represent the medians (2nd quartile or 50 percentile) and small crosses (‘x’) indicate the mean values. The ‘lower’ and ‘upper’ vertical lines extending from the boxes, also known as the ‘whiskers’, represent the lowest and highest data points, respectively, excluding any outliers (minimum and maximum values, resp.). Comparison between groups was done by the Wilcoxon Rank-Sum test. Panels A and B show the quantification results for 5-mC and 5-hmC, respectively.
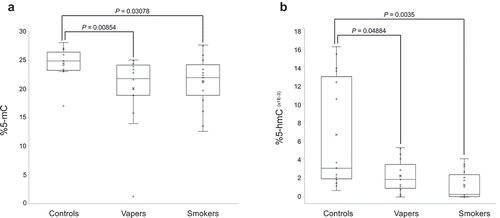
Furthermore, we measured global DNA hydroxymethylation (5-hmC) levels in leucocytes DNA from the study population. As shown in ), both vapers and smokers had significant reductions in 5-hmC levels relative to controls (P = 0.04884 and P = 0.0035, respectively). The levels of 5-hmC in vapers and smokers were reduced ~66% and 81%, respectively, relative to controls. The levels of 5-hmC did not significantly differ between vapers and smokers (P = 0.101). Moreover, there was a positive and statistically significant correlation between methylation levels of LINE-1 elements and global 5-hmC levels in the study subjects (r = 0.31696, P = 0.03389). Altogether, our data demonstrate statistically significant losses of DNA methylation and hydroxymethylation in both vapers and smokers in comparison to controls. The extent of reductions in global 5-mC and 5-hmC levels in vapers is comparable to that in smokers.
Expression analysis of enzymes catalysing DNA methylation and hydroxymethylation
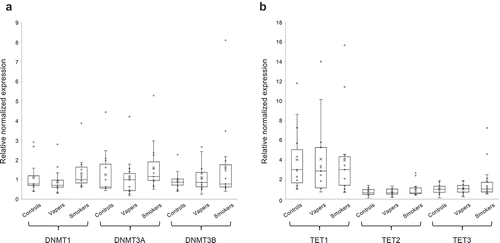
We quantitated the expression level of DNA methyltransferases (DNMT1, DNMT3A and DNMT3B) and the TET family of methylcytosine dioxygenases (TET1, TET2, and TET3), catalysing DNA methylation and hydroxymethylation, respectively [Citation14], in total RNA isolated from peripheral blood leukocytes of the study population. As shown in ), there were modest changes in expression levels of both the maintenance DNA methyltransferase (DNMT1) and de novo DNA methyltransferases (DNMT3A and DNMT3B) in vapers and smokers in comparison to controls. However, the changes in expression levels of DNMTs in vapers and smokers relative to controls did not reach a statistically significant level. Moreover, the expression levels of DNMTs were not statistically significantly different between vapers and smokers. Likewise, small but not statistically significant changes were found in transcript levels of TET1, TET2, and TET3 enzymes in both vapers and smokers as compared to controls ()). The expression levels of none of the TET enzymes were statistically significantly different between vapers and smokers. Altogether, our data show modest but not statistically significant changes in the transcription of DNMTs and TETs in both vapers and smokers as compared to controls. The observed changes in expression levels of these enzymes in vapers and smokers relative to controls are not statistically significantly different from one another.
Figure 2. Expression analysis of DNMTs and TETs. Expression level of DNMTs (DNMT1, DNMT3A, and DNMT3B) and TETs (TET1, TET2, and TET3), catalysing DNA methylation and hydroxymethylation, respectively, were measured in vapers, smokers, and controls by RT-qPCR, as described in the text. All samples were assayed in triplicate. Distribution of data within each group is shown by a combination of scatter plots and box and whisker plots, as described in the legend for . Panels A and B show the results of expression analysis for DNMTs and TETs, respectively. The modest changes in the expression level of DNMTs or TETs observed in vapers and smokers relative to controls or relative to each other did not reach a statistically significant level (P < 0.05), as determined by the Wilcoxon Rank-Sum test.
Verification of the vaping/smoking status
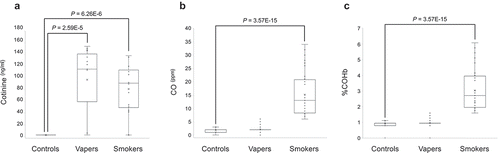
To verify the vaping/smoking status of our study population, we measured the concentrations of plasma cotinine, a major nicotine metabolite [Citation15], and the exhaled breath CO and COHb levels in the study subjects. As shown in ), plasma cotinine levels in both vapers and smokers were significantly higher than those in controls (vapers: 97.0 ± 15.5 ng/ml, smokers: 81.04 ± 11.0 ng/ml, controls: 2.5 ± 0.007 ng/ml, P = 2.59E-5 and P = 6.26E-6, respectively). The levels of plasma cotinine in vapers and smokers were not significantly different from one another (P = 0.1183).
Figure 3. Measurements of plasma cotinine, exhaled CO and COHb. Concentrations of plasma cotinine were measured by ELISA, and the exhaled CO and COHb levels were determined by a breath CO monitor, as described in the text. Distribution of data within each group is shown by a combination of scatter plots and box and whisker plots, as described in the legend for . Comparison between groups was done by the Wilcoxon Rank-Sum test. Panel A shows the results of cotinine measurement, whereas panels B and C display the quantification data for CO and COHb, respectively.
To validate the vaping/smoking history data obtained from the study participants during the screening and in-person interviews, we sought correlations between plasma cotinine levels and vaping/smoking metrics, expressed as ‘cumulative e-liquid’ and ‘cumulative e-nicotine’ for vapers and ‘pack year’ for smokers. The cumulative e-liquid and cumulative e-nicotine, respectively, were calculated as the total volume of e-liquid (in millilitre) and the total amount of nicotine present in the e-liquid (in milligrams) used by a vaper during his/her lifetime. Pack year was calculated by multiplying the number of packs of cigarette a person smoked per day by the number of years he/she smoked. We observed a positive and statistically significant correlation between plasma cotinine levels and cumulative e-liquid levels reported by the study subjects (r = 0.81315, P = 8.29E-8). Likewise, a direct and statistically significant correlation was observed between plasma cotinine levels and the reported cumulative e-nicotine levels (r = 0.73362, P = 5.95E-6). Similarly, there was a positive and statistically significant correlation between plasma cotinine levels and the reported pack years by the study participants (r = 0.81596, P = 3.90E-8). As shown in , the cumulative e-liquid/e-nicotine levels and pack-years, which represent a combination of duration and intensity of vaping and smoking, respectively, were inversely correlated to both LINE-1 methylation and global DNA hydroxymethylation levels in the study subjects. Similarly, inverse correlations were found between plasma cotinine levels and both the methylation levels of LINE-1 elements and the global DNA hydroxymethylation levels in the study participants (see, ).
Table 3. Relationship of vaping/smoking indices and effect variables.
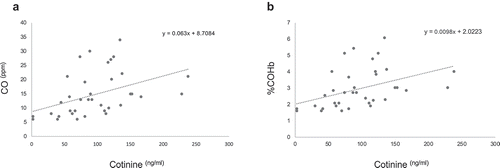
To further verify the vaping/smoking status, we quantified the exhaled breath CO levels and %COHb in our larger source population (n = 121) from which the study subjects were drawn. The source population consisted of 39 exclusive vapers, 38 cigarette smokers only, and 44 control non-vapers non-smokers. Measurement of CO levels in exhaled breath, which is an objective biomarker of recent exposure to tobacco smoke [Citation17], revealed that smokers had significantly higher levels of breath CO as compared to controls (15.16 ± 1.33 vs. 1.80 ± 0.11 ng/ml, P = 3.57E-15) ()). Conversely, vapers had almost similar levels of breath CO to those of controls (2.18 ± 0.19 vs. 1.80 ± 0.11 ng/ml, P = 2.183). The estimated percentage of COHb, which is indicative of the proportion of red blood cells carrying CO instead of oxygen [Citation17], was significantly higher in smokers than controls (3.03 ± 0.21 vs. 0.90 ± 0.03, P = 3.57E-15) ()). By contrast, vapers and controls had approximately similar percentages of COHb (0.96 ± 0.04 vs. 0.90 ± 0.03, P = 2.183). Of importance, the levels of exhaled breath CO and %COHb in smokers were directly and statistically significantly correlated to their plasma cotinine levels (see, and ). Altogether, our measurements of plasma cotinine and breath CO levels, and determination of %COHb are highly consistent with vaping/smoking status of the study subjects as reported in their questionnaires and personal interviews.
Figure 4. Correlation analysis of plasma cotinine and breath CO and COHb. The concentrations of plasma cotinine in smokers were directly and statistically significantly correlated to their exhaled breath CO (panel A) and %COHb (panel B) (r = 039,154, P = 9.78E-6 and r = 039,153, P = 9.78E-6, respectively; Spearman Rank correlation test).
Discussion
In the present study, both vapers and smokers showed significant loss of DNA methylation in LINE-1 repeat elements in comparison to controls (P = 0.00854 and P = 0.03078, respectively). The methylation levels of LINE-1 repeats were not significantly different between vapers and smokers (P = 0.80258). Because repetitive DNA elements comprise almost 50% of the human genome and account for more than one-third of genome-wide DNA methylation [Citation18], it is largely thought that the global loss of methylation that is observed in cancer is primarily due to hypomethylation at repetitive elements [Citation10]. LINE-1 repeats represent approximately 17–18% of the human genome, with a copy number estimated at roughly half a million [Citation19]. Recent studies have shown that analysis of methylation status in high copy number repeat elements, such as LINE-1, can serve as a surrogate marker for global genomic DNA methylation [Citation20,Citation21]. Comparisons of the methylation status of LINE-1 repeat elements show strong correlation with global 5-mC levels measured by high-performance liquid chromatography (HPLC), combined bisulphite restriction analysis (COBRA), and pyrosequencing [Citation20,Citation21].
Our observation that vapers have significant loss of methylation in LINE-1 repeat elements is novel and has important implications. Additionally, the finding that vapers and smokers have similar reductions in LINE-1 methylation levels is consistent with previous studies by others who have shown significantly reduced levels of LINE-1 methylation in smokers [Citation22], environmentally or occupationally exposed individuals to carcinogens [Citation23–Citation27], as well as in cells treated in vitro with cigarette smoke condensate [Citation28] or select tobacco smoke carcinogens [Citation29]. Together, the results of those studies and ours demonstrate the utility of LINE-1 hypomethylation as an informative biomarker of exposure as well as effect for known or suspected carcinogens.
There are several scenarios that may have, individually or combined, contributed to the reduced LINE-1 methylation levels in vapers and smokers alike. First, carcinogens in both e-cig vapour and cigarette smoke are known to induce a wide variety of DNA lesions [Citation1,Citation7], many of which can interfere with the binding of methyltransferases (DNMTs) to DNA, leading to genomic DNA hypomethylation [Citation30]. In addition, certain constituents of both e-cig vapour and cigarette smoke, such as heavy metals, e.g., cadmium and nickel, are known inhibitors of DNMTs activity [Citation31,Citation32]. Mammalian DNMTs contain several zinc-binding sites that appear to play a key role in regulating their function, and zinc can often be replaced by cadmium in biomolecules [Citation33]. Thus, the inhaled cadmium by vapers and smokers may potentially replace zinc-binding sites, thereby inhibiting the activity and function of DNMTs, and ultimately leading to the global loss of DNA methylation. Takiguchi et al. [Citation32] have reported that cadmium exposure of rat liver epithelial cells (TRL 1215) inhibits DNMTs activity in a concentration-dependent fashion, and, at higher doses, induces DNA hypomethylation. Of significance, lower, comparable, and in some instances, elevated levels of toxic metals (e.g., cadmium, nickel, chromium, lead, arsenic, etc.) have been found in e-cig vapour and cigarette smoke [Citation34]. Furthermore, the methyl donor, S-adenosylmethionine (SAM), is required both for DNA methylation and metabolism of chemicals, such as arsenic that is present in both e-cig liquid and vapour as well as in cigarette smoke [Citation34]. Competitive demand between metabolism of specific metals (e.g., arsenic) and DNA methylation for SAM in vapers and smokers might lead to a reduction of DNA methylation throughout the genome.
Moreover, active loss of DNA methylation may arise from impaired function of DNA repair machinery consequent to exposure to e-cig vapour or cigarette smoke [Citation2,Citation3,Citation11]. Many constituents of both e-cig vapour and cigarette smoke are known to generate reactive oxygen species (ROS) [Citation1,Citation2], which may promote DNA hypomethylation through various mechanisms [Citation30]. The ROS-induced oxidized DNA lesions, such as 8-hydroxy-2ʹ-deoxyguanosine (8-OHdG) in CpG dinucleotides, have been shown to strongly inhibit the methylation of adjacent cytosine residues [Citation35]. Also, an unrepaired 8-OHdG lesion is mis-instructional during DNA replication, thereby giving rise primarily to G to T transversion mutation, resulting in a net loss of CpG dinucleotides [Citation36]. Recently, Furlan et al. [Citation37] have demonstrated that the accumulation of oxidative DNA damage in a compromised base excision repair (BER) model of colorectal cancer is associated with significant demethylation of LINE-1 elements. Furthermore, under oxidative stress and elevated ROS burden with decreased availability of SAM, depletion of the methyl pool in a folate-deficient rat model has been shown to cause DNA hypomethylation [Citation38].
Next, we observed that vapers and smokers had significant reductions in 5-hmC levels relative to controls (P = 0.04884 and P = 0.0035, respectively). The levels of 5-hmC did not significantly differ between vapers and smokers (P = 0.101). We also observed inverse correlations between both the global 5-hmC levels and LINE-1 methylation levels and various vaping/smoking metrics, representing a combination of duration and intensity of vaping/smoking, in the study subjects (see, ). Moreover, we found a positive and statistically significant correlation between global DNA hydroxymethylation levels and DNA methylation levels in LINE-1 repeat elements in the study participants (r = 0.31696, P = 0.03389). Together, these results demonstrate, for the first time, that vapers, similarly to smokers, have significantly reduced levels of global DNA hydroxymethylation and DNA hypomethylation in LINE-1 elements. Previous studies by others have reported a significant decrease in 5-hmC levels concomitant with loss of function mutations of TETs in various types of human cancer [Citation11,Citation12,Citation14,Citation39]. More recently, it has also been shown that loss of 5-hmC may serve as a poor prognostic marker in gastric cancer, hepatocellular carcinoma, breast cancer, laryngeal squamous cell carcinoma (LSCC), and melanoma [Citation11,Citation12,Citation14,Citation39,Citation40]. Zhang et al. [Citation40] have also reported that global 5-hmC levels are negatively associated with smoking in LSCC patients (P = 0.039).
The direct and statistically significant correlation between global DNA hydroxymethylation (5-hmC) and LINE-1 DNA methylation levels (as a proxy for global 5-mC) found in our study is also in agreement with a recent study by Gosh et al. [Citation41] who have shown a positive correlation between global 5-hmC and 5-mC levels in a cohort of occupationally exposed individuals to multi-wall carbon nanotubes and unexposed controls (n = 67). Likewise, Tellez-Plaza et al. [Citation42] have demonstrated a positive correlation between global DNA methylation and global DNA hydroxymethylation in human blood samples collected in the same individuals at two time points (7–10 years apart) in 48 Strong Heart Study participants. The authors confirmed their findings in an independent population of 48 healthy men from Spain, supporting consistency in the direction of the association in two distinct human populations with different risk profiles [Citation42].
We note that measurement of 5-hmC and 5-mC in blood, as performed in this study, may differ from those in cells or tissues directly exposed to cigarette smoke or e-cig vapour or other ROS-generating chemicals/carcinogens. For example, increased levels of global 5-hmC concomitant with decreased global 5-mC levels have been observed in directly exposed cells to environmental carcinogens [Citation43–Citation45]. Coulter et al. [Citation43] have reported elevated levels of global 5-hmC together with reduced global 5-mC levels in HEK293 cells exposed in vitro to hydroquinone, a benzene metabolite and a major component of cigarette smoke. Li et al. [Citation44] have observed genome-wide DNA hypomethylation and increased global DNA hydroxymethylation in the lungs of mice exposed in vivo to an acute dose of particulate matter. Ringh et al. [Citation45] have performed genome-wide DNA methylation and hydroxymethylation analyses in bronchoalveolar lavage cells from smokers as compared to non-smokers. The authors showed that the majority of differentially methylated CpGs in smokers were hypomethylated, whereas almost all differentially hydroxymethylated regions were hyperhydroxymethylated. It is important to note that global 5-mC or 5-hmC levels may not necessarily reflect locus/gene-specific DNA methylation or hydroxymethylation, respectively. For instance, gene-specific DNA hypermethylation may co-exist with global DNA hypomethylation and vice versa. Currently, work in our laboratory is underway to initiate genome-wide DNA methylation and hydroxymethylation analyses in a large cohort of vapers and smokers.
In the mammalian genome, TET proteins are responsible for catalysing 5-mC oxidation to 5-hmC [Citation11,Citation12,Citation14]. In contrast to 5-mC, which is able to bind transcriptional repressors, 5-hmC can inhibit this binding, and therefore counteract the repressive effect of 5-mC [Citation11,Citation14]. While 5-mC is often associated with gene repression, particularly at gene promoters, 5-hmC facilitates transcription by contributing to an open chromatin state [Citation12,Citation14]. It is possible that DNA hydroxymethylation serves as a proxy for DNA methylation or vice versa (e.g., the more 5-mC, the greater potential for conversion to 5-hmC) [Citation11,Citation14]. However, demethylation of DNA also appears to be regulated by the oxidative state, where oxidative stress sequentially hydroxylates 5-mC to 5-hmC, followed by active continuous oxidation to 5-formylcytosine and 5-carboxylcytosine, which would eventually lead to the reduction of both 5-hmC and 5-mC levels [Citation12,Citation14]. Because both the DNA methylation and hydroxymethylation are regulated by redox reactions [Citation11,Citation12,Citation14], a scenario can be envisaged in which the elevated burden of oxidative stress imposed by vaping or smoking would impair both one-carbon transfer and citric acid metabolic pathways that are involved in the formation of 5-mC and 5-hmC, respectively [Citation12].
In the present study, we observed modest but not statistically significant changes in the expression level of DNMT and TET enzymes in both vapers and smokers relative to controls. There is evidence to suggest that the activity of epigenetic modifying enzymes, alone or in combination with their expression, is a good determinant of their function [Citation46]. Novakavic et al. [Citation47] have demonstrated global DNA hypomethylation in the absence of DNMT1 down-regulation in non-primate placentas and in vitro derived human cytotrophoblast stem cells, suggesting that DNMT1 down-regulation is not an absolute requirement for genomic hypomethylation in all instances. Xiao et al. [Citation48] have shown similar expression levels of DNMT3A and DNMT3B in nontumorous liver tissues of smokers and non-smokers; an earlier study by the same group found a less than twofold increase in DNMT1 expression levels in smokers as compared to non-smokers [Citation49]. However, neither study had accounted for age and gender as the relevant confounding factors [Citation48,Citation49]. Zhang et al. [Citation40] have found an association between decreased 5-hmC and reduced transcript levels of TET1, but not TET2 or TET3, in LSCC patients, albeit no confounders (e.g., age or gender) were taken into account during data analysis. Altogether, it appears that there is a ‘complex’ and not necessarily a ‘direct’ relationship between expression levels of DNMTs and TETs and DNA methylation and hydroxymethylation levels, respectively. Although our study design allowed to account for relevant confounding factors, future larger-size studies with higher statistical power would be needed to determine the impact of expression and enzymatic activity of DNMTs and TETs on DNA methylation and hydroxymethylation in vapers and smokers. Such large-scale studies with higher statistical power than ours should also allow to investigate dose–response relationships between vaping/smoking and epigenetic modifications.
Lastly, we verified the vaping/smoking status of all the study participants by demonstrating positive and statistically significant correlations between the measured plasma cotinine levels and cumulative e-liquid/e-nicotine levels reported by the vapers and the reported pack years by the smokers (). We further confirmed that smokers had significantly higher levels of breath CO and %COHb as compared to controls, whereas vapers and controls had comparable levels of CO and %COHb (). These data validate our strict inclusion & exclusion criteria for this study. The criteria were specifically chosen to avoid subject misclassification as we enrolled exclusive vapers, smokers, and control non-users into the study, whilst excluding all dual/poly users.
We acknowledge the limitations of our study, including its small size, and the use of peripheral blood leukocytes as a surrogate for target organs of tumorigenesis. However, epigenetic modifications measured in blood cells have been associated with cancer, cardiovascular disease, immunologic disease, mental disease, and a variety of other chronic diseases and conditions, which supports the utility of peripheral blood for epigenomic studies in human populations [Citation50]. Although blood is comprised of different cell types, and epigenetic marks may vary in various cell types [Citation51], Tellez-Plaza et al. [Citation42] have measured global methylation and global hydroxymethylation in blood leukocytes both before and after incorporating information on blood cell types and found highly consistent and reproducible results. Finally, we would also like to highlight the strengths of our study, which include the study design with subjects matched for age, gender, and approximately race (accounting for relevant confounding factors), availability of thoroughly detailed information on vaping/smoking history validated by measurement of objective biomarkers using biochemical assays (cotinine, CO, and %COHb), as well as data on other variables of interest (e.g., diet, lifestyle, occupation, medical history, etc.), application of validated methods for quantification of 5-mC and 5-hmC, and measurement of well-established epigenetic marks [Citation12,Citation13].
In conclusion, we have demonstrated, for the first time, key epigenetic modifications, including hypomethylation of LINE-1 repeat elements and global loss of DNA hydroxymethylation, in a well-defined population of exclusive vapers and smokers relative to controls. Our findings underline the importance of investigating the epigenetic effects of vaping in clarifying the health risks or potential benefits of e-cig use vs. smoking. Follow-up genome-wide studies in larger populations are warranted to provide urgently needed scientific evidence on which future regulations for e-cigs can be based.
Author Contributions
AWC: Performed experiments and collected data, analysed data, Co-wrote draft on M&M; AC: Performed experiments and collected data, analysed data, Co-wrote draft on M&M; ST: Performed experiments and collected data, analysed data and interpreted the results; Co-wrote the manuscript; AB: conceived and designed the study, performed experiments and collected data, analyzed data and interpreted the results, wrote the manuscript.
Acknowledgments
We would like to thank Debra E. Moreno for help with subject recruitment and enrollment and specimen collection and processing, and Niccolo Pabustan for assistance with statistical analysis. We would also like to acknowledge Dr. Seth Rubin and Dr. Michael Garbati for providing technical support for the LINE-1 DNA methylation detection assay. We are grateful to Edward Avol and Dr. Rob McConnell and Dr. Marry Ann Pentz and their staff at the USC Tobacco Center of Regulatory Science (TCORS) for facilitating subject recruitment. Also, we thank Dr. Lourdes Baez Conde for supporting our community engagement and communication plans. Special thanks to Andrew Zaw and Gregory Wilkerson for creating web forms for our online survey and providing technical support for maintenance of the database of responses. We are thankful to Dr. Alan Hiti and his staff at the USC-Norris Outpatient Clinical Laboratory for phlebotomy work.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Dinakar C, O’Connor GT. The health effects of electronic cigarettes. N Engl J Med. 2016;375:1372–1381.
- Shields PG, Berman M, Brasky TM, et al. A review of pulmonary toxicity of electronic cigarettes in the context of smoking: a focus on inflammation. Cancer Epidemiol Biomarkers Prev. 2017;26:1175–1191.
- Farsalinos K. Electronic cigarettes: an aid in smoking cessation, or a new health hazard? Ther Adv Respir Dis. 2018;12:1753465817744960.
- Besaratinia A, Tommasi S. An opportune and unique research to evaluate the public health impact of electronic cigarettes. Cancer Causes Control. 2017;28:1167–1171.
- Cheng T. Chemical evaluation of electronic cigarettes. Tob Control. 2014;23(Suppl 2):ii11–17.
- Tommasi S, Caliri AW, Caceres A, et al. Deregulation of biologically significant genes and associated molecular pathways in the oral epithelium of electronic cigarette users. Int J Mol Sci. 2019;20(3). pii: E738.
- Besaratinia A, Pfeifer GP. Second-hand smoke and human lung cancer. Lancet Oncol. 2008;9:657–666.
- You JS, Jones PA. Cancer genetics and epigenetics: two sides of the same coin? Cancer Cell. 2012;22:9–20.
- Feinberg AP, Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature. 1983;301:89–92.
- Piskareva O, Lackington W, Lemass D, et al. The human L1 element: a potential biomarker in cancer prognosis, current status and future directions. Curr Mol Med. 2011;11:286–303.
- Wu X, Zhang Y. TET-mediated active DNA demethylation: mechanism, function and beyond. Nat Rev Genet. 2017;18:517–534.
- Shi DQ, Ali I, Tang J, et al. New insights into 5hmC DNA modification: generation, distribution and function. Front Genet. 2017;8:100.
- Xiao-Jie L, Hui-Ying X, Qi X, et al. LINE-1 in cancer: multifaceted functions and potential clinical implications. Genet Med. 2016;18:431–439.
- Feinberg AP, Koldobskiy MA, Gondor A. Epigenetic modulators, modifiers and mediators in cancer aetiology and progression. Nat Rev Genet. 2016;17:284–299.
- Benowitz NL, Hukkanen J, Jacob P 3rd. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009;192:29–60.
- Besaratinia A, Tommasi S. The lambda select cII mutation detection system. J Vis Exp. 2018;134.
- Emery RL, Levine MD. Optimal carbon monoxide criteria to confirm smoking status among postpartum women. Nicotine Tob Res. 2016;18:966–970.
- Prak ET, Kazazian HH Jr. Mobile elements and the human genome. Nat Rev Genet. 2000;1:134–144.
- Kazazian HH Jr. Genetics. L1 retrotransposons shape the mammalian genome. Science. 2000;289:1152–1153.
- Yang AS, Estecio MR, Doshi K, et al. A simple method for estimating global DNA methylation using bisulfite PCR of repetitive DNA elements. Nucleic Acids Res. 2004;32:e38.
- Lisanti S, Omar WA, Tomaszewski B, et al. Comparison of methods for quantification of global DNA methylation in human cells and tissues. PloS One. 2013;8:e79044.
- Searles Nielsen S, Checkoway H, Butler RA, et al. LINE-1 DNA methylation, smoking and risk of Parkinson’s disease. J Parkinsons Dis. 2012;2:303–308.
- Tarantini L, Bonzini M, Apostoli P, et al. Effects of particulate matter on genomic DNA methylation content and iNOS promoter methylation. Environ Health Perspect. 2009;117:217–222.
- Wright RO, Schwartz J, Wright RJ, et al. Biomarkers of lead exposure and DNA methylation within retrotransposons. Environ Health Perspect. 2010;118:790–795.
- Hossain MB, Vahter M, Concha G, et al. Low-level environmental cadmium exposure is associated with DNA hypomethylation in Argentinean women. Environ Health Perspect. 2012;120:879–884.
- Lambrou A, Baccarelli A, Wright RO, et al. Arsenic exposure and DNA methylation among elderly men. Epidemiology (Cambridge, Mass). 2012;23:668–676.
- Bollati V, Baccarelli A, Hou L, et al. Changes in DNA methylation patterns in subjects exposed to low-dose benzene. Cancer Res. 2007;67:876–880.
- Liu F, Killian JK, Yang M, et al. Epigenomic alterations and gene expression profiles in respiratory epithelia exposed to cigarette smoke condensate. Oncogene. 2010;29:3650–3664.
- Teneng I, Montoya-Durango DE, Quertermous JL, et al. Reactivation of L1 retrotransposon by benzo(a)pyrene involves complex genetic and epigenetic regulation. Epigenetics. 2011;6:355–367.
- Ziech D, Franco R, Pappa A, et al. Reactive oxygen species (ROS)–induced genetic and epigenetic alterations in human carcinogenesis. Mutat Res. 2011;711:167–173.
- Lee YW, Broday L, Costa M. Effects of nickel on DNA methyltransferase activity and genomic DNA methylation levels. Mutat Res. 1998;415:213–218.
- Takiguchi M, Achanzar WE, Qu W, et al. Effects of cadmium on DNA-(Cytosine-5) methyltransferase activity and DNA methylation status during cadmium-induced cellular transformation. Exp Cell Res. 2003;286:355–365.
- Chuang LS, Ng HH, Chia JN, et al. Characterisation of independent DNA and multiple Zn-binding domains at the N terminus of human DNA-(cytosine-5) methyltransferase: modulating the property of a DNA-binding domain by contiguous Zn-binding motifs. J Mol Biol. 1996;257:935–948.
- Olmedo P, Goessler W, Tanda S, et al. Metal concentrations in e-cigarette liquid and aerosol samples: the contribution of metallic coils. Environ Health Perspect. 2018;126:027010.
- Weitzman SA, Turk PW, Milkowski DH, et al. Free radical adducts induce alterations in DNA cytosine methylation. Proc Natl Acad Sci U S A. 1994;91:1261–1264.
- Kuchino Y, Mori F, Kasai H, et al. Misreading of DNA templates containing 8-hydroxydeoxyguanosine at the modified base and at adjacent residues. Nature. 1987;327:77–79.
- Furlan D, Trapani D, Berrino E, et al. Oxidative DNA damage induces hypomethylation in a compromised base excision repair colorectal tumourigenesis. Br J Cancer. 2017;116:793–801.
- Miller JW, Nadeau MR, Smith J, et al. Folate-deficiency-induced homocysteinaemia in rats: disruption of S-adenosylmethionine’s co-ordinate regulation of homocysteine metabolism. Biochem J. 1994;298(Pt 2):415–419.
- Yang Q, Wu K, Ji M, et al. Decreased 5-hydroxymethylcytosine (5-hmC) is an independent poor prognostic factor in gastric cancer patients. J Biomed Nanotechnol. 2013;9:1607–1616.
- Zhang Y, Wu K, Shao Y, et al. Decreased 5-Hydroxymethylcytosine (5-hmC) predicts poor prognosis in early-stage laryngeal squamous cell carcinoma. Am J Cancer Res. 2016;6:1089–1098.
- Ghosh M, Oner D, Poels K, et al. Changes in DNA methylation induced by multi-walled carbon nanotube exposure in the workplace. Nanotoxicology. 2017;11:1195–1210.
- Tellez-Plaza M, Tang WY, Shang Y, et al. Association of global DNA methylation and global DNA hydroxymethylation with metals and other exposures in human blood DNA samples. Environ Health Perspect. 2014;122:946–954.
- Coulter JB, O’Driscoll CM, Bressler JP. Hydroquinone increases 5-hydroxymethylcytosine formation through ten eleven translocation 1 (TET1) 5-methylcytosine dioxygenase. J Biol Chem. 2013;288:28792–28800.
- Li Z, Li N, Guo C, et al. The global DNA and RNA methylation and their reversal in lung under different concentration exposure of ambient air particulate matter in mice. Ecotoxicol Environ Saf. 2019;172:396–402.
- Ringh MV, Hagemann-Jensen M, Needhamsen M, et al. Tobacco smoking induces changes in true DNA methylation, hydroxymethylation and gene expression in bronchoalveolar lavage cells. EBioMedicine. 2019;46:290–304.
- Benbrahim-Tallaa L, Waterland RA, Dill AL, et al. Tumor suppressor gene inactivation during cadmium-induced malignant transformation of human prostate cells correlates with overexpression of de novo DNA methyltransferase. Environ Health Perspect. 2007;115:1454–1459.
- Novakovic B, Wong NC, Sibson M, et al. DNA methylation-mediated down-regulation of DNA methyltransferase-1 (DNMT1) is coincident with, but not essential for, global hypomethylation in human placenta. J Biol Chem. 2010;285:9583–9593.
- Xiao Y, Word B, Starlard-Davenport A, et al. Age and gender affect DNMT3a and DNMT3b expression in human liver. Cell Biol Toxicol. 2008;24:265–272.
- Hammons GJ, Yan Y, Lopatina NG, et al. Increased expression of hepatic DNA methyltransferase in smokers. Cell Biol Toxicol. 1999;15:389–394.
- Terry MB, Delgado-Cruzata L, Vin-Raviv N, et al. DNA methylation in white blood cells: association with risk factors in epidemiologic studies. Epigenetics. 2011;6:828–837.
- Nestor CE, Ottaviano R, Reddington J, et al. Tissue type is a major modifier of the 5-hydroxymethylcytosine content of human genes. Genome Res. 2012;22:467–477.
