ABSTRACT
Music-listening and performance have been shown to affect human gene expression. In order to further elucidate the biological basis of the effects of music on the human body, we studied the effects of music-listening on gene regulation by sequencing microRNAs of the listeners (Music Group) and their controls (Control Group) without music exposure. We identified upregulation of six microRNAs (hsa-miR-132-3p, hsa-miR-361-5p, hsa-miR-421, hsa-miR-23a-3p, hsa-miR-23b-3p, hsa-miR-25-3p) and downregulation of two microRNAs (hsa-miR-378a-3p, hsa-miR-16-2-3p) in Music Group with high musical aptitude. Some upregulated microRNAs were reported to be responsive to neuronal activity (miR-132, miR-23a, miR-23b) and modulators of neuronal plasticity, CNS myelination, and cognitive functions like long-term potentiation and memory. miR-132 plays a critical role in regulating TAU protein levels and is important for preventing tau protein aggregation that causes Alzheimer’s disease. miR-132 and DICER, upregulated after music-listening, protect dopaminergic neurons and are important for retaining striatal dopamine levels. Some of the transcriptional regulators (FOS, CREB1, JUN, EGR1, and BDNF) of the upregulated microRNAs were immediate early genes and top candidates associated with musical traits. BDNF and SNCA, co-expressed and upregulated in music-listening and music-performance, are both are activated by GATA2, which is associated with musical aptitude. Several miRNAs were associated with song-learning, singing, and seasonal plasticity networks in songbirds. We did not detect any significant changes in microRNA expressions associated with music education or low musical aptitude. Our data thereby show the importance of inherent musical aptitude for music appreciation and for eliciting the human microRNA response to music-listening.
Introduction
Music-listening involves sensory processing of acoustic stimuli by the auditory system followed by cognitive and emotional processing in a neural network that is widely distributed in the cerebral cortex, basal forebrain, and rostral brainstem [Citation1–4]. Studies of regional cerebral blood flow [Citation5–7] and dopamine receptor-ligand binding [Citation7] in vivo have demonstrated activation of the reward system and limbic system during music listening. Music enhances motor performance during exercise in healthy adults [Citation8], and rehabilitation of motor and cognitive deficits in neurological patients [Citation9]. However, the biological background of these effects has largely been unknown.
From a genetic perspective, music is an epigenetic modulator that may affect human genes and their regulation. The regulatory roles of microRNAs are well studied in the development and synaptic plasticity of the human nervous system [Citation10,Citation11]. MicroRNAs are also involved in inner ear development and the sensory functions of the ear [Citation12]. Studies on zebra finches have indicated that song-listening regulates both novel and known microRNAs with implications on neurogenesis and neuronal differentiation [Citation13]. The song-listening response in zebra finches showed a positive correlation in transcriptomic changes of the auditory forebrain and the peripheral blood [Citation14]. We have previously shown that genes activated by music-listening and music-performance are involved in dopaminergic neurotransmission, long-term potentiation, synaptic plasticity, and memory [Citation15,Citation16]. Here, we analysed the effects of music-listening on the microRNA transcriptome using high-throughput sequencing and bioinformatics methods. We provide an integrated perspective of how music-listening affects miRNA levels by comparing the same cohort of human subjects as in the transcriptome study [Citation15], and published transcriptomic changes in songbirds including regulatory network and pathway analyses.
Results
MicroRNA response to music-listening
At a very stringent FDR threshold of 5%, we observed statistically significant upregulation of hsa-miR-132-3p, hsa-miR-361-5p, hsa-miR-421, and downregulation of hsa-miR-378a-3p in the high COMB (combined score of three tests of musical aptitude, i.e., Seashore’s test for pitch and time perception and auditory structuring ability, see Methods) Music Group compared to the Control Group. At a permissive significance threshold (FDR<10%), we also observed upregulation of hsa-miR-23a-3p, hsa-miR-23b-3p, hsa-miR-25-3p and downregulation of hsa-miR-16-2-3p in the Music Group. DE statistics for microRNAs that exhibited significant differential expression in the high-COMB Music Group compared to the Control Group are given in . Genomic information for the DE microRNAs is provided in Table S1. No statistically significant changes in microRNA expressions were found in low-COMB, high-Edu or low-Edu Music Groups (see Methods) when compared to the Control Group.
Table 1. Differentially expressed microRNAs from the music-listening versus control comparison using analysis with DESeq2. The columns 1–3 show the DE microRNA, fold change and adjusted p-value for music-listening versus control study comparison
Putative functions of DE microRNAs
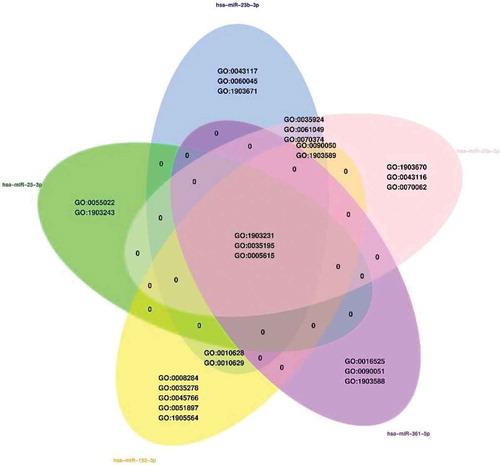
Based on the results yielded by the using TAM 2.0 tool for analysing differentially expressed microRNA (p-value<0.05), the upregulated microRNAs were found to be regulators of neuronal apoptosis (hsa-mir-23a, hsa-mir-23b), hormone-mediated signalling pathway (hsa-mir-23a, hsa-mir-23b, hsa-mir-132), neurotoxicity (hsa-mir-25, hsa-mir-132), cell death (hsa-mir-23a, hsa-mir-23b, hsa-mir-25), wound healing (hsa-mir-23a, hsa-mir-132), and glucose metabolism (hsa-mir-23a, hsa-mir-23b) (). TAM 2.0 analysis also revealed EGR1, GNRH1, USF1, and CREB1 as the top transcriptional regulators (p-value<0.05) of the upregulated microRNAs. For the downregulated microRNAs, angiogenesis (p-value = 0.00372; hsa-mir-16-2, hsa-mir-378a), cell proliferation (p-value = 0.00565; hsa-mir-16-2, hsa-mir-378a) and adiponectin signalling (p-value = 0.00755; hsa-mir-378a) were the top hits (). The comparative analysis wizard from TAM 2.0, which analyses the upregulated and downregulated microRNAs together, uncovered neuroblastoma as the topmost result. The validated TF–microRNA interactions from the TransmiR 2.0 database are provided in .
Table 2. TAM 2.0 upregulated miRNAs and their function
Table 3. TAM 2.0 downregulated miRNAs and their function
Target genes of DE microRNAs and their functions
Our goal with target gene finding was to understand the regulatory significance of the DE microRNAs in music-listening. We collected 147 validated human microRNA:target gene interactions for the DE microRNAs from the high-COMB Music Group from the miRTarBase Release 7.0 [Citation17]. Furthermore, the predicted target genes (N = 2496) from TargetScan Release 7.2 [Citation18] for these DE microRNAs were combined with the validated targets from the high-COMB Music Group. Notably, hsa-miR-132-3p and hsa-miR-25-3p showed validated targeting of CDKN1A and CDKN1B respectively (). These cell cycle inhibitors belong to the same family implying the activation of functions like cell proliferation and differentiation. Similar findings were made regarding songbird stimuli in songbirds [Citation13]. Furthermore, PTEN, which is a promoter of apoptotic mechanisms, is targeted by three of the upregulated microRNAs from this study (hsa-miR-23a-3p, hsa-miR-23b-3p, and hsa-miR-25-3p) [Citation17] suggesting neuroprotective mechanisms may be associated with music-listening (). Interestingly, this is consistent with the results of our microRNA specific enrichment analysis which indicated neuronal apoptosis as one of the functions regulated by the upregulated microRNAs.
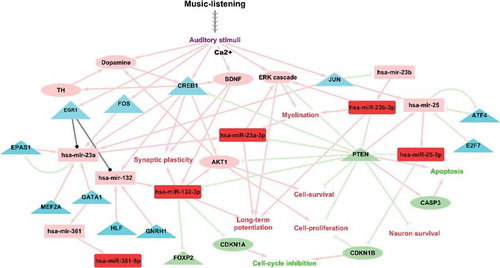
Figure 2. Schematic illustration of putative molecular mechanisms in music-listening based on findings from the current study. Mature microRNAs and microRNA transcripts are represented using rectangles, transcriptional regulators of the microRNAs using triangles and validated target genes using ellipses. The up-regulated molecules after music-listening are coloured in red. The validated transcriptional regulators of microRNAs from TransmiR 2.0 and literature are coloured in cyan. Target genes of the up-regulated microRNAs are coloured in light green and includes only validated findings from the literature. Other molecules and cascades which were implied to be up-regulated based on findings from literature and from the microRNA analyses are coloured in coral. Coral edges denote activation, light green edges indicate negative regulation and black lines show regulation where the direction is not known
Comparative analyses with songbirds
To understand the evolutionary conservation of the molecular regulatory mechanisms underlying auditory perception and vocal communication, we compared the DE microRNAs and their target genes to those identified in songbirds during song-listening and singing. Amongst the DE microRNAs, hsa-miR-25-3p, which was upregulated in the high-COMB Music Group, also showed song-responsive upregulation (tgu-miR-25) in songbirds [Citation13]. Another DE microRNA from our study, miR-132, was found to be differentially expressed across seasons in the avian song control nuclei where its target gene network regulates cell cycle inhibitors and PTEN signalling [Citation19]. Remarkably, miR-132 also promoted neurite outgrowth and radial migration of the neurons by repression of FOXP2 [Citation20]. FOXP2 is important for human language development and vocal learning [Citation21]. The downregulated hsa-miR-378a-3p has predictable interactions with TLK2, one of the predicted target genes of the song-inhibited miR-2954 in songbirds [Citation13], with roles in proliferation and neuronal differentiation. hsa-miR-378a-3p and hsa-miR-16-2-3p also show expected interactions with song-stimulated genes that are found to be upregulated during song-responsive downregulation of miR-2954 in songbirds [Citation22]. The target genes of the DE microRNAs that were found to be overlapping with the genes behaviourally regulated in songbirds [Citation23] are provided in Table S3. Consequently, the results from the comparative analysis suggest some shared molecular mechanisms relevant to the auditory perception and vocal communication processes in songbirds and humans.
Integrated results and putative regulatory network in music-listening
We observed a total of 10 upregulated genes in music-listening from the high-COMB Music Group [Citation15] to be the target genes of two of the downregulated microRNAs from the current study: hsa-miR-378a-3p shows an anticipated interaction with CREBRF and hsa-miR-16-2-3p with UBE2B, SLC4A7, MOB1A, OSBPL8, RGS2, KCTD6, MBNL1, DSTN, and TMED7. Amongst the upregulated microRNAs in our study, hsa-miR-132-3p was predicted to target PSMD13, which was found downregulated after music-listening in the high-COMB Music Group [Citation15]. Furthermore, DICER1 was upregulated in the high-COMB Music Group [Citation15] and is crucial for the biogenesis of microRNAs and functions of multiple systems [Citation24].
proposes a gene regulatory network activated by music listening based on the integrated analysis, expanded with transcriptional regulatory data for microRNAs (TF-microRNA), TF-gene regulatory data for the DE genes, microRNA-TF interactions, and findings related to auditory perception and vocal communication. Notably, from the merged network, we observed that hsa-miR-132-3p and hsa-miR-25-3p, which were upregulated in the high-COMB Music Group, have interactions, respectively, with MAPT (Microtubule-associated protein tau) and TNFSF10 (a cytokine), two of the upstream regulators of the downregulated genes from the same group [Citation15]. MAPT is predicted to activate the downregulated ATP5J, HSPE1, and STIP1 as MAPT expression has been attributed to reduced connectivity in the brains of patients with Parkinson’s Disease [Citation25]. TNFSF10 is predicted to activate the downregulated HLA-A, IFI6, and TNFRSF10B. miR-132 plays a critical role in regulating TAU protein levels [Citation26] and is important for preventing tau protein aggregation that causes Alzheimer’s disease. Furthermore, one of the downregulated microRNAs from the high-COMB Music Group hsa-miR-16-2-3p is anticipated to target HOXA9, one of the up-stream regulators of the genes upregulated n the high-COMB Music Group [Citation15]. The functional interactions between the upregulated genes [Citation15] are shown in . The genes and pathways previously reported to be associated with song-perception and human musical aptitude are provided in Figure S1.
Figure 3. Putative gene regulatory network in music-listening. A putative gene regulatory network was constructed using Cytoscape version 3.7.1 [Citation66] extending the results from the integrated analyses of microRNA and gene expression data after music-listening from the high COMB group, validated transcriptional regulators of DE microRNAs from TransmiR 2.0, validated target genes of the DE microRNAs and functions based on microRNA over-representation analysis and literature findings. Legend: Rectangles indicate microRNAs and ellipses indicate genes. The up-regulated and down-regulated molecules after music-listening are coloured respectively in red and green. The validated transcriptional regulators of microRNAs from TransmiR 2.0 are coloured in cyan and the upstream regulators of the DE genes from the gene expression study [Citation10] in blue. The target genes of up-regulated microRNAs which are not differentially regulated after music-listening are coloured in light green and includes only validated findings from the literature. Likewise, the target genes of down-regulated microRNAs which are not differentially regulated after music-listening are coloured in coral and includes also some predicted target genes which are mentioned in the discussion. Solid lines denote direct interactions, dotted lines indicate indirect interactions, coral lines denote positive regulation, light-green lines for negative regulation and black lines show regulation where the direction is not known
![Figure 3. Putative gene regulatory network in music-listening. A putative gene regulatory network was constructed using Cytoscape version 3.7.1 [Citation66] extending the results from the integrated analyses of microRNA and gene expression data after music-listening from the high COMB group, validated transcriptional regulators of DE microRNAs from TransmiR 2.0, validated target genes of the DE microRNAs and functions based on microRNA over-representation analysis and literature findings. Legend: Rectangles indicate microRNAs and ellipses indicate genes. The up-regulated and down-regulated molecules after music-listening are coloured respectively in red and green. The validated transcriptional regulators of microRNAs from TransmiR 2.0 are coloured in cyan and the upstream regulators of the DE genes from the gene expression study [Citation10] in blue. The target genes of up-regulated microRNAs which are not differentially regulated after music-listening are coloured in light green and includes only validated findings from the literature. Likewise, the target genes of down-regulated microRNAs which are not differentially regulated after music-listening are coloured in coral and includes also some predicted target genes which are mentioned in the discussion. Solid lines denote direct interactions, dotted lines indicate indirect interactions, coral lines denote positive regulation, light-green lines for negative regulation and black lines show regulation where the direction is not known](/cms/asset/e62705cf-4509-4985-9a81-3edae9d42e63/kepi_a_1809853_f0003_oc.jpg)
Discussion
We have previously shown that listening to music and music performance affects human gene expression [Citation15,Citation16]. The present study demonstrated that music listening alters human microRNA profiles. Of the identified miRNAs, miR-132 is an activity-dependent microRNA which responds immediately to neuronal stimulation [Citation27], is seasonally regulated in avian song control nuclei, and is important for sensorimotor neuronal plasticity [Citation19]. In parallel, of our convergent analysis of genes identified correlating with musical traits and the effects of music, several of the top candidate genes [EGR1, FOS, ARC, BDNF, DUSP1] are known to be activity-dependent immediate early genes [IEGs] [Citation28]. At the molecular level, neural stimulation is conducted via calcium channel activity and neurotransmitters, which activate immediate early genes (IEG) thereby regulating gene and microRNA expression patterns [Citation29]. miR-132 is also activated by CREB [Citation27], BDNF – a neurotrophin which is a target of CREB [Citation29]- and external stimulants like cocaine. miR-132 and CREB are important for the maturation and plasticity of dendrites [Citation30]. CREB is also critical for consolidation of long-term memory and is stimulated by song-learning of songbirds [Citation31]. Interestingly, ARC, which is co-expressed with miR-132 after induction of long-term potentiation, is also activated by BDNF [Citation32]. BDNF augments neurogenesis and cognition [Citation33] and is found to be activated after music exposure [Citation34] and songbird singing [Citation35]. FOS is activated after music-performance in musicians [Citation16] and has roles in neurotransmission and experience-dependent neuroplasticity [Citation36]. In addition, miR-132 protects dopaminergic neurons by its regulation of caspase3 (CASP3) [Citation37], and its expression has been linked to dopaminergic neuronal loss of Parkinson’s disease patients [Citation38]. Individuals with Alzheimer’s and mild cognitive impairment had lower expression levels of miR-132 in the hippocampal and cortical areas [Citation26] whereas music-listening seems to upregulate neuroprotective microRNAs and molecules linked to neurodegenerative diseases.
It is noteworthy that miR-23a, another candidate microRNA, is also induced by long-term potentiation with implications in memory consolidation [Citation39]. More importantly, brain expressions of BDNF that are connected to behavioural activation of dopaminergic neurons showed a positive correlation with that of SNCA [Citation40,Citation41], the candidate gene upregulated in the high-COMB Music Group and high Edu Music Groups [Citation15] and in musicians after music-performance [Citation16]. Furthermore, SNCA also activates BDNF [Citation42] and BDNF and SNCA are regulated by GATA2, which is located in the strongest associated region for musical aptitude [Citation43,Citation44]. miR-23a and miR-23b have been experimentally confirmed to show neuroprotective effects via repression of APAF1, which is an activator of caspases and neuronal apoptotic processes [Citation45]. This finding is in line with the results of the transcriptome study where downregulated genes were responsible for mammalian neuronal apoptosis and deficits in dopaminergic neurotransmission [Citation15].
The upregulated miR-25 and its cluster members inhibit the pro-apoptotic TP53 and its mediators and reduce the neuronal apoptotic process [Citation45]. miR-25 also promotes neurogenesis and differentiation of adult neurons by regulating the TGFB-signalling pathway, which was previously identified to repress neurogenesis and neuronal cell proliferation, and by activating insulin-like growth factor-1 (IGF) signalling via its targeting of PTEN [Citation46]. This is consistent with the findings of the gene expression study of music-listening that indicated the downregulated genes from the high-COMB Music Group as activators of peptidase, endopeptidase, and caspase activities [Citation15]. For instance, upregulation of miR-25 is consistent with findings from songbirds where it was found activated in response to song-learning and listening [Citation13].
miR-23b and miR-23a are involved in feedback regulatory circuits with their transcriptional regulator EGR1 which is an IEG that is induced in songbird learning and singing [Citation35]. Furthermore, DE microRNAs from this study (hsa-miR-23a-3p, hsa-miR-23b-3p, hsa-miR-132-3p, hsa-miR-25-3p) show validated and predicted targeting of FOXP2. Interestingly, FOXP2 was one of the top 10 candidates associated with musical abilities including the recognition and production of sound [Citation28], and was found to be positively selected for during human evolution [Citation47]. In songbirds, FOXP2 is enriched in corticostriatal circuits and shows downregulation during the sensorimotor learning period, during vocal practice, and after undirected singing [Citation21,Citation48]. This behavioural regulation of FOXP2 plausibly finetunes neural structures for learning [Citation21] and vocal complexity [Citation48]. This suggests a regulatory role of the candidate microRNAs in the plasticity circuits associated with music-listening.
Interestingly, PTEN is targeted by other upregulated microRNAs: miR-132, miR-23b, and miR-23a. Of these, miR-23a activates AKT (Protein Kinase B) signalling, PI3K (phosphatidylinositol 3-kinase) signalling, MAPK activity and promotes the expression of myelin genes through its regulation of PTEN [Citation49]. The MAPK signalling pathway has a crucial role in the regulation of neuronal transcription, synaptic plasticity, memory consolidation [Citation50], and was previously reported to be activated by microRNA regulation in response to song-listening in songbirds [Citation13]. The upregulation of microRNAs that are regulators of neuronal apoptosis and neurotoxicity may raise a question about clinical findings of the neuroprotective role of music [Citation9].
Putative activation of the pro-survival PI3K/AKT signalling cascade indirectly by music-induced miR-23a is one candidate mechanism which explains dopaminergic neurotransmission in our study. PI3K/AKT signalling is activated in response to growth factors and neurotrophins and when coupled with dopaminergic signalling, it protects adult dopaminergic neurons from apoptosis [Citation51]. Moreover, genetic variations in the AKT1 gene affect neural structures of the frontostriatal dopaminergic brain network as well as bioavailable dopamine levels and cognitive functions [Citation52].
We found DICER to be upregulated after music-performance [Citation16]. DICER is important for the biogenesis of microRNAs. It acts on various systems including those in the inner ear and brain that are important for the reception and perception of auditory signals [Citation53]. A sensory neuronal Dicer knockout reduced the expressions of the music-induced miR-23a and miR-23b [Citation54] and DICER ablation in the inner ear hair cells led to hair cell degeneration and hearing loss [Citation55]. DICER protects adult dopaminergic neurons [Citation56] and is critical for the maintenance of proper levels of striatal dopamine [Citation24]. These findings might explain the prior observations of music-listening-responsive dopamine release and of the activation of reward pathways [Citation57].
In this study, music-listening affected microRNA regulation only in subjects with relatively high music test scores (high COMB). This is in accordance with findings from a previous transcriptome study which identified more changes in the high-COMB Music Group than in the high Edu Music Group [Citation15].
Conclusions
We provide evidence that listening to music has an effect on human gene regulation. The identified microRNAs were shown to affect dopamine metabolism and to prevent neurodegeneration. Some of the human DE microRNAs shared signalling pathways with songbirds suggesting an evolutionary conservation of the molecular regulatory mechanisms underlying auditory perception. MicroRNA expression patterns in the human brain and blood have been published previously [Citation58,Citation59]. Future studies are needed to experiment with the duration of listening, genre of music, and personal preferences of the participants, as well as ambience in different combinations to get further insight into the effects of each of these factors on microRNA expression levels.
Methods
Study participants
MicroRNA samples were obtained from the same cohort, and during the same music exposure (concert) as described in transcriptome study [Citation15]. Briefly, the participants were invited to listen to Wolfgang Amadeus Mozart’s Violin Concerto No. 3 in G major, K.216 which lasts about 20 minutes, typical duration for a concerto in the Western Classical Period. Before listening and immediately after listening, blood samples were drawn from each participant. The participants were unaware of the type of music intended for the listening session. Given electroencephalographic evidence that humans differentiate and categorize musical instrument sounds and voices within 100 ms [Citation60], we expected that the duration of listening session would be sufficient to affect microRNA regulation.
Blood samples from 43 volunteers who met our inclusion criteria were analysed. Thirty-seven were in the Music Group (i.e., the test group), and seven in the Control Group. In the control condition participants did not listen to the Mozart concerto the day their bloods were drawn and did not listen to music or exercise vigorously the day before blood sampling. During the 20 minutes between the two phlebotomies, participants in the Control Group were permitted to read, take a leisurely walk outside, and/or converse. Peripheral blood samples were collected from the participants just before and after 20 min in the control session.
The analysed phenotypes and their classification have been described in Kanduri et al. [Citation15] (see also Supplementary Methods). In short, we sub-phenotyped the participants based on their level of music education and musical aptitude using COMB score distributions (range: 0–148). The data regarding the level of music education of the participants were collected using a questionnaire. Based on the answers, participants were allocated to four different Edu classes (class 1–4) [Citation15]. Participants in Edu classes 3 and 4 are referred to as the high-Edu Music Group and those in Edu classes 1 or 2 have been categorized as low-Edu Music Group.
The study was approved by the ethical committee of Helsinki University Central Hospital (permission #13/03/2013) and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all the subjects.
MicroRNA extraction, sequencing, and pre-processing of microRNA sequencing reads
Details of the microRNA extraction are provided in the Supplementary Methods. Sequencing libraries were prepared at the High-Throughput Genomics department of The Welcome Trust Centre for Human Genetics followed by sequencing with Illumina HiSeq. We assessed the quality of the microRNA sequencing reads with FastQC version 11.3 (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Next, we trimmed sequencing adapters from the 3ʹ end of 50 bp reads requiring an adapter overlap of 5 bp, error rate of 0.1 and then we filtered shorter (<15 bp) and low-quality reads (Phred score <20) with Trim Galore! version 0.3.7 (http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/). Trimmed reads were quality checked again using FastQC and aligned with the human genome reference (GRCh38, Ensembl release 76) with bowtie version 1.1.2 [Citation61]. Only unique alignments were selected from the best alignments (–best – strata), requiring a complete match for a seed length of 18. Afterwards, we quantified microRNA expression using HTSeq version 0.6.1p1 [Citation62] according to miRBase release 21 annotations for human microRNAs [Citation63].
Read and microRNA statistics
All the reads of the Music Group and Control group samples passed the FastQC quality check for A) basic statistics, B) per sequence quality scores and C) per base N content. The median number of raw reads per sample was 13,521,377.5 for the music study (range: 8,577,637–19,549,067) and 9,355,225 for the control study (range: 8,423,477–11,094,252). On average, 97.75% and 96.35% of reads from the music-listening study and control study, respectively, were trimmed for sequencing adapters. Mean alignment percentage of the trimmed reads to the human genome reference (Ensembl 76/GRCh38) were 83.6% (range: 65.69–90.81) and 85.35% (range: 79.85–87.88) respectively, for the music-listening and control studies. All alignments from both the studies passed quality control.
Differential expression analyses of microRNA
To understand the effects of music-listening on microRNA expression, we used DESeq2 (version 1.20.0) [Citation63] and analysed the differential expression of microRNAs over time (Post versus (vs.) Pre) in the music-listening group compared to the control group. DESeq2 has high sensitivity for experiments with a wide range of sample numbers (small to large) and for those with a small fold change [Citation64,Citation65]. Furthermore, a benchmark comparison of statistical tools for analysing differential expression supports the use of DESeq2 as it shows that the DESeq2 false-positive rate can be as low as 0 and the true positive rate above 80%, even with a log fold threshold and a replicate number as low as 0.5 and 6, respectively, [Citation66].
We then performed generalized linear model-based differential expression analyses with DESeq2, implementing likelihood ratio tests with a design matrix which controls for paired experimental design. False discovery rate (FDR) adjusted p-values were calculated using the Benjamini–Hochberg method which accounts for multiple testing correction. MicroRNAs were considered to be differentially expressed when the FDR adjusted p-values were less than 10% [Citation19]. We kept the fold-change threshold of 1.2 in accordance with gene-environmental interaction studies where moderate changes in microRNA expressions have been observed [Citation67,Citation68]. We chose differentially expressed (DE) microRNAs that showed a Post-Pre threshold of at least 10% for the music-listening session for further analyses [Citation15]. The control samples were used as one reference group, without sub-phenotype divisions, to compare music-listening responsive microRNA expressions. To facilitate this, we estimated expression differences in microRNA between high-Edu Music Group (N = 3) and low-Edu Music Group (N = 4) from the Control Group and used it as an indicator of homogeneity of the control samples. From this analysis, we did not observe any significant differences in microRNA expression between these groups thereby showing homogeneity of the control samples.
Functional analysis of microRNAs
We performed a functional enrichment analysis of DE microRNAs using TAM 2.0 [Citation69]. For a given microRNA dataset, TAM 2.0 analyzes the over-representation of functional and disease annotations by comparing the input microRNAs to a high quality, manually annotated reference microRNA dataset. TAM 2.0 then applies a hyper-geometric test to determine whether the given microRNA dataset is over-represented or under-represented for functions, diseases, transcription factors (upstream-regulators) etc. The TAM 2.0 analysis addresses the bias previously noted to be associated with the over-represented functions reported for microRNAs, when the over-representation analysis was performed solely based on target genes [Citation70,Citation71]. Additionally, TAM 2.0 performs a comparative analysis of the upregulated and downregulated microRNAs together to correlate them to those dysregulated in disease conditions. We then collected the validated transcriptional regulators of the DE microRNAs from TransmiR 2.0 [Citation72]. We targeted candidate genes previously associated with musical traits in humans [Citation43], some of which may reflect convergent gene expression specialization for auditory-motor integration sufficient to support vocal communication in humans and songbirds [Citation73], and those which were found to be positively selected for in accordance with human musical aptitude [Citation47]. Comparing to the genes obtained from the same human cohort was done in order to reduce the genetic heterogeneity of complex human musical traits. Next, we obtained validated ontology annotations from miRBase, which is derived from experimentally verified miRNA:target interaction data [Citation74]. Annotations for the closest orthologs of our DE microRNAs, as indicated by Alliance of Genome Resources as Rattus norvegicus, were collected from the Rat Genome Database (RGD) [Citation75]. Furthermore, to correlate blood microRNA expression to the brain, we obtained tissue-wide expression patterns for DE microRNAs from the miRWalk2.0 [Citation76], miRIAD [Citation77], BBBomics [Citation78], and literature.
Identification of microRNA-target genes
To understand the post-transcriptional gene regulatory mechanisms involving microRNAs, validated target genes supported by strong evidence (based on reporter assay or western blot) were obtained for the DE microRNAs using the miRTarBase database (Release 7.0) [Citation17]. We also collected predicted target genes for the DE microRNAs from TargetScan (Release 7.2) [Citation18] and applied the filtering criteria below to reduce false-positive target genes. For the conserved and broadly conserved microRNA families, only target genes with conserved sites having an aggregate probability of conserved targeting at least 0.2 and a total context++ score at most −0.15 were selected. For the poorly conserved DE microRNA families and those with other miRBase annotations, target genes with a total context++ score of less than −0.15 were selected [Citation18]. Predicted target genes of the DE microRNAs with non-canonical binding were not considered for the analyses. Both the predicted and validated target genes of the DE microRNAs were then combined for further analysis and functional interpretation.
Comparative analyses
We then compared the DE microRNAs to the song-responsive and singing-regulated microRNAs in zebra finches [Citation13,Citation22,Citation79] to understand of the microRNA regulatory mechanisms in human music cognition. We also compared target genes of the DE microRNAs to singing-regulated genes in songbirds [Citation23], and to the target genes of song-listening and singing responsive microRNAs [Citation13,Citation22,Citation79]. Next, we calculated the significance of the overlap between the target genes of our DE microRNAs and the genes regulated by songbird singing using random sampling (without replacement) of our datasets (N = 10,000) and overlap estimation for each of the re-sampled datasets. To this end, we created a dataset with behaviourally (singing) stimulated genes from songbird brain [Citation23,Citation35,Citation80] and labelled the gene set as the song production cum perception gene set. For the songbird set sampling, we used all the annotated genes from Taeniopygia guttata (N = 17,926) as Universe and sampling was performed for the same size as song production cum perception gene set. Human genes were sampled for the same size as the number of predicted and validated target genes of the downregulated microRNAs using all annotated human genes as the Universe (N = 20,219). Similarly, we analysed the overlap significance between the target genes of the upregulated microRNAs from the high-COMB Music Group and the singing-inhibited genes from the songbird brain [Citation23] using resampling (N = 10,000).
Integrated analysis and putative regulatory network construction
To understand the microRNA-gene regulatory mechanisms underlying music-listening in listeners with high musical aptitude, we integrated our microRNA findings with the music responsive gene expression findings from the same group [Citation15] using IPA and the microRNA–gene interactions gathered from TargetScan, miRTarBase and literature. Only target genes of the DE microRNAs from this study which showed an inverse direction of regulation in the gene expression findings (from the same music-performance and control activity as this study) [Citation15] were considered as microRNA–gene interactions in music-listening.
We further created a putative gene regulatory network in music-listening using Cytoscape 3.7.1 by merging our integrated results (above) with transcriptional regulatory data for microRNAs (TF-microRNA) from TransmiR 2.0 including previously reported [Citation15] statistically significant up-stream regulators of the DE genes (TF-gene), the microRNA-TF regulatory information from TargetScan/literature and findings related to song and music perception. Here, it is important to highlight the fact that microRNA can simultaneously regulate the expression of multiple genes through direct interactions or indirectly through the regulation of their transcriptional regulators (microRNA-TF) [Citation81,Citation82]. In this study, we examined the regulatory effects (activation/inhibition) of the upstream regulator on the DE genes (TF-gene) and included only those TFs which were targeted by DE microRNA (microRNA-TF) to the putative regulatory network. From the validated TF-microRNA regulatory data from TransmiR 2.0, only those TFs which met our criteria described in the functional analysis were included in the network. This putative regulatory network was further extended with some of the functions from the microRNA enrichment analysis, literature findings, and putative connecting molecules between the microRNAs and the functions. Functional interactions between the upregulated molecules, putatively up-regulated molecules and some of the transcriptional regulators in this network were also inferred with STRING [Citation83].
Author contributions
IJ conceived the idea of the study. IJ, PR, LUV, and PSN designed the study. LUV recruited the participants. AKP and MA performed the laboratory analyses. PSN performed the bioinformatic analyses, interpreted the data and wrote the manuscript. IJ helped in interpreting the data and writing the manuscript.
Supplemental Material
Download Zip (343 KB)Acknowledgments
We thank the participants for their generous collaboration. We are grateful to Petri Myllynen, Sanna Pyy, Laura Salmela, Sonja Suhonen, Jaana Oikkonen, and Kai Karma for their help in organizing the study. We are grateful to Dr Eija Korpelainen, CSC, Finland for the insightful comments that helped to improve the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Tramo MJ. Biology and music. Music of the hemispheres. Science. 2001;291(5501):54–56.
- Peretz I, Zatorre RJ. Brain organization for music processing. Annu Rev Psychol. 2005;56:89–114.
- Bradley W, Vines BW, Krumhansl CL, et al. Cross-modal interactions in the perception of musical performance. Cognition. 2006;101(1):80–113.
- Koelsch S. Brain correlates of music-evoked emotions. Nat Rev Neurosci. 2014;15:170–180.
- Blood AJ, Zatorre RJ. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc Natl Acad Sci USA. 2001;98:11818–11823.
- Menon V, Levitin DJ. The rewards of music listening: response and physiological connectivity of the mesolimbic system. NeuroImage. 2005;28:175–184.
- Salimpoor VN, Benovoy M, Larcher K, et al. Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat Neurosci. 2011;14:257–262.
- Karageorghis CI, Terry PC, Lane AM, et al. The BASES Expert Statement on use of music in exercise. J Sports Sci. 2012;30:953–956.
- Sihvonen AJ, Särkämö T, Leo V, et al. Music-based interventions in neurological rehabilitation. Lancet Neurol. 2017;16:648–660.
- Fineberg SK, Kosik KS, Davidson BL. MicroRNAs potentiate neural development. Neuron. 2009;64:303–309.
- Cohen JE, Lee PR, Chen S, et al. MicroRNA regulation of homeostatic synaptic plasticity. Proc Natl Acad Sci USA. 2011;108:11650–11655.
- Patel M, Hu BH. MicroRNAs in inner ear biology and pathogenesis. Hearing Res. 2012;287:6–14.
- Gunaratne PH, Lin Y-C, Benham AL, et al. Song exposure regulates known and novel microRNAs in the zebra finch auditory forebrain. BMC Genomics. 2011;12:277.
- Louder MIM, Hauber ME, Balakrishnan CN. Early social experience alters transcriptomic responses to species-specific song stimuli in female songbirds. Behav Brain Res. 2018;347:69–76.
- Kanduri C, Raijas P, Ahvenainen M, et al. The effect of listening to music on human transcriptome. PeerJ. 2015;3:e830.
- Kanduri C, Kuusi T, Ahvenainen M, et al. The effect of music performance on the transcriptome of professional musicians. Sci Rep. 2015;5:9506.
- Chou C-H, Shrestha S, Yang C-D, et al. miRTarBase update 2018: A resource for experimentally validated microRNA-target interactions. Nucleic Acids Res. 2018;46:D296–D302.
- Agarwal V, Bell GW, Nam J-W, et al. Predicting effective microRNA target sites in mammalian mRNAs. eLife. 2015;4. DOI:10.7554/eLife.05005
- Larson TA, Lent KL, Bammler TK, et al. Network analysis of microRNA and mRNA seasonal dynamics in a highly plastic sensorimotor neural circuit. BMC Genomics. 2015;16:905.
- Clovis YM, Enard W, Marinaro F, et al. Convergent repression of Foxp2 3ʹUTR by miR-9 and miR-132 in embryonic mouse neocortex: implications for radial migration of neurons. Development. 2012;139:3332–3342.
- Teramitsu I, Poopatanapong A, Torrisi S, et al. Striatal FoxP2 is actively regulated during songbird sensorimotor learning. PLoS ONE. 2010;5:e8548.
- Lin Y-C, Balakrishnan CN, Clayton DF. Functional genomic analysis and neuroanatomical localization of miR-2954, a song-responsive sex-linked microRNA in the zebra finch. Front Neurosci. 2014;8:409.
- Whitney O, Pfenning AR, Howard JT, et al. Core and region-enriched networks of behaviorally regulated genes and the singing genome. Science (New York, NY). 2014;346:1256780.
- Pang X, Hogan EM, Casserly A, et al. Dicer expression is essential for adult midbrain dopaminergic neuron maintenance and survival. Mol Cell Neurosci. 2014;58:22–28.
- Rittman T, Rubinov M, Vértes PE, et al. Regional expression of the MAPT gene is associated with loss of hubs in brain networks and cognitive impairment in Parkinson disease and progressive supranuclear palsy. Neurobiol Aging. 2016;48:153–160.
- Smith PY, Hernandez-Rapp J, Jolivette F, et al. miR-132/212 deficiency impairs tau metabolism and promotes pathological aggregation in vivo. Hum Mol Genet. 2015;24:6721–6735.
- Nudelman AS, DiRocco DP, Lambert TJ, et al. Neuronal activity rapidly induces transcription of the CREB‐regulated microRNA‐132, in vivo. Hippocampus. 2010;20:492–498.
- Oikkonen J, Onkamo P, Järvelä I, et al. Convergent evidence for the molecular basis of musical traits. Sci Rep. 2016;6:39707.
- Tao X, Finkbeiner S, Arnold DB, et al. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726.
- Magill ST, Cambronne XA, Luikart BW, et al. microRNA-132 regulates dendritic growth and arborization of newborn neurons in the adult hippocampus. Proc Natl Acad Sci USA. 2010;107:20382–20387.
- Sakaguchi H, Wada K, Maekawa M, et al. Song-induced phosphorylation of cAMP response element-binding protein in the songbird brain. J Neurosci. 1999;19:3973–3981.
- Wibrand K, Pai B, Siripornmongcolchai T, et al. MicroRNA regulation of the synaptic plasticity-related gene Arc. PloS One. 2012;7:e41688.
- Strait DL, Kraus N. Biological impact of auditory expertise across the life span: musicians as a model of auditory learning. Hearing Res. 2014;308:109–121.
- Xing Y, Chen W, Wang Y, et al. Music exposure improves spatial cognition by enhancing the BDNF level of dorsal hippocampal subregions in the developing rats. Brain Res Bull. 2016;121:131–137.
- Wada K, Howard JT, McConnell P, et al. A molecular neuroethological approach for identifying and characterizing a cascade of behaviorally regulated genes. Proc Nat Acad Sci USA. 2006;103:15212–15217.
- Kaczmarek L, Nikołajew E. C-fos protooncogene expression and neuronal plasticity. Acta Neurobiol Exp. 1990;50:173–179.
- Sun -Z-Z, Lv Z-Y, Tian W-J, et al. MicroRNA-132 protects hippocampal neurons against oxygen-glucose deprivation-induced apoptosis. Int J Immunopathol Pharmacol. 2017;30:253–263.
- Hartmann A, Hunot S, Michel PP, et al. Caspase-3: A vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson’s disease. Proc Nat Acad Sci USA. 2000;97:2875–2880.
- Ryan B, Logan BJ, Abraham WC, et al. MicroRNAs, miR-23a-3p and miR-151-3p, Are regulated in dentate gyrus neuropil following induction of long-term potentiation in vivo. PloS One. 2017;12:e0170407.
- Aid-Pavlidis T, Pavlidis P, Timmusk T. Meta-coexpression conservation analysis of microarray data: A “subset” approach provides insight into brain-derived neurotrophic factor regulation. BMC Genomics. 2009;10:420.
- Kudryavtseva NN, Bondar NP, Boyarskikh UA, et al. Snca and Bdnf gene expression in the VTA and raphe nuclei of midbrain in chronically victorious and defeated male mice. PloS One. 2010;5:e14089.
- Kohno R, Sawada H, Kawamoto Y, et al. BDNF is induced by wild-type alpha-synuclein but not by the two mutants, A30P or A53T, in glioma cell line. Biochem Biophys Res Comm. 2004;318:113–118.
- Oikkonen J, Huang Y, Onkamo P, et al. A genome-wide linkage and association study of musical aptitude identifies loci containing genes related to inner ear development and neurocognitive functions. Mol Psychiatry. 2015;20:275–282.
- Park H, Lee S, Kim H-J, et al. Comprehensive genomic analyses associate UGT8 variants with musical ability in a Mongolian population. J Med Genet. 2012;49:747–752.
- Kumar M, Lu Z, AaL T, et al. Negative regulation of the tumor suppressor p53 gene by microRNAs. Oncogene. 2011;30:843–853.
- Brett JO, Renault VM, Rafalski VA, et al. The microRNA cluster miR-106b~25 regulates adult neural stem/progenitor cell proliferation and neuronal differentiation. Aging (Albany NY). 2011;3:108–124.
- Liu X, Kanduri C, Oikkonen J, et al. Detecting signatures of positive selection associated with musical aptitude in the human genome. Sci Rep. 2016;6:21198.
- Heston JB, White SA. Behavior-linked FoxP2 regulation enables zebra finch vocal learning. J Neurosci. 2015;35:2885–2894.
- Lin S-T, Huang Y, Zhang L, et al. MicroRNA-23a promotes myelination in the central nervous system. Proc Natl Acad Sci USA. 2013;110:17468–17473.
- Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183.
- Nair VD, Olanow CW, Sealfon SC. Activation of phosphoinositide 3-kinase by D2 receptor prevents apoptosis in dopaminergic cell lines. Biochem J. 2003;373:25–32.
- Tan H-Y, Nicodemus KK, Chen Q, et al. Genetic variation in AKT1 is linked to dopamine-associated prefrontal cortical structure and function in humans. J Clin Invest. 2008;118:2200–2208.
- Pannese A, Grandjean D, Frühholz S. Amygdala and auditory cortex exhibit distinct sensitivity to relevant acoustic features of auditory emotions. Cortex. 2016;85:116–125.
- Zhao J, Lee M-C, Momin A, et al. Small RNAs control sodium channel expression, nociceptor excitability, and pain thresholds. J Neurosci. 2010;30:10860–10871.
- Friedman LM, Dror AA, Mor E, et al. MicroRNAs are essential for development and function of inner ear hair cells in vertebrates. Proc Natl Acad Sci USA. 2009;106:7915–7920.
- Chmielarz P, Konovalova J, Najam SS, et al. Dicer and microRNAs protect adult dopamine neurons. Cell Death Dis. 2017;8:e2813.
- Moraes MM, Rabelo PCR, Pinto VA, et al. Auditory stimulation by exposure to melodic music increases dopamine and serotonin activities in rat forebrain areas linked to reward and motor control. Neurosci Lett. 2018;673:73–78.
- Liang Y, Ridzon D, Wong L, et al. Characterization of microRNA expression profiles in normal human tissues. BMC Genomics. 2007;8:166.
- Liew -C-C, Ma J, Tang H-C, et al. The peripheral blood transcriptome dynamically reflects system wide biology: A potential diagnostic tool. J Lab Clin Med. 2006;147:126–132.
- Rigoulot S, Pell MD, Armony JL. Time course of the influence of musical expertise on the processing of vocal and musical sounds. Neuroscience. 2015;290:175–184.
- Langmead B, Trapnell C, Pop M, et al. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25.
- Anders S, Pyl PT, Huber W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169.
- Kozomara A, Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014;42:D68–73.
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550.
- Zhou X, Lindsay H, Robinson MD. Robustly detecting differential expression in RNA sequencing data using observation weights. Nucleic Acids Res. 2014;42:e91.
- Schurch NJ, Schofield P, Gierliński M, et al. How many biological replicates are needed in an RNA-seq experiment and which differential expression tool should you use? RNA. 2016;22:839–851.
- Chilton WL, Marques FZ, West J, et al. Acute exercise leads to regulation of telomere-associated genes and microRNA expression in immune cells. PloS One. 2014;9:e92088.
- Tonevitsky AG, Maltseva DV, Abbasi A, et al. Dynamically regulated miRNA-mRNA networks revealed by exercise. BMC Physiol. 2013;13:9.
- Li J, Han X, Wan Y, et al. TAM 2.0: tool for MicroRNA set analysis. Nucleic Acids Res. 2018;46:W180–W185.
- Bleazard T, Lamb JA, Griffiths-Jones S. Bias in microRNA functional enrichment analysis. Bioinformatics. 2015;31:1592–1598.
- Godard P, van Eyll J. Pathway analysis from lists of microRNAs: common pitfalls and alternative strategy. Nucleic Acids Res. 2015;43:3490–3497.
- Tong Z, Cui Q, Wang J, et al. TransmiR v2.0: an updated transcription factor-microRNA regulation database. Nucleic Acids Res. 2019;47:D253–D258.
- Pfenning AR, Hara E, Whitney O, et al. Convergent transcriptional specializations in the brains of humans and song-learning birds. Science (New York, NY). 2014;346:1256846.
- Griffiths-Jones S, Grocock RJ, van Dongen S, et al. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–144.
- Shimoyama M, De Pons J, Hayman GT, et al. The rat genome database 2015: genomic, phenotypic and environmental variations and disease. Nucleic Acids Res. 2015;43:D743–750.
- Dweep H, Gretz N. miRWalk2.0: A comprehensive atlas of microRNA-target interactions. Nat Methods. 2015;12:697.
- Hinske LC, França GS, Torres HAM, et al. miRIAD-integrating microRNA inter- and intragenic data. Database: J Biol Databases and Curation. 2014;2014.
- Kalari KR, Thompson KJ, Nair AA, et al. BBBomics-human blood brain barrier transcriptomics hub. Front Neurosci. 2016;10:71.
- Shi Z, Luo G, Fu L, et al. miR-9 and miR-140-5p target FoxP2 and are regulated as a function of the social context of singing behavior in zebra finches. J Neurosci. 2013;33:16510–16521.
- Hilliard AT, Miller JE, Fraley ER, et al. Molecular microcircuitry underlies functional specification in a basal ganglia circuit dedicated to vocal learning. Neuron. 2012;73:537–552.
- Otaegi G, Pollock A, Hong J, et al. MicroRNA miR-9 modifies motor neuron columns by a tuning regulation of FoxP1 levels in developing spinal cords. J Neurosci. 2011;31:809–818.
- Li C, Zhang K, Chen J, et al. MicroRNAs as regulators and mediators of forkhead box transcription factors function in human cancers. Oncotarget. 2017;8:12433–12450.
- Szklarczyk D, Morris JH, Cook H, et al. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45:D362–D368.