ABSTRACT
Vitamin D could be beneficial for healthy ageing in humans. We previously found that vitamin D supplementation may slow down epigenetic ageing in young African American adults. We tested new epigenetic clocks developed for neonates among a multiethnic population, and tested the hypothesis that maternal vitamin D supplementation would slow down the epigenetic gestational age acceleration (GAA) in newborn babies. Ninety-two pregnant women (aged 29.6 ± 4.8 y; 21% African Americans, 28% Hispanics) were randomized to receive 4000 IU/day vitamin D3 or placebo, plus prenatal vitamins containing 400 IU vitamin D3 during pregnancy in a randomized controlled trial (RCT). Cord blood genome-wide methylation analysis was performed on the Illumina Infinium MethylationEPIC Beadchip. DNA methylation gestational age was calculated based on two calculations developed by Knight and Bohlin. DNA methylation gestational ages calculated by Knight’s clock and Bohlin’ clock were highly correlated with the gestational age in the placebo group (correlation coefficients = 0.88, p s< 0.001, respectively). GAA was associated with higher birth weight (p = 0.039). In the entire cohort, vitamin D3 supplementation was not associated with GAA (p > 0.05). However, vitamin D3 supplementation decreased GAA by both Knight’s clock (β = −0.89, p = 0.047) and Bohlin’s clock (β = −0.71, p = 0.005) in the African American participants. Maternal vitamin D3 supplementation may slow down the epigenetic gestational ageing process in African American neonates. Long-term follow-up studies are warranted to determine the role of epigenetic age acceleration in the growth and development of offspring.
Introduction
Epigenetic clock has emerged as a new marker of biological ageing. In 2013, Horvath’s epigenetic clock was developed by Horvath et al., which predicts DNA methylation (DNAm) age based on 353 CpG sites [Citation1]. In the same year, Hannum et al. also formulated a quantitative model of ageing based on the methylation levels of 71 CpG sites [Citation2]. Epigenetic age acceleration in adults has been associated with several ageing-related diseases [Citation3–Citation8], poor health outcomes and predicts all-cause mortality [Citation9–Citation12]. Recently, two epigenetic clocks were developed to estimate gestational age of neonates. Knight’s clock calculates the DNA methylation gestational age (DNAmGA) using 148 CpG sites selected through elastic net regression based mainly on the white population (82% whites, 18% African Americans) [Citation13]. Bohlin’s clock estimates the DNAmGA using 96 CpG sites selected by Lasso-regression models based solely on the white population [Citation14]. Both Knight’s and Bohlin’s DNAmGAs were proven to be highly correlated with chronological gestational age in their testing datasets (Knight γ = 0.91; Bohlin γ = 0.81) [Citation13,Citation14].
Unlike the wide applications of the Horvath’s and Hannum’s epigenetic clocks in adults [Citation1,Citation2], studies of epigenetic clock of gestational age are limited. In 814 Finnish mother-neonate pairs, Girchenko et al. showed that DNAmGA acceleration (GAA), was associated with maternal age of over 40 y at delivery, preeclampsia and foetal demise in a previous pregnancy, lower neonatal birth size, being born small-for-gestational age, lower 1-min Apgar score, and female sex; whereas DNAm GA deceleration (GAD) was associated with insulin-treated gestational diabetes in a previous pregnancy and Sjogren’s syndrome [Citation15]. Räikkönen’s research team calculated Knight’s DNAmGA of the subjects from the Prediction and Prevention of Preeclampsia and Intrauterine Growth Restriction (PREDO) study [Citation16], and found that maternal antenatal depression was associated with lower DNAmGA in offspring [Citation17]. Another study calculated DNAmGA based on Bohlin’s clock and found that both higher maternal body mass index (BMI) and larger birth size were associated with greater GAA [Citation18]. Epigenetic age acceleration has been associated with negative health outcomes and can predict all-cause mortality in adult populations. However, the clinical implications of GAA are unclear given that the existing evidence is contradictory. Moreover, the existing studies were conducted solely on the white populations. Whether these two epigenetic clocks of gestational age can be applied to other ethnic groups, i.e., African Americans or Hispanics, remains unknown.
Vitamin D is considered to be involved in epigenetic regulation in humans. We previously conducted a series of epigenome-wide association studies in youth and young adults and showed that vitamin D deficiency was associated with global DNA hypomethylation; vitamin D supplementation in individuals with vitamin D deficiency increased the level of global DNA methylation in leukocytes [Citation19]. In addition, vitamin D deficiency also was associated with locus-specific leukocyte DNA methylation change [Citation20]. Recently, we have shown that vitamin D supplementation may slow down epigenetic ageing by Horvath’s clock in young African American adults in a small randomized controlled trial (RCT) [Citation21]. Pregnancy represents a time of rapid bodily change, which includes changes in physical proportions, physiology, and responsibility. Environmental adversities in prenatal life may induce life-long consequences by compromising the foetal developmental milieu [Citation22]. Maternal nutrition status may affect the prenatal programming [Citation22]. Therefore, vitamin D status in pregnancy is more important than any other time period during the life span, affecting not only the mother but also her growing foetus and later, her growing infant [Citation23]. However, whether maternal vitamin D3 supplementation has any effect on epigenetic GAA is unknown.
Studies about DNAmGA are very limited and all observational. Whether those two gestational clocks can be a sensitive biomarker in response to intervention is unclear. Therefore, we conducted an epigenome-wide methylation study in cord blood DNA from the offspring of mothers who participated in a RCT of vitamin D3 supplementation (placebo vs. 4000 IU/day) beginning at 10–14 weeks of gestation until delivery. We have previously shown that vitamin D3 supplementation was associated with decreased epigenetic ageing in young African American adults. Therefore, we tested the hypothesis that vitamin D3 supplementation would slow down the epigenetic GAA of the neonates. We further tested Knight’s clock and Bohlin’s clock among a multiethnic population, which included whites, African Americans and Hispanics.
Results
General characteristics of the study population
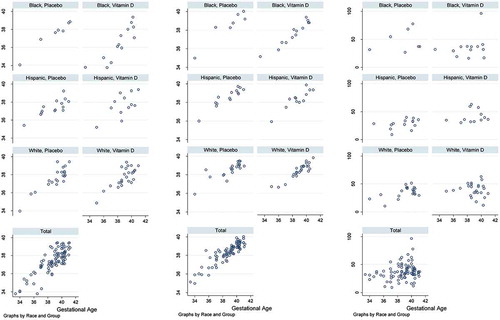
There was no significant difference in most maternal and neonatal characteristics between the placebo group and the treatment group who received 4000 IU/day vitamin D3 supplementation (ps > 0.05), except that the serum 25-hydroxyvitamin D3 [25(OH)D] concentrations in mothers at the second and the third trimester and in cord blood were significantly higher in the treatment group compared with the placebo group (ps < 0.001) as expected. There was no difference in gestational age between treatment and placebo groups (, Supplementary Table S1 and S2).
Table 1. Comparisons of general characteristics of the study population (N = 92).
Correlations of three epigenetic gestational age clocks with gestational age in placebo group
presents the correlations between DNAmGA and chronological gestational age stratified by race and group. In the placebo group, which had no potential deviation induced by vitamin D3 supplementation, Knight DNA methylation gestational age (DNAmGAKnight) and Bohlin DNA methylation gestational age (DNAmGABohlin) were highly correlated to gestational age (γ = 0.88 for both clocks, ps < 0.001). Horvath DNA methylation gestational age (DNAmGAHorvath) was much less correlated to gestational age (γ = 0.35, p = 0.025). Both DNAmGAKnight and DNAmGABohlin clocks performed well in African Americans (γ = 0.98 and 0.94, ps < 0.001) and the Hispanics (γ = 0.80 and 0.93, ps < 0.001), other than the whites (γ = 0.88 and 0.82, ps < 0.001) (Supplementary Table S3).
Associations between general characteristics and DNA methylation-based gestational age
presents the associations between general characteristics and DNAmGA. There were no gender differences in gestational age or DNAmGA clocks. Higher maternal BMI was associated with lower gestational age, but not DNAmGA. Birth weight and head circumference were positively associated with gestational age and both DNAmGA clocks.
Table 2. Associations between general characteristics and DNA methylation-based gestational age (N = 92).
Associations between general characteristics and DNA methylation-based gestational age acceleration
presents the associations between general characteristics and GAA. GAAresidual by Bohlin’s clock was positively associated with birth weight (β = 0.22, p = 0.039) and maternal BMI (β = 0.01, p = 0.049). According to Bohlin’s clock, the Hispanics and whites were associated with higher GAA compared to the African Americans (β = 0.33, p = 0.013 in Hispanics; β = 0.22, p = 0.062 in whites). Similarly, the Hispanics and whites also had higher Knight’s GAA compared to the African Americans (β = 0.54, p = 0.020 in Hispanics; β = 0.50, p = 0.017 in whites).
Table 3. Associations between general characteristics and DNA methylation-based gestational age acceleration.
Effects of vitamin D3 supplementation on DNA methylation-based gestational age acceleration
In the entire cohort, multiple linear regression models adjusted for maternal age, race, BMI, smoking status and sex of the newborns showed that the vitamin D3 supplementation was not associated with GAA (p > 0.05) (). However, there were significant interactions between race and vitamin D3 supplementation on GAAresidual (ps < 0.05). Stratified by race, the vitamin D3 supplementation was associated with decreased GAAresidual by both Knight’s clock (β = −0.89, p = 0.047) and Bohlin’s clock (β = −0.71, p = 0.005) in African American participants only. The effects of the vitamin D3 supplementation on GAA were not significant either in Hispanic or in white participants (ps > 0.05) (). We further conducted sensitivity analysis adjusting for newborns’ birthweight, instead of maternal BMI in the model (Supplementary Table S4 and S5). Similarly, in the entire cohort, vitamin D3 supplementation was not associated with GAA (p > 0.05). But when stratified by race, the vitamin D3 supplementation was associated with decreased GAA by Bohlin’s clock (β = −0.53, p = 0.038) in African American participants only.
Table 4. Effects of vitamin D3 supplementation on epigenetic gestational age acceleration based on multiple linear regression in all participants (N = 92).
Table 5. Effects of vitamin D3 supplementation on epigenetic gestational age acceleration based on multiple linear regression stratified by race.
Discussion
In this study, both Knight’s and Bohlin’s epigenetic gestational age were highly correlated with gestational age in this multiethnic cohort including whites, Hispanics and blacks. Epigenetic GA was positively associated with birth weight and head circumference. In addition, Bohlin’s GAA was positively associated with maternal BMI and birth weight. Vitamin D3 supplementation was associated with decreased Knight’s and Bohlin’s GAA only in African Americans.
This study validated Knight’s and Bohlin’s epigenetic clocks in an independent cohort and found that the predicted methylation gestational ages were highly correlated with chronological gestational age in white, African American and Hispanic populations (with correlation coefficients of 0.80–0.98 in the placebo group). To our knowledge, this is the first study to test both Knight’s and Bohlin’s epigenetic gestational age clocks in a multiethnic cohort. Both Knight’s and Bohlin’s clocks performed well in both African American and Hispanic populations despite that both clocks were developed mainly in a white population. The Knight’s clock was developed based on multiple cohorts including whites and African Americans. The 148 CpG sites selected were not enriched or depleted for the sites previously reported to associate with African American or white race [Citation13]. Our results suggest that the accuracy of both Knight’s and Bohlin’s epigenetic gestational clocks is not confounded by race or ethnicity. Also, we found no sex difference in epigenetic gestational age estimation, which is in agreement with the study by Knight et al [Citation13]. While Horvath’s clock also has been used in studies on newborn babies [Citation24], it was developed based on 0–100-year-old individuals. In our cohort, Horvath’s clock did not perform as well as Knight’s and Bohlin’s clocks, which were developed just for neonates.
Up to date, limited studies reported inconsistent findings regarding the relationships between epigenetic gestational age acceleration and offspring characteristics at birth, which makes the clinical implication of epigenetic gestational age acceleration uncertain. Neonates with higher DNAmGA may be more developmentally mature [Citation13]. In consistent with this assumption, Knight and Khouja et al. reported that greater GAA was associated with higher birth weight, birth length and head circumference of the child [Citation13,Citation18]. Similarly, our study showed that GAA was positively associated with birth weight.
On the other hand, GAA may reflect epigenetic programming by early-life environmental exposures such as maternal prenatal stress or pregnancy disorders, which may compromise neonatal health status. Girchenko et al. evaluated 814 mother-neonate pairs and observed that GAA was associated with lower birth weight, birth length, ponderal index at birth, birth head circumference, placenta weight, being small for gestational age, and a lower 1-min Apgar score [Citation15]. Moreover, some maternal disadvantages were also associated with GAA, including maternal age of over 40 y at delivery, preeclampsia and foetal demise in a previous pregnancy [Citation15]. On the contrary, epigenetic gestational age deceleration can also be interpreted as a sign of epigenetic immaturity and was associated with maternal depression [Citation17]. As aforementioned, the difference between DNAmGA and clinically estimated GA can be influenced by neonatal developmental stage, by prenatal and perinatal exposures, or by the accuracy of the clinical GA estimations [Citation13]. Therefore, the interpretation should be cautious and more studies are needed.
The effects of nutrition during development were recently found to be related to altered programming of epigenetic markers [Citation25]. A study in mice demonstrated that maternal vitamin D depletion can have long-term effects on the epigenome of subsequent generations [Citation26]. Moreover, a small genome-wide scale methylation study comparing healthy newborns with high to low cord blood 25(OH)D concentrations identified DNA methylation differences in multiple CpG sites [Citation27].
In this study, we showed that vitamin D3 supplementation during pregnancy was associated with decreased GAA only in African American neonates, suggesting that the epigenetic gestational clock could be a marker in response to intervention. The different effects of vitamin D supplementation among different ethnic groups may be partially explained by the fact that African American populations are prone to suboptimal vitamin D status [Citation28]. In our prior vitamin D pregnancy studies, African American women had significantly greater vitamin D deficiency at baseline than white or Hispanic women [Citation29–Citation32]. In this study, the circulating concentration of 25(OH)D in African Americans was significantly lower than Hispanics and whites (p = 0.001) (Supplementary Table S1). We have recently found that vitamin D3 supplementation also slowed down the epigenetic ageing among young African American adults [Citation21]. As discussed above, GAA is associated with both favourable and unfavourable birth outcomes and early life exposures. In addition, the sample size of African American group in the present study was relatively small, which could result in chance findings. Large independent replication studies are warranted.
There are several unique aspects of our study. First, DNA methylation gestational age was calculated based on three recently developed calculations, which were compared to one another for the first time. Second, we are the first to apply these gestational epigenetic clocks in African American and Hispanic populations. Third, to the best of our knowledge, this is the first study to evaluate the effect of a nutritional intervention on epigenetic gestational ageing utilizing a randomized controlled clinical trial design. The exploratory nature, and relative small sample size, especially in the subgroup analyses are limitations in this present study. Among the African Americans, the estimated powers were 46.1% and 47.2% (α = 0.05) to detect the differences in GAAresidual between the placebo and the treatment group according to Bohlin’s clock and Knight’s clock, respectively. The estimated power in white (Knight: 17%, Bohlin: 7%) and Hispanic population (Knight: 13%, Bohlin: 19%) were even lower, which may also explain the null findings in these two populations. In addition, this is a post-hoc analysis of a subset sample of a randomized controlled trial, such that there is a likelihood of either false-positive or false-negative results. Further large-scale studies are needed to validate our results. Third, we employed the updated Infinium MethylationEPIC Kit (850 K) instead of Infinium HumanMethylation450 BeadChip, which resulted in 6 CpG sites missing from the 142 CpG sites that were used in Knight’s clock, and 8 CpG sites missing from the 88 CpG sites that were used in Bohlin’s clock. However, the performances of the two clocks were satisfactory as demonstrated by their high correlations to the chronological gestational age. Moreover, Dhingra et al. found that 850 K array platforms did not cause deviations in Horvath’s clock, and the missing 17 CpG sites did not affect the residuals of epigenetic age on chronological age [Citation33].
For the first time, we show that both Knight’s and Bohlin’s epigenetic gestational clocks work well in Hispanic and African American populations in addition to the white population. In this exploratory study, we observed that maternal vitamin D3 supplementation seems associated with decreased GAA in African American neonates, which requires further validation in large independent samples. The clinical implication of accelerated or decelerated epigenetic gestational ageing requires further longitudinal follow-up studies.
Materials and methods
Participants
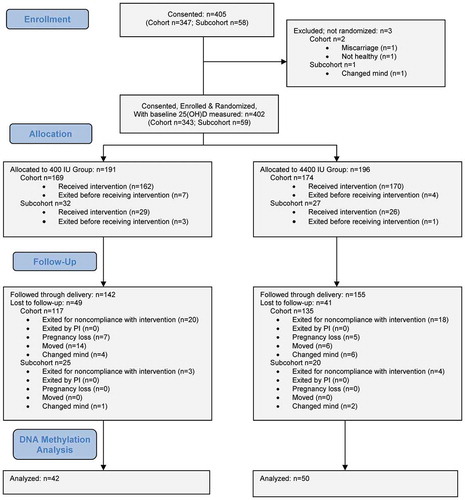
A total of 405 pregnant women were enrolled in a randomized, placebo-controlled clinical trial between 10–14 weeks of gestation and followed until delivery (). Two hundred ninety-seven women completed the trial. A subset of 92 participants (mean age ± SD, 29.6 ± 4.8 y; 21% African Americans, 28% Hispanics) were randomly selected from this trial for the post-hoc analyses in the present study. Each mother was randomized to receive either 4000 IU/day vitamin D3 or placebo, plus the standard prenatal vitamins containing 400 IU vitamin D3. Blood samples were collected at each trimester. Cord blood also was collected at delivery. Written consent was obtained from all participants. The study was approved by the Institutional Review Board of Medical University of South Carolina (HR #PRO00020570; clinicaltrials.gov #NCT01932788).
Maternal and neonatal assessments
Height and weight of the mothers were obtained according to standard procedures and body mass index (BMI) was calculated as weight (kg) divided by height (m2). Gestational age was estimated by a combined method, which was based on last menstrual period, first-trimester ultrasound and 20-week ultrasound. Weight (grams) and head circumference (centimetres) were measured at birth according to standard procedure. Blood samples were obtained at the end of each trimester, and cord blood samples were obtained during delivery. Blood samples were frozen and stored at −80°C until assayed. Maternal serum 25(OH)D concentrations were measured at each trimester using an established radioimmunoassay with a minimal of detection of 2 ng/mL (RIA, Diasorin, Stillwater, MN) as previously described. Cord blood 25(OH)D concentration also was measured by RIA. The inter- and intra-assay coefficient of variation was ≤10%. History of maternal smoking history was obtained at the first visit. At each subsequent visit using a standardized questionnaire, each participant was asked if she smoked, and if so, how many cigarettes per day was ascertained.
Genome-wide DNA methylation
DNA methylation data were obtained from the in this cohort. DNA was extracted from stored cord blood buffy coat samples using the QIAamp DNA Mini Kit (QIAGEN, Valencia, CA). The DNA quantity and quality were checked by Nanodrop and PicoGreen methods. Genome-wide DNA methylation levels were analysed by the Infinium MethylationEPIC Kit (Illumina Inc., Denver, CO) on 92 cord blood DNA samples and quality control (QC) samples. In the quality control stage, DNA methylation data were processed using the Minfi package [Citation34]. Detectable probes were defined as the probes with detection p < 1E-16 in more than 95% samples; detectable samples were those with detection p < 1E-16 in more than 95% CpG sites. The quality control report is included in supplementary file.
DNA methylation gestational age calculation
DNA methylation gestational age (DNAmGA) was determined based on three calculations that were developed by Knight, Bohlin and Horvath, respectively. Horvath’s clock was based on 0–100-year-old individuals [Citation1], whereas Hannum’s clock was based on 10–101-year-old individuals [Citation2]. Therefore, we only included the Horvath’s clock in addition to the Knight’s and the Bohlin’s clocks.
DNAmGAKnight was based on the DNA methylation levels of 148 CpG sites from HumanMethylation450 BeadChip using the statistical pipeline developed by Knight research team where background-corrected β values were pre-processed using the calculator’s internal normalization method [Citation1]. Since we employed the Infinium MethylationEPIC Kit instead of Infinium HumanMethylation450 BeadChip, some CpG sites in the DNAmGA calculation were missing in our platform. DNA methylation values were missing in 6 CpG sites, and a total of 142 CpG sites were used to predict DNAmGAKnight.
DNAmGABohlin was based on the DNA methylation levels of 96 CpG sites using the predictive function developed by the Bohlin research team. DNA methylation values were missing in 8 CpG sites in the Infinium MethylationEPIC Kit, and a total of 88 CpG sites were used to predict DNAmGABohlin.
Horvath DNA methylation age (DNAmAge) was defined as a prediction of age based on the DNA methylation levels of 353 CpG sites using the statistical pipeline developed by Horvath where background-corrected β values were pre-processed using the calculator’s internal normalization method [Citation1]. DNA methylation values were missing in 19 CpG sites, and a total of 334 CpG sites were used to predict DNAmAge. DNAmGAHorvath was then calculated as DNAmAge×365.25/7 to be comparable to gestational age.
DNA methylation-based gestational age acceleration (GAA) was defined as the difference between DNAmGA and chronological gestational age in weeks. GAA was calculated in two different ways as recommended [Citation15]. Raw GAA (GAAraw) was calculated by subtracting the chronological gestational age from the DNAmGA. GAA residual (GAAresidual) was extracted from a linear regression of DNAmGA on chronological gestational age.
Statistical analysis
Participant characteristics are presented as mean ± SD for continuous variables, while N (%) for categorical variables. Normality of each continuous variable was tested based on a combination test statistics of skewness and kurtosis. Maternal characteristics, neonatal characteristics, gestational age and DNAmGAs were compared between placebo group and treatment group. Two-group mean-comparison t-test was performed to compare the normally distributed characteristics between placebo and treatment groups, while otherwise the Wilcoxon rank-sum test was performed. Pearson’s chi-squared test was used for categorical variables. Pearson correlation coefficients (γ) were calculated to measure the correlations between DNAmGAs and chronological gestational age.
Associations between general characteristics and GAA were examined in linear regression models that adjusted for group assignment. The effects of vitamin D3 supplementation on GAA were estimated in linear regression that adjusted for maternal age, race, BMI, smoking status and sex of the babies. Associations between GAA and serum 25(OH)D concentrations from blood at the end of the first, second and third trimester, as well as cord blood, were also studied in linear regression models that adjusted for the same covariates. A p < 0.05 was considered statistically significant. All statistical analysis was performed using Stata version 12.0 (College Station, Texas 77845 USA).
Clinical trial registration
#NCT01932788 at https://clinicaltrials.gov/
Supplemental Material
Download PDF (840 KB)Supplemental Material
Download MS Word (24.8 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:R115.
- Hannum G, Guinney J, Zhao L, et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49:359–367.
- Horvath S, Erhart W, Brosch M, et al. Obesity accelerates epigenetic aging of human liver. Proc Natl Acad Sci U S A. 2014;111:15538–15543.
- Nevalainen T, Kananen L, Marttila S, et al. Obesity accelerates epigenetic aging in middle-aged but not in elderly individuals. Clin Epigenetics. 2017;9:20.
- Horvath S, Garagnani P, Bacalini MG, et al. Accelerated epigenetic aging in Down syndrome. Aging Cell. 2015;14:491–495.
- Horvath S, Langfelder P, Kwak S, et al. Huntington’s disease accelerates epigenetic aging of human brain and disrupts DNA methylation levels. Aging (Albany NY). 2016;8:1485–1512.
- Maierhofer A, Flunkert J, Oshima J, et al. Accelerated epigenetic aging in Werner syndrome. Aging (Albany NY). 2017;9:1143–1152.
- Voisey J, Lawford BR, Morris CP, et al. Epigenetic analysis confirms no accelerated brain aging in schizophrenia. NPJ Schizophr. 2017;3:26.
- Horvath S, Gurven M, Levine ME, et al. An epigenetic clock analysis of race/ethnicity, sex, and coronary heart disease. Genome Biol. 2016;17:171.
- Chen BH, Marioni RE, Colicino E, et al. DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging (Albany NY). 2016;8:1844–1865.
- Marioni RE, Harris SE, Shah S, et al. The epigenetic clock and telomere length are independently associated with chronological age and mortality. Int J Epidemiol. 2016;45:424–432.
- Perna L, Zhang Y, Mons U, et al. Epigenetic age acceleration predicts cancer, cardiovascular, and all-cause mortality in a German case cohort. Clin Epigenetics. 2016;8:64.
- Knight AK, Craig JM, Theda C, et al. An epigenetic clock for gestational age at birth based on blood methylation data. Genome Biol. 2016;17:206.
- Bohlin J, Haberg SE, Magnus P, et al. Prediction of gestational age based on genome-wide differentially methylated regions. Genome Biol. 2016;17:207.
- Girchenko P, Lahti J, Czamara D, et al. Associations between maternal risk factors of adverse pregnancy and birth outcomes and the offspring epigenetic clock of gestational age at birth. Clin Epigenetics. 2017;9:49.
- Girchenko P, Lahti M, Tuovinen S, et al. Cohort profile: prediction and prevention of preeclampsia and intrauterine growth restriction (PREDO) study. Int J Epidemiol. 2017;46:1380–1g.
- Suarez A, Lahti J, Czamara D, et al. The epigenetic clock at birth: associations with maternal antenatal depression and child psychiatric problems. J Am Acad Child Adolesc Psychiatry. 2018;57:321–8.e2.
- Khouja JN, Simpkin AJ, O’Keeffe LM, et al. Epigenetic gestational age acceleration: a prospective cohort study investigating associations with familial, sociodemographic and birth characteristics. Clin Epigenetics. 2018;10:86.
- Zhu HD, Bhagatwala J, Huang Y, et al. Race/ethnicity-specific association of vitamin D and global DNA methylation: cross-sectional and interventional findings. PloS One. 2016;11:e0152849.
- Zhu HD, Wang XL, Shi HD, et al. A genome-wide methylation study of severe vitamin D deficiency in African American adolescents. J Pediatr-US. 2013;162:1004–U165.
- Chen L, Dong Y, Bhagatwala J, et al. Effects of vitamin D3 supplementation on epigenetic aging in overweight and obese African Americans with suboptimal vitamin D status: a randomized clinical trial. J Gerontol A Biol Sci Med Sci. 2019;74:91–98.
- Raikkonen K, Pesonen AK, Roseboom TJ, et al. Early determinants of mental health. Best Pract Res Clin Endocrinol Metab. 2012;26:599–611.
- Hollis BW, Wagner CL. Vitamin D supplementation during pregnancy: improvements in birth outcomes and complications through direct genomic alteration. Mol Cell Endocrinol. 2017;453:113–130.
- Javed R, Chen W, Lin F, et al. Infant’s DNA methylation age at birth and epigenetic aging accelerators. Biomed Res Int. 2016;2016:4515928.
- Heindel JJ, Vandenberg LN. Developmental origins of health and disease: a paradigm for understanding disease etiology and prevention. Curr Opin Pediatr. 2015;27:248–253.
- Xue J, Schoenrock SA, Valdar W, et al. Maternal vitamin D depletion alters DNA methylation at imprinted loci in multiple generations. Clin Epigenetics. 2016;8:107.
- Junge KM, Bauer T, Geissler S, et al. Increased vitamin D levels at birth and in early infancy increase offspring allergy risk—evidence for involvement of epigenetic mechanisms. J Allergy Clin Immunol. 2016;137:610–613.
- Dong Y, Pollock N, Stallmann-Jorgensen IS, et al. Low 25-hydroxyvitamin D levels in adolescents: race, season, adiposity, physical activity, and fitness. Pediatrics. 2010;125:1104–1111.
- Hollis BW, Johnson D, Hulsey TC, et al. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res. 2011;26:2341–2357.
- Johnson DD, Wagner CL, Hulsey TC, et al. Vitamin D deficiency and insufficiency is common during pregnancy. Am J Perinatol. 2010;28:7–12.
- Hamilton SA, McNeil R, Hollis BW, et al. Profound vitamin D deficiency in a diverse group of women during pregnancy living in a sun-rich environment at latitude 32 degrees N. Int J Endocrinol. 2010;2010:917428.
- Wagner CL, McNeil R, Hamilton SA, et al. A randomized trial of vitamin D supplementation in 2 community health center networks in South Carolina. Am J Obstet Gynecol. 2013;208:137 e1–e13.
- Dhingra R, Kwee LC, Diaz-Sanchez D, et al. Evaluating DNA methylation age on the illumina MethylationEPIC bead chip. PloS One. 2019;14:e0207834.
- Aryee MJ, Jaffe AE, Corrada-Bravo H, et al. Minfi: a flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics. 2014;30:1363–1369.