ABSTRACT
A stressor can induce resilience in another, different stressor, a phenomenon known as cross-tolerance. To learn if cross-tolerance is governed by epigenetic regulation, we used embryonic heat conditioning (EHC) in chicks, during the development of the hypothalamus, to increase the immunization response. Indeed, EHC induced a lifelong systemic antibody response to immunization, in addition to reduced hypothalamic IL6 inflammatory expression following LPS challenge. Since the outcome of EHC was long-term cross-tolerance with the immune system, we studied possible epigenetic mechanisms. We first analysed the methylation and hydroxymethylation patterns of IL6. We found reduced hydroxymethylation on IL6 intron 1 in the EHC group, a segment enriched with CpGs and NFkB-binding sites. Luciferase assay in cell lines expressing NFkB showed that IL6 intron 1 is indeed an enhancer. ChiP in the same segment against NFkB in the hypothalamus presented reduced binding to IL6 intron 1 in the EHC group, before and during LPS challenge. In parallel, EHC chicks’ IL6 intron 1 presented increased H3K27me3, a repressive translational modification mediated by EZH2. This histone modification occurred during embryonic conditioning and persisted later in life. Moreover, we showed reduced expression of miR-26a, which inhibits EZH2 transcription, during conditioning along with increased EZH2 expression. We demonstrate that stress cross-tolerance, which was indicated by EHC-induced inflammatory resilience and displayed by attenuated inflammatory expression of IL6, is regulated by different epigenetic layers.
Introduction
Environmental changes in early life can determine an individual’s well-being later in life [Citation1,Citation2]. Numerous studies have shown that adult stress responses are influenced by childhood adversity [Citation3,Citation4], but also by the function of the immune system [Citation5]. However, stress in early life can induce resilience, as well as vulnerability, later in life [Citation6]. In addition, conditioning for one stressor can induce resilience to another, different one, an effect known as cross-tolerance. For example, thermal conditioning induces neuroprotection against traumatic brain injury [Citation7] as well as a positive metabolic cross-tolerance effect [Citation8]. The embryonic period is extremely susceptible to environmental changes, as suggested by Barker’s hypothesis which postulates that poor embryonic development may lead to adult heart disease, as well as diabetes, in middle age [Citation9,Citation10].
Here we studied the mechanism underlying cross-tolerance of embryonic heat conditioning (EHC) and immune tolerance, along the chicks’ life. The chick offers an optimal model system to study environmental stress during development, because the embryo can be easily manipulated and pharmacologically treated, these birds do not depend on maternal guidance and are able to learn immediately after hatch and, more importantly, their behavioural repertoire after hatching can be easily measured [Citation11–13]. At the same time, their hypothalamic thermal control system is very similar to that in mammals.
Cross-tolerance is attributed to tissue-specific activation of stress response. The hypothalamus is a key regulator of energy and thermal homoeostasis [Citation14], but it is also a major contributor to the development of stress responses [Citation15], and influences the immune system [Citation16]. Here we used heat conditioning during the period of chick incubation during hypothalamic development [Citation17], hypothesizing that the epigenetic mechanisms involved in thermal regulation will also affect immune and inflammatory targets.
Hypothalamic inflammation is related to the development of diet-induced obesity [Citation18–20], which is also influenced by early life stress [Citation21]. Hypothalamic inflammation also contributes to the neurological damage associated with ischaemic stress due to stroke, heatstroke, or traumatic brain injury [Citation22–25]. CNS inflammation involves macrophages and resident microglia and is characterized by increased secretion of proinflammatory cytokines, such as TNFα, IL1β and IL6, via TLR4/MyD88-dependent activation of NFκB, as well as increased nitric oxide production [Citation26,Citation27]. As a proof of concept, in this work, we studied the epigenetic regulation of pro-inflammatory expression of IL6. IL6 is a cytokine, a glycosylated protein which regulates immune activation. Membrane-bound IL6 receptors are expressed on a specific set of cells, and operate their anti-inflammatory action via JAK/STAT signalling, which is considered the classic IL6 signalling. However, upon inflammatory IKK/NFKB dependent transcription of IL6, non-soluble IL6 activation occurs increasing the inflammatory response [Citation28]. In this work, we used lipopolysaccharide (LPS) to induce TLR4/MyD88-dependent activation of NFκB in order to study the epigenetic regulation of the inflammatory activation of IL6 transcription in the hypothalamus.
Increased IL6 expression is evident in the hypothalamus of offspring from resistin, a TLR4 activating hormone, treated dams [Citation29], indicating that IL6 expression can be regulated by epigenetic mechanisms. Furthermore, various forms of epigenetic regulation influences IL6 expression: from several miRs binding to its 3ʹUTR [Citation28], to a single CpG site in the IL6 promoter associate with rheumatoid arthritis [Citation30], and by H3K27 methylation affecting seizure susceptibility during hyperthermia [Citation31], and increased promoter methylation of in obese Korean women [Citation32].
Epigenetic mechanisms regulating hypothalamic gene expression have been shown to be critical for heat-stress resilience or vulnerability [Citation33–39]. Here we studied different layers of hypothalamic, thermal-induced, epigenetic mechanisms regulating inflammatory IL6 expression and possible inflammatory resilience.
Results
Cross-tolerance: EHC induces immune tolerance later in life, and reduced expression of IL6 following LPS challenge
Chicks were heat-conditioned between embryonic days (ED) 7 and 16. After hatch (posthatch), chicks were raised in their optimal environmental temperature of 30°C. Fourteen days posthatch, both EHC and control (unconditioned) chicks were intravenously (IV) injected with BSA (500µgr/ml; BSA group) mixed with complete Freund's adjuvant (CFA) or CFA mixed with PBS (CFA group). Seven days later (day 21 posthatch), blood samples were taken from the wing’s vein and BSA antibody was measured ()). Indeed, as a result of EHC, the concentration of BSA antibody was increased in the BSA injected EHC group 3 weeks after hatch (n = 10, P < 0.001) compared to the unconditioned, BSA injected group (n = 10). There was no difference in antibody concentration between EHC (n = 10) and CTRL (n = 9) CFA groups (p = 0.95). Nevertheless, both CTRL (P < 0.001) and EHC BSA groups (P < 0.001) presented significant increase in antibody concentration compared to their CFA counterparts. Overall, both conditioning (F1, 35 = 252.2, P < 0.001) and immunization (F1, 35 = 2305, P < 0.001) presented a significant effect, in addition to significant interaction (F1, 35 = 228.3, P < 0.001; )). These results suggest that embryonic heat conditioning enabled increased antibody production later in life.
Figure 1. Cross-Tolerance: EHC induces immune tolerance later in life, and reduced expression of IL6 following LPS challenge. (a) Schematic representation of the experiment. EHC on embryonic days (ED) 7–16, followed by BSA/CFA IV–injection on day 14 posthatch. BSA systemic antibody concentration was measured a week later on day 21 poshatch. (b) BSA systemic antibody concentration, CTRL group (nCFA = 9, nBSA = 10, ***P = <0.001); EHC group group (nCFA = 10, nBSA = 10, ***P = <0.001). (c) Experiment scheme, 10-day-old chicks were ICV-injected with LPS (0.3 µg) or saline vehicle (LPS challenge). (d) IL6 mRNA expression was measured in the hypothalamus 6 and 24 hours after LPS challenge, results are presented as fold change relative to HMBS expression and normalized to the average of saline-injected groups. 6 hours (nCTRL = 25, nEHC = 20, **P = 0.009), 24 hours (nCTRL = 15, nEHC = 16, P = 0.23). Data are presented as mean ± SEM, and significant effects between groups are indicated as *0.01 < P < 0.05, **0.001 < P < 0.01, ***P < 0.001 using ANOVA test with LSD for multiple comparisons. CTRL, control
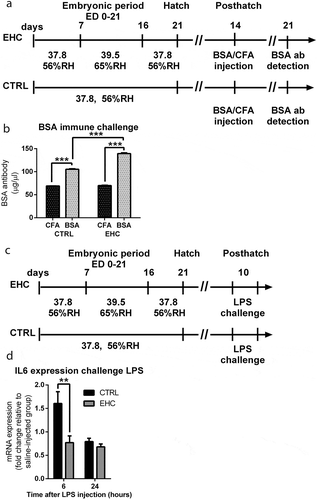
Since we are interested not only in immune resilience but also in inflammation resilience, especially the hypothalamic inflammatory response, we measured the IL6 cytokine mRNA expression in the hypothalamus 6 and 24 hours following LPS/saline intracerebroventricular (ICV) injection (i.e., LPS challenge), on day 10 posthatch ()). We chose the hypothalamus as it is a key regulator of thermal response and present changes in gene expression and protein abundance during thermal conditioning [Citation6,Citation34,Citation40]. Day 10 post hatch was chosen for the LPS challenge, because the critical period for the establishment of the thermoregulatory system in chicks is between days 3 and 5 post hatch [Citation41]. Therefore, it was essential to apply the immunological challenge after the hypothalamic thermal regulatory properties are stable.
IL6 expression displayed a 52% decrease in the EHC group compared to controls (t43 = 2.71, P = 0.009), 6 hours into LPS challenge, but not 24 hours later, as the EHC group presented a nonsignificant 10% decrease in expression (t29 = 1.23, P = 0.23; )). This reduction in IL6, inflammatory cytokine, expression following LPS challenge, indicates reduced inflammatory response, along with the results suggesting increased antibody production following BSA challenge, we posit that EHC induces immune cross-tolerance, later in life.
IL6 intron 1, an enhancer, presents reduced demethylation and NFκB Binding in EHC chicks
Since we showed that EHC has a long-term effect, downregulating the expression of IL6, we wanted to study changes in its CpG methylation (% 5-methylcytosine; %5mC) and hydroxymethylation (% 5-hydroxymethylcytosine; %5hmC) in regulatory regions. We extracted DNA from the hypothalamus of 10-day-old EHC and control chicks and performed next-generation sequencing for specific IL6 amplicons enriched with NFκB-binding sites. The methylation levels along the IL6 sequence were significantly different between the promoter, first intron and first exon (15%, 1.4%, and 8.7%, respectively, F2, 184 = 31.35, P < 0.0001); however, there was no difference between the EHC and control groups (F1, 184 = 0.24, P = 0.62; )). Variations in %5hmC in the three different regions of IL6 – promoter, first exon and first intron (2.6%, 1.2%, 3.5%, respectively) – were apparent, with higher hydroxymethylation in the promoter and first intron (F2, 185 = 24.31, P < 0.0001; )). In the IL6 first intron, %5hmC was significantly reduced in the EHC vs. control group, according to Sidak’s multiple comparisons (P = 0.004; )). Specific CpG hydroxymethylation of IL6 intron 1 is presented in ), where the CpGs are numbered downstream of the transcription start site. Repeated-measures ANOVA of all CpG sites within IL6 intron 1 displayed a significant embryonic conditioning effect whereby hydroxymethylation was downregulated in the EHC group (F1, 8 = 5.86, P = 0.04; )).
Figure 2. IL6 Intron 1, an Enhancer Suppressed by Methylation, Presents Reduced Demethylation and NFκB Binding in EHC Chicks (a) Schematic representation of IL6 gene. (b) Percentage of methylation (%5mC) in the IL6 promoter, 1st exon (exon 1) and 1st intron (intron 1), n = 5. (c) Percentage of hydroxymethylation (%5hmC) in IL6 promoter exon 1 and intron 1, n = 5. IL6 intron 1 (*P = 0.02). (d) CpG-specific hydroxymethylation of IL6 intron 1. (e) Luciferase assay of IL6 intron 1 inserted into HEK293-HTLR4-MD-CD14 cells (pGL3 + IL6_int1) and normalized to the basic plasmid (pGL3-basic), not containing the insert (n = 8, ***P < 0.0001). (f) ChIP against NFκB on day 10 posthatch before (t0; n = 6) and 6 hours into LPS challenge (n = 6). t0 (***P < 0.0001), LPS challenge (***P < 0.0001). IgG ChIP (n = 3), t0 (P = 0.8), LPS challenge (P = 0.22). Data are presented as mean ± SEM. Significant effect between groups is indicated by *0.01 < P < 0.05, **0.001 < P < 0.01, ***P < 0.001 using ANOVA test with LSD for multiple comparisons. CTRL, control
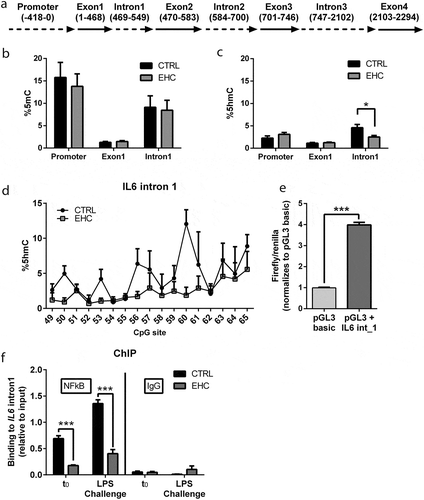
Since we identified hyper-hydroxymethylation in the control group in intron 1, and hydroxymethylation is the first step during active demethylation [Citation42], we wanted to verify its role in regulating IL6 expression. Therefore, we performed a luciferase assay in which IL6 intron 1 (469–549) was inserted into HEK293-HTLR4-MD-CD14 cells, which constitutively express NFκB ()). Cells containing IL6 intron 1 plasmids displayed almost four times the activity of the control, empty plasmids (t14 = 24.4, P < 0.0001; )). To test NFκB binding to IL6 intron 1 in vivo, we performed chromatin immunoprecipitation (ChIP) with NFκB antibody on day 10 posthatch before (t0), and 6 hours into the LPS challenge ()). LPS significantly increased NFκB binding to IL6 intron 1 in the control group (P = 0.009). Moreover, in the LPS-injected groups, 6 hours after injection, NFκB binding to IL6 intron 1 was lower in the EHC group compared to controls (P = 0.005; )). These results not only indicated that IL6 intron 1 is an enhancer, but also presents a reduction in demethylation of IL6 intron 1, which corresponded with the reduced binding of NFκB in the EHC group.
EZH2 and H3K27me3 both bind to IL6 intron 1 during conditioning, yet, only the histone methylation persists later in life
Since we proved that IL6 intron 1 is an enhancer, we wanted to explore additional epigenetic regulatory mechanisms, of this genetic region, with a focus on histone post-translational modifications. Repressive H3K27 trimethylation (H3K27me3) has been previously shown to be related to reduced hypothalamic gene expression following thermal conditioning [Citation38]. In addition, it was shown to be involved in macrophage polarization and inflammatory activation [Citation43]. We, therefore, performed ChIP with anti-H3K27me3 antibody and measured its binding to IL6 intron 1. We found that H3K27me3 binding during the embryonic period, 2 days after conditioning, on ED-18, is increased in the EHC group compared to control, normally incubated, chick embryos (t10 = 3.32, P = 0.008). This difference in IL6 intron 1 methylation was specific to H3K27me3, as there was no difference in the IgG binding to this intron (t10 = 0.41, P = 0.7; )). Furthermore, in order to verify the specificity of the histone modification at IL6 intron 1, we checked using the same samples for two more modifications and performed ChIP using H3K27me2 and H3K27acetyl on ED-18. Both histone modifications did not differ between groups (H3K27me2; t10 = 1.11, P = 0.29, ); H3K27acetyl; t8 = 0.58, P = 0.29, )). Methylation of H3K27 is mediated by polycomb repressive complex (PRC) 2/3 which employs the enhancer of zeste homolog 2 (EZH2) methyltransferase [Citation44], which is also regulated in the hypothalamus during thermal conditioning [Citation36]. Therefore, we performed additional ChIP with anti-EZH2 antibody and measured its binding to the same segment of IL6 intron 1. EZH2 binding to IL6 intron 1 was significantly higher during the embryonic period in the EHC group vs. the control group (t12 = 3.94, P = 0.002). IgG binding to IL6 intron 1 did not differ between groups (t4 = 0.99, P = 0.38, )).
Figure 3. EZH2 and H3K27me3 both bind to IL6 intron 1 during conditioning, yet only the histone methylation persists later in life. (a) Experimental scheme. Midbrain samples were taken during the embryonic period (embryonic day ED-18) and hypothalamic samples on day 10 posthatch. (b) ChIP against H3K27me3 on ED-18 (n = 6, **P = 0.008); IgG ChIP (n = 4, P = 0.7). (c) ChIP against EZH2 on ED-18 (n = 7, **P = 0.002); IgG ChIP (n = 3, P = 0.38). (d) ChIP against H3K27me2 on ED-18 (n = 6, P = 0.29); IgG ChIP (n = 6, P = 0.89). (e) ChIP against H3K27acetyl on ED-18 (n = 5, P = 0.58); IgG ChIP (n = 4, P = 0.7). (f) ChIP against H3K27me3 on day 10 posthatch (n = 6, *P = 0.02); IgG ChIP (n = 3, P = 0.24). (g) ChIP against EZH2 on day 10 posthatch (n = 6, P = 0.46); IgG ChIP (n = 3, P = 0.28). Data are presented as mean ± SEM. Significant effect between groups is indicated by *0.01 < P < 0.05, **0.001 < P < 0.01, ***P < 0.001. IL6 IL6 intron 1, CTRL, control
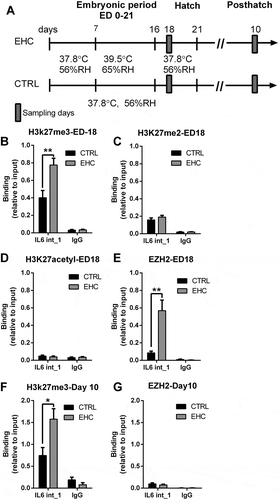
To study the long-term histone-modification effects, we measured H3K27me3 on IL6 intron 1 on day 10 posthatch, 2 weeks after conditioning. We found a long-lasting effect. H3K27me3 binding to IL6 intron 1 remained higher in the EHC group (t10 = 2.76, P = 0.02). The control IgG binding did not differ between groups (t4 = 1.37, P = 0.25, )). Although histone methylation was apparent, later in life, on day 10 posthatch, EZH2 binding to IL6 intron 1 did not differ between EHC chicks and controls (t5 = 1.21, P = 0.28; )). These results indicate that the EZH2 activity was a result of the thermal manipulation and despite the fact it was not apparent later in life, the H3k27me3 was maintained.
EZH2 expression during EHC is regulated by miR26a
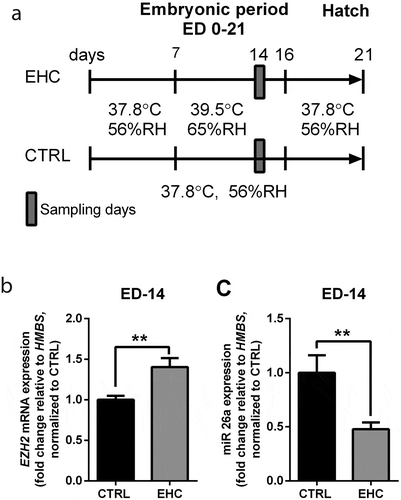
To further understand the multi-level epigenetic regulation of IL6 inflammatory expression during cross-tolerance establishment, we studied the regulation of EZH2 during conditioning. First, we measured EZH2 mRNA expression on ED-14, during conditioning ()) and found it to present a 50% increase in the EHC group (t17 = 3.24, P = 0.005; )). Previous study published that EZH2 expression is regulated by miR-26a [Citation45]. Looking into chicken (Gallus-Gallus) miR 26-a target genes, we found a target score of 90 with EZH2 3ʹ UTR, according to miRDB target prediction database (http://www.mirdb.org/). Therefore, we measured the expression of miR-26a, in the midbrain on ED-14, during conditioning (results are displayed as fold change relative to HMBS and normalized to CTRL group). We found a 50% reduction in miR-26a expression in the EHC group t17 = 3.16, P = 0.006; )), indicating a possible another level of epigenetic regulation of IL6 expression due to EHC.
Figure 4. EZH2 expression during EHC is regulated by miR26a. (a) Experimental scheme. Midbrain samples were taken during the embryonic period (embryonic day ED-14). (b) EZH2 mRNA expression was measured in the midbrain during ED-14, results are presented as fold change relative to HMBS expression and normalized to the average of CTRL group (nCTRL = 9, nEHC = 10, **P = 0.005). (c) miR26a mRNA expression was measured in the midbrain during ED-14, results are presented as fold change relative to HMBS expression and normalized to the average of CTRL group (nCTRL = 9, nEHC = 10, **P = 0.006). Data are presented as mean ± SEM. Significant effect between groups is indicated by *0.01 < P < 0.05, **0.001 < P < 0.01, ***P < 0.001, CTRL, control
Discussion
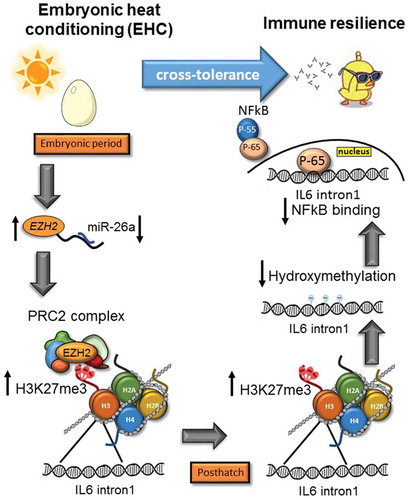
Early-life stressors and changes in the environment have long-term effects on organisms’ functioning later in life [Citation1]. These long-term effects are thought to be regulated by epigenetic mechanisms. In this work, we suggest a role for multilevel epigenetic regulation in the establishment of stress cross-tolerance. Cross-tolerance is a beneficial outcome of resilience of a completely different nature to another, unconditioned, stressor [Citation8]. To demonstrate the epigenetic role in the establishment of stress cross-tolerance, we tested the long-term effects of EHC on both antibody abundance, and IL6 inflammatory expression in the hypothalamus. We proposed that IL6 inflammatory expression depends on different layers of epigenetic alterations that occur during the embryonic thermal manipulation and persist throughout life (illustrated by the model in ).
Figure 5. Stress cross-tolerance: EHC induces multi-level, epigenetic mechanisms leading to reduced expression of IL6 and inflammation. From the embryonic period and conditioning to posthatch chick life. During the embryonic period: downregulation of miR-26a and increased EZH2 mRNA expression, followed by increased binding of PRC2 complex and H3K27me3 by EZH2 on IL6 intron1. H3K27me3 persisted to posthatch life, along with reduced IL6 intron1 hydroxymethylation and reduced the NFkB binding to this enhancer. NFkB is presented as two sub-units P55 (regulatory) and P65 (active). In order to activate transcription P65 separates from P55 and moves to the nucleus
From plants to humans, heat acclimation has been shown to protect against different stressors [Citation8,Citation46–51]. In relation to heat–inflammation-related networks, numerous findings point to the hypothalamus as a potential convergent site [Citation14,Citation25,Citation52,Citation53]. Early-life exposure to thermal stress has been shown to induce resilience to thermal stress [Citation54–59]. Furthermore, heat manipulation during early life has been shown to induce either resilience or vulnerability, depending on the intensity of the stressor. The ‘switch’ between resilience and vulnerability depends on epigenetic regulation of common stress-related genes, such as those encoding corticotropin-releasing hormone [Citation6,Citation33,Citation35] and heat-shock protein 70 [Citation34].
To elaborate on our understanding of the induction of stress responses, in this manuscript we suggest that the phenomenon of stress cross-tolerance is also regulated by epigenetic mechanisms. First, we studied immune-system activity in EHC chicks. Since stress resilience and vulnerability interact with the immune system [Citation60], we wanted to determine, before exploring the inflammatory reaction, whether the immune response is induced by EHC. We immunized the chicks with BSA and measured the concentration of circulating BSA antibodies a week later. We found that the immune system is not only intact, but much more reactive to the BSA immunization, with increased antibody concentration in the EHC chicks compared to controls.
To test the concept that inflammatory resilience, induced by EHC, is regulated by multilevel epigenetic marks, we concentrated on studying the regulation of IL6 inflammatory activation of transcription. IL6 expression can be either pro- or anti-inflammatory. Classic anti-inflammatory IL6 expression, leading to IL6 protein secretion and activation of membrane-bound IL6 receptors, is specific to a limited cell population. However, IL6 inflammatory activation and transcription, achieved by soluble IL6 receptors and triggered by the TLR4/MyD88-dependent activation of NFκB, can affect a broad cell population [Citation28]. To study the regulation of IL6 inflammatory expression, we used LPS from Salmonella, which activates the TLR4 inflammatory pathway. Moreover, the TLR4/MyD88 pathway was chosen to prove inflammatory resilience in the hypothalamus because it has been previously shown to be induced during hypothalamic inflammation [Citation24,Citation61,Citation62].
We performed an LPS challenge on day 10 posthatch by direct ICV injection of LPS into the brain. This approach was used so that the LPS effect would be directed to the hypothalamus, rather than to peripheral inflammation. Moreover, sickness behaviour in birds was much more pronounced following ICV administration of LPS, compared with IP injection [Citation63]. IL6 expression in the hypothalamus increased 6 h into the LPS challenge, corresponding with the same phenotype observed during ischaemic and high-fat diet-induced hypothalamic inflammation [Citation24,Citation61]. It should be stressed that EHC was indeed effective and significantly reduced LPS-dependent IL6 expression throughout life.
Since the embryonic heat treatment had a lifelong effect on IL6 expression, we looked for potential areas along this gene that might be affected by epigenetic regulation. Methylome analysis of IL6 revealed that its first intron has significantly less 5hmC in EHC chicks than in controls. Furthermore, intron 1 is enriched with binding sites for NFκB, which is known to regulate IL6 expression [Citation64,Citation65]. To confirm the regulatory role of IL6 intron 1 and its relationship with NFκB, we performed a luciferase assay which, indeed, demonstrated that this segment induces the expression of IL6 in the presence of NFκB. Methylation of the IL6 promoter has been associated with rheumatoid arthritis, lifestyle risk factors, and obesity [Citation30,Citation32,Citation66]. Moreover, pharmacological inhibition of IL6 expression by intron methylation has been shown to reduce stress and increase synaptic plasticity in the nucleus accumbens [Citation67]. DNA hydroxymethylation is the first step in a series of oxidations of methylated cytosines, performed by TET family enzymes and leading to active demethylation [Citation42]. It should be noted that although we found changes in IL6 intron 1 hydroxymethylation, we did not find changes in its methylation. These findings could be attributed to the fact that DNA methylation is much more abundant than hydroxymethylation, which spans less than 1% of total DNA [Citation68]. Interestingly, we found high %5hmC in IL6 intron 1, in both control (4.6%) and EHC (2.5%) groups, indicating possible dynamic changes in the availability of CpG methylation. In addition, hydroxymethylation is only the first step in demethylation, followed by 5hmC transformation to 5-formylcytosine (5fC), 5-carboxylcytosine (5-caC) and base excision repair to restore nonmethylated cytosines [Citation68]. Therefore, it is possible that while the control group presented higher %5hmC, final demethylation by base excision repair had not occurred at the time that we measured the methylation.
Since CpG methylation influences transcriptional activity [Citation69], we assessed whether the reduction in IL6 intron 1 hydroxymethylation makes it less accessible to NFkB. We used ChIP with NFkB antibody and found that not only was NFkB baseline binding reduced, but also, when challenged with LPS, there was less binding of NFkB to IL6 intron 1. Therefore, we suggest that these changes in CpG hydroxymethylation in this genetic region are important for NFkB binding and IL6 inflammatory expression.
Our next step was to study additional epigenetic modifications, such as histone post-translational modifications of IL6 intron 1. As a proof of concept, we evaluated the role of H3K27 methylation, because thermal conditioning of chicks posthatch has been shown to induce H3K27 methylation. [Citation36,Citation38,Citation39]. Moreover, H3K27 methylation is also associated with macrophage polarization and immune activation [Citation43]. Considering the convergence of thermal conditioning and reduced inflammation, we performed ChIP with H3K27me3 antibody and found increased binding to IL6 intron 1 in EHC vs. control embryos, as well as in chicks, later in life. To test the specificity of H3K27me3 in the establishment and maintenance of cross-tolerance, we assessed two additional modifications using the same samples, i.e., H3K27me2 and H3K27acetyl, and found no difference in these modifications between groups. H3K27me3’s long-term effect of repressive methylation further corroborates the role of IL6 intron 1 regulation, but also displays another epigenetic layer affecting IL6 expression. These results can also contribute to the cross-tolerance effect of EHC, since it is the thermal manipulation that enabled H3K27 methylation of IL6 intron 1 in the midbrain.
Since H3K27 is methylated by the EZH2, which is part of the PRC2 complex, to elaborate on the role of this modification, we also performed ChIP with EZH2 antibody during the embryonic period and later in life. We found increased EZH2 binding to IL6 intron 1 in the EHC embryonic period, but not later in life, indicating that H3K27 methylation occurs during the embryonic period, induced by the thermal manipulation, and persists long after the embryonic period. In previous studies, EZH2 inhibition has been shown to trigger NFκB activation and inflammation [Citation70,Citation71]. Moreover, inhibition of H3K27me3 by hyperthermia, as well as EZH2 knockdown, promoted an increase in IL6 expression and seizure susceptibility in hyperthermia-prone rats [Citation31]. We suggest that it is the thermal induction of EZH2 during the embryonic period that causes the trimethylation of IL6 intron 1, contributing to reduced inflammatory expression later in life.
We chose to explore the possible epigenetic regulation of EZH2 expression as a follow up to the heat- and EZH2-dependent methylation that occurs in the midbrain during EHC. EZH2 expression has been shown to be downregulated by miR-26a in gastric cancer [Citation45], and therefore we wanted to see if such regulation occurs during EHC and might affect EZH2 in its regulation of inflammatory cross-tolerance. Indeed, we found an increased EZH2 expression along with decreased miR-26a expression during conditioning, indicating another level of EHC-induced epigenetic regulation contributing to the reduced expression of IL6 intron 1.
Taken together, these results led us to suggest a simple model presenting different layers of EHC-induced epigenetic mechanisms. During the embryonic period, cross-tolerance is manifested by reduced expression of miR-26a and increased expression of EZH2, leading to an increase in H3K27me3 at IL6 intron 1 by the PRC2 complex. H3K27me3 persists later in life. Similarly, 10 days posthatch, there is also a reduction in hydroxymethylation at IL6 intron 1, in parallel with reduced binding of NFkB (). Although the flow of events is suggested with arrows in our model, the order of the epigenetic modifications leading to stress cross-tolerance might differ.
Materials and methods
Experimental model and subject details
All procedures in this study were approved by the Volcani Center animal experimentation ethics committee (licence no 800/18 IL_14293) and performed according to the guidelines of the European Community Council.
EHC
EHC was performed as previously described [Citation72]. Briefly, we used fertile Cobb strain broiler (Gallus domesticus) eggs from one breeder flock of hens. The eggs were randomly divided into two incubation treatments: control – eggs were incubated at 37.8°C and 56% relative humidity throughout the 21 days of the incubation period; EHC – eggs were incubated at 39.5°C and 65% relative humidity for 12 h/day on ED-7 to ED-16. The eggs were incubated in two type 65Hs automatic incubators (Masalles, Barcelona, Spain).
Bird housing
Hatched chicks were randomly divided into 8 chicks per pen in climate-controlled rooms at 32°C under a 22/2 h cycle of artificial illumination with ad libitum access to food and water. On day 7 posthatch, the temperature was changed to 30°C and chicks were further divided into 5 chicks per pen. On day 10 posthatch, the chicks were subjected to either heat or LPS challenge. Previous experiment did not present sexual diversity, so we did not distinguish between sexes in this work.
BSA immune challenge and antibody response
Intramuscular (IM) immunization was performed as previously described [Citation73], with minor changes. Briefly, 14-day old chicks were immunized with bovine serum albumin (BSA; Sigma Aldrich, Rehovot, Israel) emulsified in Freund’s complete adjuvant (CFA; Sigma Aldrich, Rehovot, Israel). Antigen was dissolved in sterile PBS (2 mg/ml) and the solution was then emulsified in an equal volume of CFA. As controls, PBS emulsified in an equal volume of CFA, was injected and designated as CFA group. Each injection (0.5 ml containing 250 μg antigen) was delivered into four IM sites: left and right shank and breast muscles. Blood samples, from the wing’s vein of BSA and CFA chicks, were obtained 7 days later. Serum samples were collected from centrifuged clotted blood and stored at −20°C until used.
BSA antibodies in chicks’ sera were detected and quantified using ‘Chicken Bovine Serum Albumin (BSA) ELISA Kit’ according to the manufacturer’s protocols (MyBioSource, San Diego, CA, USA).
ICV injection
Injection was performed according to Johnston and Rose (2001) [Citation74] and as performed previously in our laboratory [Citation33–36], with slight modifications. In brief, a 28-gauge needle was connected via PE20 tubing to a Hamilton microsyringe. The injection was into the third ventricle area, with coordinates A = 8.4 mm from the ear position in the middle of the skull (coordinates according to Kunzel and Masson, 1988). The injection needle was fitted with a stop that limited penetration to a maximum of 9 mm. The injection location was verified in preliminary experiments by 2% Evans-blue injection dissolved in saline. The free-hand injection to the third ventricle is facile and precise because the location is into the Sutura sagittalis. This procedure does not require anaesthetic and is routinely performed without administration of analgesics (Johnston and Rose, 2001). The chicks were not overly distressed by the injection, and their behaviour seemed normal as they immediately after injection walk, eat, do not make any Startled squawks and their Tb does not change. LPS (0.3 µg; Sigma Aldrich, Rehovot, Israel) originated from salmonella and its vehicle solution (0.9% NaCl, designated as saline) was ICV injected. The chicks’ tb was measured and they were sacrificed by decapitation 0, 6 hours and 24 hours into the LPS challenge.
Tissue collection
For total RNA and DNA isolation, the brain area matching the anterior hypothalamus was dissected as previously described [Citation33–35]. In brief, the skull was cut along the Lambda Suture and the sagittal suture, the brain was removed from the skull, it was set on a small plastic cube with the lateral side upwards; then, the anterior hypothalamus was dissected. The boundaries of the dissections were determined by the optic chiasma and the clear boundaries of the hypothalamus (A 8–10 L 0–1.4 on both hemispheres coordinates according to Kunzel and Masson, 1988 [Citation75]). The dissected anterior hypothalamus was immediately immersed in RNALater pH 5.2 (5.3 M Ammonium sulphate, 25 mM Sodium citrate, 20 mM EDTA).
To study hypothalamic development during the embryonic period, midbrains were isolated on embryonic day (ED) 14 and 18, briefly: the embryo was extracted from the egg into cold phosphate-buffered saline (PBS; Sigma Aldrich, Rehovot, Israel), the head was removed using scissors and a vertical incision was cut along the skull, including the beak. Using forceps, the skull was peeled of the brain, and the area between the optical chiasm and the cerebellum, not including the optic lobe was removed and designated as midbrain. Isolated midbrains and anterior hypothalamus used for ChIP assays were immediately frozen in liquid nitrogen and stored at −80°C.
Total RNA isolation and qPCR
Total RNA was isolated using TRI Reagent (Molecular Research Center, Cincinnati, OH, USA) according to the manufacturer’s instructions. Hypothalamic RNA – 0.5 µg was reverse transcribed to single-stranded cDNA by Super Script II Reverse Transcriptase and oligo (dT) plus random primers (Thermo-Fisher Scientific, Waltham, MA, USA). qPCR was performed with 10 ng cDNA in a StepOnePlus Real Time PCR System (Applied Biosystems) with PerfeCta SYBR Green FastMix, ROX (Quanta BioSciences, Gaithersburg, MD, USA). Dissociation curves were analysed following each real-time PCR to confirm the presence of only one product and the absence of primer dimer formation. The threshold cycle number (Ct) for each tested gene (X) was used to quantify the relative abundance of that gene using the formula 2 (Ct gene X – Ct standard). HMBS was used as the standard for mRNA expression. The primers used for real-time PCR were as follows (5ʹ→3ʹ): HMBS, F-CGTTTGGAGGGTGGCTGTAG, R-TGTCAAGTACAACTGGCCATCTTT [Citation33]; IL6 (exon 4) F-TTTATGGAGAAGACCGTGAGG, R-TGTGGCAGATTGGTAACAGAG [Citation76]; EZH2 F‐CACTGAACAGCAGCTTCCAGG, R‐AAGAATGCAGGCTTTGCTCC [Citation38]
CpG specific DNA methylation and hydroxymethylation
DNA was isolated together with RNA from the same sample by TRI Reagent according to the manufacturer’s protocol (Molecular Research Center). The purified DNA (100 ng of each sample) was oxidized using KRuO4 and further processed for bisulfite modification using the Imprint DNA Modification Kit according to the manufacturer’s instructions (Sigma). We chose to evaluate the DNA methylation status of IL6 region −418 → +520, relative to the transcription start site (TSS), because it was enriched with NFκB-binding motifs and CpG sites. IL6 was sequenced in three consecutive amplicons according to NCBI Reference Sequence NM_204628.1
Primers for this region were designed as follows: (5ʹ→3ʹ): IL6-1, F- TATATTAATGGATAAGATGTATAAAATATA, R-AAAAATCCAACATAAAAACA; IL6-2, F- GTGTTTTTATGTTGGATTTTTT, R-CCTCCAACCCAACCTCTC; IL6-3, F-AGAGGTTGGGCTGGAGG, R-TCACCTTAAACAAATTAAAATTATTC.
Amplified samples were sent for next-generation sequencing by the Crown Institute for Genomics at the Nancy and Stephen Grand Israel National Center for Personalized Medicine, Weizmann Institute of Science. Sequencing libraries were prepared using the INCPM DNA-Seq protocol (ChIP-Seq protocol). Long reads were sequenced on an Illumina MiSeq machine. Trimming of Illumina adapters, as well as quality trimming, were performed with Trimmomatic, using the following options: -phred33, TruSeq3-PE.fa:2:30:10, SLIDINGWINDOW:4:15, LEADING:10, TRAILING:10 and MINLEN:36. Reads were mapped to the provided amplicon sequences using Bismark (version 0.13.1) using the options – bowtie2 and – non_directional. Methylation calls were extracted using the MethylKit package (version 1.6.1). The Methylkit command processBismarkAln was used to read the aligned files using the option nolap set to TRUE.
Different barcodes were used to distinguish between different chicks and oxidative bisulphite or bisulphite conversion of samples. A total of 95 CpGs were measured in 10 chicks. Different amplicons from different barcodes produced average coverage of 2140 ± 43 (SEM) per CpG.
The average %C/T for each CpG per animal was calculated from the analysis, followed by the average of all five animals per group. For %5mC we used %C/T of the oxidative bisulphite amplicons. For %5hmC we subtracted %C/T of the oxidative bisulphite amplicons from the %C/T of the bisulphite corresponding amplicons.
CpGs were distributed according to the genetic region (promoter, exon, and intron), total CpG methylation/hydroxymethylation per region between groups was analysed using Two-way ANOVA with Sidak’s multiple comparisons between CTRL and EHC groups. CpG-specific methylation of IL6 intron1 was analysed using Two-way ANOVA repeated measures with Sidak’s multiple comparisons between CTRL and EHC groups.
Plasmid constructs
IL6 intron 1 construct was generated by PCR amplification of the following fragment (5ʹ→3ʹ): 266–383 bp relative to the transcription start site F-ATAGAGCTCCAGGACGAGGTGGGTACG, R-ATATCTCGAGGTTCTCGCACACGGTGAAC. Forward primers included restriction site SacI and reverse primers were constructed with restriction site XhoI. PCR products were cloned into pGL3-basic vector and digested with SacI and XhoI restriction enzymes upstream of the firefly luciferase reporter gene.
Luciferase reporter assays
pGL3*/9-basic vector containing the PCR-amplified IL6 intron 1 fragment was transfected into hek293-htlr4-md-cd14 cells (Invivogen, San Diego, CA, USA) grown in 24-well plates using lipofectamine 2000 (Invitrogen; 500 ng vector/well), according to the manufacturer's protocol, and as previously reported 34. To monitor transfection efficiency, the IL6 reporter was co-transfected with 10 ng of pRL-TK vector (Promega); 12 h later, the medium was replaced and cells were incubated under normal (37°C) conditions for 24 h. Thereafter, the cells were washed with phosphate-buffered saline and lysed in Passive Lysis Buffer (Promega). Luciferase activity in the cell lysates was determined with a dual-luciferase reporter assay system (Promega) according to the manufacturer’s instructions and read in a VictorLight 1420 luminescence counter (PerkinElmer, Waltham, MA, USA). The luciferase reporter signal of the analysed fragment was normalized to that of empty pGL3-basic vector.
ChIP assays
ChIP assays were performed using the Magna Chip A/G (Merck, Millipore, Sigma, Burlington, MA, USA) Kit, according to the manufacturer’s protocol, with antibodies directed against: NFκB p65 (4 µg/sample; Abcam, Cambridge, UK), H3K27me3 (3 µg/sample; Cell signalling, Temecula, CA, USA), H3K27me2 (3 µg/sample; Cell signalling, Temecula, CA, USA), H3K27acetyl (3 µg/sample; Cell signalling, Temecula, CA, USA), EZH2 (3 µg/sample; Merck Millipore, Sigma, Burlington, MA, USA). Normal rabbit IgG (3 µg/sample; Cell signalling, Temecula, CA, USA) was used for mock immunoprecipitation (background). DNA was isolated from each immunoprecipitate and subjected to q-PCR using the following primers (5ʹ→3ʹ): IL6 intron 1 F-AAAATGACTTCATGCCTCTGC, R-GGCATCCCTGAACGTGTATT. Results were normalized to input samples that were not precipitated.
miR26-a relative quantification
miR26-a relative quantification in the midbrain of chick embryos on ED-14 was measured using the has-mir-26a Real-time RT-PCR Kit according to the manufacturer’s protocols (Cohesion Bioscience, London, UK).
Quantification and statistical analysis
Statistical analysis was performed using GraphPad Prism 6 software (GraphPad Software, San Diego, CA, USA). All data were examined for normality by the goodness-of-fit test and by the Bartlett test for variance equality. Means of two groups were compared by two-tailed unpaired t-test. Comparisons of LPS and conditioning effects were analysed by two-way ANOVA followed by Sidak’s or LSD multiple-comparison tests. Figure data are presented as mean ± SEM, with exact n and P values reported in the legends. The Results section provides the F and t-test expressions.
Acknowledgments
We are grateful to the Volcani Center’s chicken farm staff for their dedicated work. We would also like to thank Dr. Yuval Cinnamon for his instruction and guidance regarding the extraction of chick embryo midbrain. Additional thanks to the staff of the Crown Institute for Genomics of the Nancy and Stephen Grand Israel National Center for Personalized Medicine, Weizmann Institute of Science, Israel, and especially to Gilgi Friedlander for her skilled bioinformatics work.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Taylor SE. Mechanisms linking early life stress to adult health outcomes. Proc Natl Acad Sci U S A. 2010;107(19):8507–8512.
- Labonté B, Suderman M, Maussion G, et al. Genome-wide epigenetic regulation by early-life trauma. Arch Gen Psychiatry. 2012;69(7):722–731.
- Franklin TB, Saab BJ, Mansuy IM. Neural mechanisms of stress resilience and vulnerability. Neuron. 2012;75:747–761.
- Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nat Neurosci. 2004;7:841–846.
- Chen T, Liu HX, Yan HY, et al. Developmental origins of inflammatory and immune diseases. Mol Hum Reprod. 2016;22:558–565.
- Cramer T, Kisliouk T, Yeshurun S, et al. The balance between stress resilience and vulnerability is regulated by corticotropin-releasing hormone during the critical postnatal period for sensory development. Dev Neurobiol. 2015;75:842–853.
- Horowitz M, Umschweif G, Yacobi A, et al. Molecular programs induced by heat acclimation confer neuroprotection against TBI and hypoxic insults via cross-tolerance mechanisms. Front Neurosci. 2015;9:256.
- Horowitz M. Heat acclimation-mediated cross-tolerance: origins in within-life epigenetics? Front Physiol. 2017;8:548.
- Barker D. The fetal and infant origins of adult disease The womb may be more important than the home. BMJ. 1990;301:1111.
- Hales C, Barker D. Diabetologia Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis*. Diabetologia. 1992;35(7):595–601.
- Goodfellow FT, Tesla B, Simchick G, et al. Zika virus induced mortality and microcephaly in chicken embryos. Stem Cells Dev. 2016;25(22):1691–1697.
- Duman R, Ertekin T, Duman R, et al. Comparison of the efficacy of different hydrocortisone-induced cataract models in developing chick embryos. J Pharm Res Int. 2018;21:1–7.
- Haller S, Ametamey SM, Schibli R, et al. Investigation of the chick embryo as a potential alternative to the mouse for evaluation of radiopharmaceuticals. Nucl Med Biol. 2015;42(3):226–233.
- Morrison SF. Central control of body temperature. F1000 Res. 2016;5: F1000Faculty Rev-880.
- Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8:383–395.
- Soto-Tinoco E, Guerrero-Vargas NN, Buijs RM. Interaction between the hypothalamus and the immune system. Exp Physiol. 2016;101(12):1463–1471.
- Piestun Y, Halevy O, Yahav S. Thermal manipulations of broiler embryos—The effect on thermoregulation and development during embryogenesis1. Poult Sci. 2009;88(12):2677–2688.
- Valdearcos M, Xu AW, Koliwad SK. Hypothalamic inflammation in the control of metabolic function. Annu Rev Physiol. 2015;77(1):131–160.
- Jais A, Brüning JC. Hypothalamic inflammation in obesity and metabolic disease. J Clin Invest. 2017;127(1):24–32.
- André C, Guzman-quevedo O, Rey C, et al. Inhibiting microglia expansion prevents diet-induced hypothalamic and peripheral in fl ammation. Diabetes. 2017;66:908–919.
- Entringer S. Impact of stress and stress physiology during pregnancy on child metabolic function and obesity risk. Curr Opin Clin Nutr Metab Care. 2013;16:320–327.
- Arvin B, Nevile LF, Barone FC, et al. The role of inflammation and cytokines in brain injury. Neurosci Biobehav Rev. 1996;20:445–452.
- Emsley HCA, Smith CJ, Tyrrell PJ, et al. Inflammation in acute ischemic stroke and its relevance to stroke critical care. Neurocrit Care. 2008;9:125–138.
- Macrez R, Ali C, Toutirais O, et al. Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol. 2011;10:471–480.
- Chao C-M, Cheng B-C, Chen C-Y, et al. Proteomic analysis of hypothalamic injury in heatstroke rats. Proteomics. 2015;15:1921–1934.
- Wang X, Stridh L, Li W, et al. Lipopolysaccharide sensitizes neonatal hypoxic-ischemic brain injury in a MyD88-dependent manner. J Immunol. 2009;183(11):7471–7477.
- Tang Y, Le W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol. 2016;53(2):1181–1194.
- Wolf J, Rose-John S, Garbers C. Interleukin-6 and its receptors: a highly regulated and dynamic system. Cytokine. 2014;70(1):11–20.
- Poizat G, Alexandre C, Al Rifai S, et al. Maternal resistin predisposes offspring to hypothalamic inflammation and body weight gain. PLoS One 2019;14.
- Nile CJ, Read RC, Akil M, et al. Methylation status of a single CpG site in the IL6 promoter is related to IL6 messenger RNA levels and rheumatoid arthritis. Arthritis Rheum. 2008;58:2686–2693.
- Wang Z, Zhang Y, Fang J, et al. Decreased methylation level of H3K27me3 increases seizure susceptibility. Mol Neurobiol. 2017;54:7343–7352.
- Na YK, Hong HS, Lee WK, et al. Increased methylation of interleukin 6 gene is associated with obesity in Korean women. Mol Cells. 2015;38:452–456.
- Cramer T, Rosenberg T, Kisliouk T, et al. Early-life epigenetic changes along the corticotropin-releasing hormone (CRH) gene influence resilience or vulnerability to heat stress later in life. Mol Psychiatry 2018;24:1013–26.
- Kisliouk T, Cramer T, Meiri N. Methyl CpG level at distal part of heat-shock protein promoter HSP70 exhibits epigenetic memory for heat stress by modulating recruitment of POU2F1-associated nucleosome-remodeling deacetylase (NuRD) complex. J Neurochem. 2017;141:358–372.
- Cramer T, Rosenberg T, Kisliouk T, et al. PARP inhibitor affects long-term heat-stress response via changes in DNA methylation. Neuroscience. 2019;399:65–76.
- Kisliouk T, Yosefi S, Meiri N. MiR-138 inhibits EZH2 methyltransferase expression and methylation of histone H3 at lysine 27, and affects thermotolerance acquisition. Eur J Neurosci. 2011;33:224–235.
- Yossifoff M, Kisliouk T, Meiri N. Dynamic changes in DNA methylation during thermal control establishment affect CREB binding to the brain-derived neurotrophic factor promoter. Eur J Neurosci. 2008;28:2267–2277.
- Kisliouk T, Meiri N. A critical role for dynamic changes in histone H3 methylation at the Bdnf promoter during postnatal thermotolerance acquisition. Eur J Neurosci. 2009;30:1909–1922.
- Kisliouk T, Ziv M, Meiri N. Epigenetic control of translation regulation: alterations in histone H3 lysine 9 post-translation modifications are correlated with the expression of the translation initiation factor 2B (Eif2b5) during thermal control establishment. Dev Neurobiol. 2009;70:NA–NA.
- Kisliouk T, Cramer T, Meiri N. Heat stress attenuates new cell generation in the hypothalamus: A role for miR-138. Neuroscience. 2014;277:624–636.
- Yahav S, McMurtry JP. Thermotolerance acquisition in broiler chickens by temperature conditioning early in life — The effect of timing and ambient temperature. Poult Sci. 2001;80:1662–16666.
- Kohli RM, Zhang Y. TET enzymes, TDG and the dynamics of DNA demethylation. Nature. 2013;502:472–479.
- Liu P, Wang H, Li X, et al. a -ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat Immunol. 2017;18:985–974.
- Viré E, Brenner C, Deplus R, et al. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2005;439:871.
- Deng M, Zhang R, He Z, et al. TET-mediated sequestration of miR-26 drives EZH2 expression and gastric carcinogenesis. Cancer Res. 2017;77:6069–6082.
- Hossain MA, Li Z-G, Hoque TS, et al. Heat or cold priming-induced cross-tolerance to abiotic stresses in plants: key regulators and possible mechanisms. Protoplasma. 2018;255:399–412.
- Assayag M, Saada A, Gerstenblith G, et al. Mitochondrial performance in heat acclimation—a lesson from ischemia/reperfusion and calcium overload insults in the heart. Am J Physiol Integr Comp Physiol. 2012;303:R870–81.
- Umschweif G, Shabashov D, Alexandrovich AG, et al. Neuroprotection after traumatic brain injury in heat-acclimated mice involves induced neurogenesis and activation of angiotensin receptor type 2 signaling. J Cereb Blood Flow Metab. 2014;34:1381–1390.
- Umschwief G, Shein NA, Alexandrovich AG, et al. Heat acclimation provides sustained improvement in functional recovery and attenuates apoptosis after traumatic brain injury. J Cereb Blood Flow Metab. 2010;30:616–627.
- Pollak A, Merin G, Horowitz M, et al. Heat acclimatization protects the left ventricle from increased diastolic chamber stiffness immediately after coronary artery bypass surgery: a lesson from 30 years of studies on heat acclimation mediated cross tolerance. Front Physiol. 2017;8:1022.
- Shein NA, Grigoriadis N, Alexandrovich AG, et al. Differential neuroprotective properties of endogenous and exogenous erythropoietin in a mouse model of traumatic brain injury. J Neurotrauma. 2008;25:112–123.
- Klett H, Rodriguez-Fernandez M, Dineen S, et al. Modeling the inflammatory response in the hypothalamus ensuing heat stroke: iterative cycle of model calibration, identifiability analysis, experimental design and data collection. Math Biosci. 2015;260:35–46.
- Audet GN, Dineen SM, Quinn CM, et al. Altered hypothalamic inflammatory gene expression correlates with heat stroke severity in a conscious rodent model. Brain Res. 2016;1637:81–90.
- Tzschentke B. Attainment of thermoregulation as affected by environmental factors. Poult Sci. 2007;86:1025–1036.
- Tzschentke B, Halle I. Influence of temperature stimulation during the last 4 days of incubation on secondary sex ratio and later performance in male and female broiler chicks. Br Poult Sci. 2009;50:634–640.
- Nassar M, Halle I, Plagemann A, et al. Detection of long-term influence of prenatal temperature stimulation on hypothalamic type-II iodothyronine deiodinase in juvenile female broiler chickens using a novel immunohistochemical amplification protocol. Comp Biochem Physiol Part A Mol Integr Physiol. 2015;179:120–124.
- Tona K, Onagbesan O, Bruggeman V, et al. Effects of heat conditioning at d 16 to 18 of incubation or during early broiler rearing on embryo physiology, post-hatch growth performance and heat tolerance. Eur Poult Sci. 2008;72:75–83.
- Loyau T, Hennequet-antier C, Coustham V, et al. Thermal manipulation of the chicken embryo triggers differential gene expression in response to a later heat challenge. BMC Genomics. 2016;17:1–15.
- Loyau T, Bedrani L, Berri C, et al. Cyclic variations in incubation conditions induce adaptive responses to later heat exposure in chickens: a review. Animal. 2014;9:76–85.
- Ménard C, Pfau ML, Hodes GE, et al. Immune and neuroendocrine mechanisms of stress vulnerability and resilience. Neuropsychopharmacology. 2017;42:62–80.
- Roesslein M, Froehlich C, Jans F, et al. Hypothalamic inflammation in the control of metabolic function. Comp Biochem Physiol - A Mol Integr Physiol. 2015;9:475–483.
- Ropelle ER, Flores MB, Cintra DE, et al. IL-6 and IL-10 anti-inflammatory activity links exercise to hypothalamic insulin and leptin sensitivity through IKKβ and ER stress inhibition. PLoS Biol. 2010;8:31–32.
- Johnson RW, Curtis SE, Dantzer R, et al. Sickness behavior in birds caused by peripheral or central injection of endotoxin. Physiol Behav. 1993;53:343–348.
- Matsusaka T, Fujikawa K, Nishio Y, et al. Transcription factors NF-IL6 and NF-kappa B synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc Natl Acad Sci U S A. 1993;90:10193–10197.
- Ryffel B, Schnyder B, Quesniaux J, et al. IL-1R1/MyD88 signaling dependent inflammation is TLR4/MyD88 and cigarette smoke-induced pulmonary and isabelle couillin. J Immunol Ref. 2019;180:1169–1178.
- Zhang FF, Santella RM, Wolff M, et al. White blood cell global methylation and IL-6 promoter methylation in association with diet and lifestyle risk factors in a cancer-free population. Epigenetics. 2012;7:606–614.
- Wang J, Hodes GE, Zhang H, et al. Epigenetic modulation of inflammation and synaptic plasticity promotes resilience against stress in mice. Nat Commun. 2018;9(1):477.
- Shi DQ, Ali I, Tang J, et al. New insights into 5hmC DNA modification: generation, distribution and function. Front Genet. 2017;8:1–11.
- Jang HS, Shin WJ, Lee JE, et al. CpG and non-CpG methylation in epigenetic gene regulation and brain function. Genes (Basel). 2017;8:2–20.
- Barroso M, Kao D, Blom HJ, et al. S-adenosylhomocysteine induces inflammation through NFkB: A possible role for EZH2 in endothelial cell activation. Biochim Biophys Acta. 2016;1862:82–92.
- Zhang X, Wang Y, Yuan J, et al. Macrophage/microglial Ezh2 facilitates autoimmune inflammation through inhibition of Socs3. J Exp Med. 2018;215:1365–1382.
- Piestun Y, Yahav S, Halevy O. Thermal manipulation during embryogenesis affects myoblast proliferation and skeletal muscle growth in meat-type chickens. Poult Sci. 2015;94:2528–2536.
- Bar-Shira E, Sklan D, Friedman A. Establishment of immune competence in the avian GALT during the immediate post-hatch period. Dev Comp Immunol. 2003;27:147–157.
- Johnston ANB, Rose SPR. Memory consolidation in day-old chicks requires BDNF but not NGF or NT-3; an antisense study. Mol Brain Res. 2001;88(1–2):26–36.
- Kuenzel WJ. A stereotaxic atlas of the brain of the chick (Gallus domesticus)/. Baltimore: Johns Hopkins University Press; 1988.
- Tan J, Liu S, Guo Y, et al. Dietary l-arginine supplementation attenuates lipopolysaccharide-induced inflammatory response in broiler chickens. Br J Nutr. 2014;111:1394–1404.
