ABSTRACT
Acetylation of histone and non-histone proteins is a post-translational modification mostly associated with activation of gene transcription. The first histone acetyltransferase (HAT) identified as modifying newly synthesized histone H4 in yeast was a type B HAT named HAT1. Although it was the first HAT to be discovered, HAT1 remains one of the most poorly studied enzymes in its class. In addition to its well-established role in the cytoplasm, recent findings have revealed new and intriguing aspects of the function of HAT1 in the nucleus. Several studies have described its involvement in regulating different pathways associated with a wide range of diseases, including cancer. This review focuses on our current understanding of HAT1, highlighting its importance in regulating chromatin replication and gene expression. This previously unknown role for HAT1 opens up novel scenarios in which further studies will be required to better understand its function.
Introduction
Post-translational modifications (PTMs) of histone and non-histone proteins are known to be essential for the regulation of gene transcription. All chromatin modifications that can influence gene expression without any change in DNA sequence are defined as ‘epigenetic modifications’[Citation1]. Such alterations result from the activity of specific enzymes, grouped into three categories referred to ‘writers’, ‘readers’, and ‘erasers’, responsible for adding, recognizing, or removing epigenetic marks, respectively [Citation2].
Histone modifications (acetylation, phosphorylation, methylation, ubiquitination, and SUMOylation) are well-studied PTMs that affect a wide range of biological processes and play an important role in disease development [Citation3]. Therefore, the regulation of gene expression of healthy cells depends not only on non-mutated genetic sequences but also on proper histone modifications; a single alteration is potentially sufficient for the development of disease, including cancer [Citation4,Citation5].
One of the most dynamic modifications controlling chromatin regulation is lysine acetylation, mediated by the balance between two enzymatic classes, histone acetyltransferases (HATs) and histone deacetylases (HDACs). The transfer of an acetyl group onto ε-amino groups of specific lysine residues by HATs results in the loss of a positive charge on the histones, thereby increasing chromatin accessibility to RNA polymerase and transcription factors [Citation6,Citation7]. Conversely, removal of the acetyl moiety by HDACs results in gene silencing [Citation8,Citation9]. Apart from changing chromatin structure, acetylated histones can also be ‘read’ by bromodomain proteins, allowing additional functionality such as recruitment of other factors for regulating transcription [Citation10]. However, histone acetylation is not only associated with activation of gene expression, but also with DNA replication [Citation11] and DNA repair [Citation12,Citation13].
Histone synthesis mainly occurs during S-phase of the cell cycle in conjunction with DNA replication, and newly synthesized histones are rapidly modified in the cytoplasm before their assembly into the nucleosome [Citation14–16]. Specifically, stable dimers of histone H3/H4 are escorted into the nucleus following acetylation of newly synthesized histone H4 at Lys5 and Lys12 in the cytoplasm. Two H2A/H2B heterodimers enter the nucleus as part of a different histone-chaperone complex, but remain unacetylated and are only then associated with the H3/H4 tetramers. When the histone octamer is completely formed, histone acetylation marks are removed from H3 and H4 by HDACs and the linker histone H1 binds the nucleosome, increasing its stability during chromatin maturation [Citation15,Citation17,Citation18]. Subsequently, histone N-terminal tails protruding outwards from the nucleosome particle undergo different histone modifications, including acetylation, involved in gene regulation and overall chromatin structure remodelling [Citation19]. Acetylation masks positive charges leading to a change in chromatin conformation through different histone-DNA or histone-protein interactions [Citation16].
Unlike newly synthesized histone H3 acetylation patterns, which appear to differ between organisms [Citation20–22], the acetylation pattern of histone H4 is highly evolutionarily conserved [Citation20,Citation22–24]. Specifically, in all eukaryotes (protozoan, fly, yeast, human) acetylation of histone H4 at Lys5 and Lys12 was found to be the major histone modification conserved (corresponding to positions 4 and 11 in protozoan Tetrahymena) [Citation20,Citation22–24]. Further, the bulk of histones subjected to acetylation differs between histones H3 and H4; while histones H4 are highly acetylated, only a low percentage of H3 molecules are modified after their synthesis [Citation22,Citation24,Citation25,Citation26]. A recent finding showed that histone H3 and H4 lysine acetylation can be influenced by polyamines which stimulate HATs (GCN5 and HAT1) at translational level via the binding of microRNA to the 5ʹ-UTR of GCN5 mRNA and a possible enhancement of initiation complex formation on HAT1 mRNA [Citation27].
Mammalian HATs, also named lysine acetyltransferases (KATs) for their capacity to acetylate non-histone proteins, are grouped into three main families based on their structural homology and substrate binding: Gcn5-related N-acetyltransferases (GNAT), p300 and CREB-binding proteins (p300/CBP), and MOZ, HBO1, Sas2, Tip60 (MYST). In addition, some transcription factors and nuclear receptor coactivators exhibit HAT activity [Citation28,Citation29]. ().
Table 1. Classification of mammalian type A and type B HATs, their substrates, and cellular localization
HATs are also classified into two types, type A and B, which differ in substrate recognition and cellular compartment localization. Specifically, type A HATs perform their function uniquely in the nucleus and acetylate nucleosomal histones, while type B HATs are mainly cytoplasmic and responsible for the acetylation of newly synthesized histones [Citation1,Citation22]. ().
Since nuclear type A HATs are also involved in the acetylation of non-histone proteins, they have been more extensively studied than type B HATs. Aberrant type A HATs are linked to several diseases and their structural domains are therefore well characterized [Citation30–32]. However, increasing insights into the role and function of type B HAT1 have prompted research efforts to further investigate this enzyme.
In this review we will describe the increasingly broad role of HAT1, the first type B HAT identified but still the most poorly understood, in normal development and disease. Several functions of HAT1 associated with histone metabolism including histone deposition, nucleosome formation, and chromatin assembly during DNA replication and repair will also be discussed.
Identification of HAT1
HAT1 was originally isolated in the eukaryotic Saccharomyces cerevisiae by two different experimental approaches highlighting its histone acetyltransferase activities [Citation33,Citation34]. Subsequently, a homologue of yeast, Hat1p, with 29% identical and 55% similar sequence, was found in human cells [Citation35]. Further characterization led to the identification of two polypeptides of 46 kDa and 50 kDa, namely the catalytic subunit HAT1 and regulatory subunit retinoblastoma-binding protein 46 (RbAp46, RBBP7) respectively, which formed the HAT1 holoenzyme.
RbAp46, as well as its homologue in yeast, Hat2p, is a protein with tryptophan-aspartic acid (WD) repeats, which in association with HAT1 determines a 10-fold increase in acetyltransferase activity in vitro [Citation34,Citation36], explained by the conformational change of HAT1 during binding. Specifically, the side chain of histone H4 was shown to be closer to the enzyme and to its cofactor acetyl coenzyme A (AcCoA) in a crystal structure forming the ternary complex HAT1-AcCoA-H4. Moreover, the primary binding between RbAp46 and histone H4 occurs through amino acid residues within helix 1 of the N-terminal of histone H4. The interaction between RbAp46 and the histone H3/H4 dimer (or tetramer) in fact leads to an unfolding of helix 1 of histone H4, facilitating its binding [Citation37].
The HAT1/RbAp46 complex was found to be highly conserved across a variety of eukaryotes [Citation35,Citation38,Citation39], but was shown not to be necessary for cell proliferation or viability [Citation22,Citation33,Citation34].
A combination of different biochemical approaches classified HAT1 as type B HAT. Specifically, a binding assay using GST-histone fusion proteins purified from Escherichia coli for histones H2A, H2B, H3, and H4 revealed that the substrates of HAT1/RbAp46 activity were free H4 and, to a lesser degree, H2A histones [Citation35]. Indeed, when equal amounts of H2A/H2B dimers and H32/H42 tetramers were added in the mixture, HAT1 holoenzyme preferentially acetylated H4 rather than H2A [Citation35].
HAT1 holoenzyme acetylates recombinant H2A at Lys5, and di-acetylates recombinant histone H4 at Lys5 and Lys12. Based on the acetyltransferase activity of yeast Hat1p, a common consensus sequence GxGKxG was identified on histones H4 and H2A, also recognized by human HAT1 [Citation34–36].
The crystal structure of HAT1 revealed that it has an elongated shape formed by three domains: N-terminal, central, and C-terminal [Citation36]. These last two domains form a canyon that can be occupied by its substrate H4 peptide and its cofactor AcCoA. Although AcCoA is positioned in the same site in both human and yeast HAT1 homologue, molecular binding is different. In human HAT1, molecular binding is stronger due to interactions of its Phe288 and Lys284 residues (in the C-terminal domain) with the ribose and adenine rings of the cofactor; in yeast Hat1p, these amino acids are substituted by Arg267 and Asp263, preventing any interactions between the protein and the adenine ring of the cofactor. In addition, Ile186, Pro278 and Tyr282 of human HAT1 facilitate the acetyl group of the cofactor to be in proximity with the ε-amino group of Lys12 of the histone H4 peptide [Citation36]. Binding of the histone H4 peptide to the enzyme results in Lys12 to be positioned in the deep canyon between the central and C-terminal domains, and to be very close to Glu187, Glu276, and Asp277 of the enzyme; while the side chain of the histone can interact with Ile186 of HAT1, considered important for proper orientation of the peptide [Citation36].
In order to investigate the role of these specific amino acid residues and how they might influence HAT1 activity, Glu187, Glu276, and Asp277 were mutated and kinetic properties were evaluated. Only the Glu276 mutant displayed a strong decrease in kinetic activity compared to the wild type, suggesting that this amino acid residue may be involved in the deprotonation of the ε-amino group of Lys12 of histone H4. This crystal model strengthened the findings that HAT1 preferentially acetylates Lys12 of histone H4 [Citation36].
Cellular localization and function of HAT1
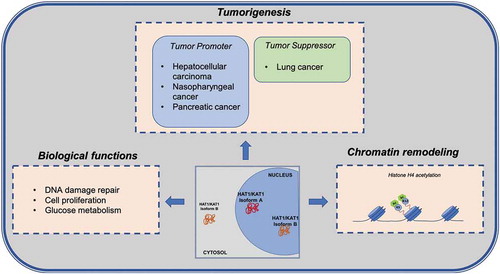
HAT1 has two transcriptional isoforms, named a and b, in normal human keratinocytes (NHKs) [Citation40]. Isoform a is exclusively nuclear (nuclear matrix) and encompasses 418 amino acids, whereas isoform b is both cytoplasmic and nuclear and encompasses 334 amino acids [Citation40]. Interestingly, distribution of the isoforms was found to change upon stress treatments. NHKs treated with hydrogen peroxide or 1000 MeV/n iron ions presented enhanced HAT1 nuclear immunostaining compared to untreated cells. At protein level, the amount of nuclear HAT1 in untreated and treated cells was the same, suggesting that the increased staining was due to a redistribution of the protein within the nucleus, and not to enhanced HAT1 translocation after treatment [Citation40].
As a type B HAT, the role of HAT1 in the cytoplasm was long recognized. However, later studies showed that it was predominantly nuclear [Citation41–43], prompting efforts to investigate its translocation into the nucleus and whether partner proteins are involved.
Unlike other enzymes, which usually leave their substrates soon after catalysis, HAT1 stably binds histones H3/H4. HAT1 holoenzyme has been in fact observed in a complex with histones H3/H4 and karyopherin/importin in yeast [Citation44]. The same finding was subsequently reported in other organisms such as human, chicken, and Physarum polycephalum, in which HAT1 holoenzyme also binds histone chaperones [Citation45–48].
Although the epigenetic marker, histone H4 Lys5 and Lys12 acetylation was discovered several years ago, its function in chromatin assembly remains to be clarified [Citation22,Citation42]. As one of the main steps of chromatin assembly is histone complex import, it was speculated that the highly conserved HAT1 and its principal target (histone H4 Lys5 and Lys12 acetylation) are implicated in facilitating histone nuclear import/deposition and chromatin assembly/maturation.
The first histone-chaperone complex found to bind HAT1 holoenzyme and histones H3/H4 is the mammalian nuclear autoantigenic sperm protein (NASP). A recent study corroborates the link between testicular NASP (tNASP) and newly synthesized histones H3 and H4. Specifically, reduction of JMJD1B (histone demethylase) increases the expression levels of tNASP which leads to the accumulation of histones H3 and H4 at the early phase of their maturation [Citation49]. Although it has a low degree of homology with the budding yeast protein Hat1-interacting factor 1 (Hif1) [Citation50], both are able to bind the H3/H4 heterodimer [Citation43]. In human, the HAT1/RbAp46/NASP/H3/H4 complex interacts with the histone chaperone anti-silencing function protein 1 (ASF1), an evolutionarily conserved protein [Citation45,Citation46,Citation51], via the carboxyl region of histone H3 [Citation22,Citation46]. The mechanism by which histones H3/H4 are recruited to ASF1 is still unclear. ASF1 is responsible for nucleosome assembly and localizing histones to DNA repair sites. It has a high affinity to nuclear transport receptors and H3/H4 can therefore be shuttled from the cytoplasm to the nucleus by specific karyopherins/importins, and subjected to further acetylation and histone chaperone bindings such as those leading to nucleosome and chromatin assembly [Citation44,Citation45,Citation52,Citation53] ().
Figure 1. Representative model of the mechanism by which HAT1/KAT1 cytoplasmic complexes shuttle into the nucleus
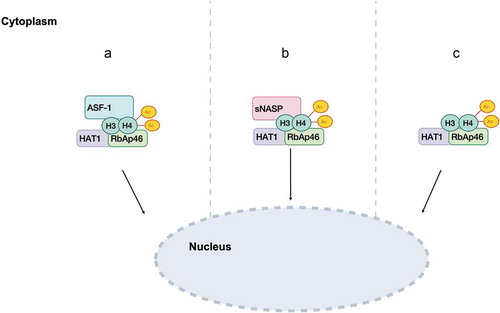
It was postulated that HAT1 could enter the nucleus through different pathways [Citation22,Citation54] (: A, B, C). The first hypothesis is that HAT1/RbAp46 enters the nucleus remaining bound to H3/H4 and ASF1. Upon release from ASF1, HAT1/RbAp46 might still bind histones H3/H4, perhaps even after their deposition [Citation46,Citation51]. The second model proposes that HAT1/RbAp46/H3/H4 could be transferred into the nucleus while binding to NASP and localize to DNA within this complex [Citation22,Citation42,Citation46]. Thirdly, it was suggested HAT1 complex may enter the nucleus by itself [Citation22,Citation54] ().
Although HAT1-mediated di-acetylation of histone H4 at Lys5 and Lys12 was initially shown to facilitate histone nuclear import in human and yeast [Citation47,Citation53], later reports showed that acetylation of histone H4 N-terminal tail seemed to be insufficient for sustaining histone nuclear import, and in some cases might interfere with histone nuclear shuttling [Citation55–57].
Recent studies support the notion that HAT1 may not only have a catalytic activity in the cytoplasm, but association of HAT1 complexes to histones H3/H4 may be involved in their delivery to chromatin assembly factors [Citation56,Citation58].These findings are supported by studies using HAT1−/- and HAT1+/+ mouse embryonic fibroblasts (MEFs), in which HAT1 is not required for either nuclear transportation or histone deposition (H2A, H2B, H3, H4) onto newly replicated DNA [Citation58]. However, HAT1/RbAp46 might control chromatin maturation since this process begins when histone H4 Lys5 and Lys12 is deacetylated, and the latter event does not take place until HAT1/RbAp46 is displaced from chromatin [Citation56,Citation58]. It was further shown that acetylation of histone H4 (Lys5 and Lys12) leads to reorganization of bromodomain-containing proteins such as Brg1, Baz1A, and Brd3 on nascent DNA, and that HAT1 is involved in their recruitment [Citation56].
HAT1 and DNA replication
The interaction between HAT1 and the replication fork was found to be transient and higher in HAT1+/+ compared to HAT1−/- MEF cells using a method for identifying proteins associated with, or in close proximity, to newly replicated DNA. However, this association became more stable when replication forks stalled [Citation54]. It was suggested that HAT1 binds to nascent chromatin and may promote acetylation of non-histone substrates, as reported in a recent study on the acetylome of HAT1+/+ and HAT1−/- MEFs grown in both glucose- and galactose-containing media, in which HAT1−/- cells displayed a decrease in acetylated proteins involved in chromatin structure, transcriptional regulation, and mitochondrial function [Citation59].Interestingly, a link between Hat1p/Hat2p and DNA replication was previously shown in yeast, where loss of either HAT1 or HAT2 (RbAp46) genes combined with origin recognition complex (ORC) proteins Orc-2 and Orc-5 temperature-sensitive mutant yeast cells led to growth defects. Specifically, it was demonstrated that the ORC subunits interacted with Hat1p/Hat2p during S-phase, and that this association did not change throughout the cell cycle. Although the underlying mechanism is not yet understood, histone H4 Lys5 and Lys12 acetylation may remodel the chromatin structure of some specific Orc proteins [Citation60].
Role of HAT1 in DNA repair and telomeric silencing
Loss of HAT1 in MEF cells increased its sensitivity to single- and double-strand break DNA damage, suggesting a role for this enzyme in DNA damage repair. Specifically, HAT1−/- and HAT1+/+ MEFs were treated with methyl methanesulfonate or hydroxyurea to induce double-strand breaks, or with UV irradiation, which causes single-strand breaks. MEF cells depleted of HAT1 were shown to be susceptible to all these agents. These findings were confirmed by expression analysis of γH2AX; HAT1−/- MEFs displayed a higher level of this mark than HAT1+/+MEFs, even in the absence of any DNA-damaging treatments. Furthermore, HAT1 depletion led to chromosomal abnormalities, such as aneuploidy, polyploidy, and chromatin breaks and fusions [Citation58].
HAT1 depletion was also found to affect homologous recombination, but not non-homologous end joining repair of double-strand breaks [Citation61]. To investigate the role of HAT1 in DNA repair, co-immunoprecipitation of FLAG-tagged HAT1 in HeLa cells was performed and identified several DNA repair proteins including RAD50, RAD51, HIRA, and HIRIP3 as HAT1 interactors [Citation61]. A possible mechanism proposed was that HAT1 facilitates DNA repair through histone H4 Lys5 and Lys12 acetylation, which in turn recruits repair proteins [Citation61].
Although depletion of HAT1 did not result in either DNA damage sensitivity or telomeric silencing in S. cerevisiae [Citation62,Citation63], observable effects were reported in mammals and in other organisms [Citation58,Citation61,Citation64–66]. For instance, HAT1 was shown to be necessary for viability in mammalians because its deletion led to neonatal lethality in mice due to cellular hyperproliferation, giving rise to defects in lung development [Citation58]. Unlike functional mutant of HAT1 in Drosophila Melanogaster which only showed a slight effect in viability and no effects on fertility [Citation57].
A study on the involvement of HAT1 in telomeric silencing investigated a possible functional redundancy with histone H3 and histone H4 [Citation67]. Different combinations of histone H3 tail mutants combined with HAT1 deletion were tested in yeast while growing in the presence or absence of 5-Fluoroorotic acid (5-FOA). Specifically, the telomeric URA3 gene allowed yeast cells to grow when 5-FOA was present in the medium, while when telomeric silencing was disrupted the cells became sensitive to this agent and died. A major effect on reduction of telomeric silencing was shown to occur when deletion of HAT1 was combined with histone H3K14R substitution. Importantly, the region of silent chromatin had a recognizable acetylation pattern, in which histone H4 remained unacetylated at Lys5, Lys8, and Lys16, while it was highly acetylated at Lys12 [Citation62]. The role of histone H4 acetylation was therefore also examined. By using specific H3 and H4 mutants (H3K9/K14R and H4K12R), Hat1p function in telomeric silencing was shown to be due to its ability to mediate histone H4 Lys12 acetylation [Citation67].
An in vivo study into the role of Hat1p in telomeric silencing was carried out in S. cerevisiae [Citation68]. To investigate how Hat1p enzymatic activity and cellular localization could affect this process, catalytic mutants, which showed a decrease in enzymatic activity, were tested in the presence or absence of specific histone H3 mutants (H3K9R/K14R) by evaluating expression of the telomeric URA3 gene. Catalytic Hat1p mutants showed defects in telomeric silencing compared to yeast strains with HAT1 wild type combined with H3K9R/K14R mutants. Furthermore, it was shown that Hat1p catalytic activity in the cytoplasm was not sufficient for proper cellular function in vivo: when its translocation into the nucleus was inhibited by the presence of a nuclear export signal fused to Myc-tagged HAT1 protein, a reduction in yeast viability occurred as a result of defective telomeric silencing, suggesting that both catalytic activity and nuclear localization are necessary to maintain telomeric silencing [Citation68].
The function of HAT1 in the immune system and in viral replication
The HDAC/HAT ratio regulates expression of several genes including those involved in inflammatory diseases. Type A HATs, such as CBP and p300, act towards several interleukins and regulate the pathway mediated by nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), which is responsible for modulating inflammatory response [Citation69]. It was recently shown that HAT1 is involved in the acetylation of a non-histone substrate, transcription factor promyelocytic leukaemia zinc finger (PLZF), thus regulating an NF-κB-mediated transcriptional programme[Citation70]. After toll-like receptor (TLR) and tumour necrosis factor (TNF) receptor signalling, HAT1 interacted with PLZF and in this process calcium/calmodulin-dependent protein kinase (CaMK2) had a functional role [Citation70].
A previous study identified a phosphorylation site at serine 361 of HAT1 which is preferentially recognized by CaMK2 [Citation71]. Since CaMK2 is involved in regulating NF-κB activity during innate immune response, it might directly or indirectly phosphorylate and trigger HAT1 activation during this response. Indeed, gene silencing of CaMK2 in human monocytic cell lines hampered HAT1/PLZF association upon TNF-α stimulation, indicating that NF-κB activity was regulated by HAT1 acetylation signalling, following its phosphorylation on serine 361 [Citation70].
Experiments performed on exogenous PLZF after immunoprecipitation in TLR4-HEK293T cells treated with lipopolysaccharide revealed that it was acetylated in two different lysine residues (Lys271 and Lys277) of the repressor domain [Citation70]. Specifically, the immunoprecipitation of mutated PLZF at these specific lysine residues showed that only acetylation of Lys277 was involved in repression of NF-κB activity after stimulation with lipopolysaccharide and TNF-α [Citation70].
Since PLZF was already reported to interact with HDACs to form a complex with NF-κB [Citation72,Citation73], it was speculated whether the formation of this complex might explain the repression and inactivation of NF-κB. A transcriptional study using luciferase assays demonstrated the presence of a complex formed by PLZF/HDAC3/NF-κB. Specifically, this complex was able to inhibit NF-κB activity, resulting in reduced inflammatory cytokine production, following TLR and TNF receptor signalling.
HAT1 and hepatitis B virus replication
Hepatitis B covalently closed circular DNA (cccDNA) is stably maintained in hepatocyte nuclei and is responsible for infection. Understanding its regulatory mechanism could therefore help to develop novel therapeutic treatments. It was recently shown that the host cell’s machinery may affect the nucleosome assembly of histones into cccDNA, forming cccDNA minichromosome [Citation74]. Given the well-known function of HAT1 in nucleosome assembly [Citation22,Citation56], studies investigated its role in viral infection and in particular whether it could also enhance hepatitis B virus (HBV) replication. One possible involvement of HAT1 in HBV comes from evidence that HAT1 mRNA and protein levels were higher in HBV-infected human liver-chimeric mice than in control human liver-chimeric mice, and that its subsequent depletion led to diminished levels of intracellular HBV-DNA and accumulation of cccDNA in HBV-infected human cells [Citation74]. Moreover, depletion of HAT1 in HBV-infected human cells led to a deregulation in histone H3/H4 assembly onto cccDNA. The mechanism by which HAT1 binds to cccDNA was elucidated, demonstrating an interaction between HAT1 and hepatitis B core protein (HBc), a structural protein of HBV important for viral replication. GST pull-down assays followed by purification of GST-HAT1 and His-HBc revealed the presence of an interaction between HAT1 and HBc in HBV-infected dHepaRG cells.
Since previous studies showed that long non-coding RNAs (lncRNAs) can function as scaffolds to regulate gene expression [Citation75], it was hypothesized that hepatocellular carcinoma up-regulated lncRNA (HULC) may allow the interaction between HAT1 and HBc in HBV-infected human liver-chimeric mice [Citation74]. Hepatitis B e-antigen (HBeAg) is commonly secreted into the supernatant at high levels during viral replication, and HULC knockdown was shown to lead to decreased levels of HBV-DNA and HBeAg in HBV-infected cells, indicating a decrease in replication. HAT1 was recruited onto cccDNA minichromosome following HBc binding, in a system in which lncRNA-HULC plays an important role, and this HAT1/HULC/HBc complex was proposed to regulate histone acetylation resulting in HBV transcription activation [Citation74].
HAT1 in human immunodeficiency virus infection
An additional role of HAT1 in immune system was described in human immunodeficiency virus (HIV) infection. A possible correlation between deregulated function of monocytes in HIV patients and epigenetic modifications had already been reported [Citation76]. Evidence of a possible involvement of HAT1 in HIV was put forward after observing increased levels of genes related to transcription activation, including HAT1, in HIV+ patients with high soluble sCD163, a marker of monocyte and macrophage activation [Citation76]. Cells from sCD163-high HIV patients stimulated with Mycobacterium tuberculosis displayed an increased production of TNF-α, IL-6, IL-1β, and CCL2 compared to sCD163-low HIV patients and non-infected controls, indicating that HAT1 gene activation correlates with monocyte activity. In particular, it was shown for the first time that HAT1 is a good progression biomarker, since its level was considerably different between sCD163-low and scCD163-high HIV+ patients. Although the exact role of HAT1 in the process is still unclear, it was suggested as a potential target for restoring correct systemic immune activation following viral and opportunistic infections, such as tuberculosis [Citation76].
HAT1 and cancer
The involvement of HAT1 in chromatin assembly and DNA damage repair, whose alteration can induce tumour development, invasion, and metastasis, prompted investigations into the role of HAT1 in tumorigenesis. The acetylation of histones leads to a different regulation of gene expression involved in distinct pathways, with a consequent change in glucose metabolism, promoting glycolysis, and activation of cell death pathway [Citation77,Citation78]. To date, little evidence directly implicates HAT1 function with cancer development. The contradictory role of HAT1 in carcinogenesis lies in its varying expression levels observed in different types of solid tumours. Specifically, lower expression of HAT1 is associated with the pathogenesis of lung cancer [Citation78], while in hepatocellular carcinoma (HCC), nasopharyngeal cancer, and pancreatic cancer [Citation77,Citation79,Citation80] it was found highly expressed, acting as an oncogene, and was linked to poor prognosis [Citation80]. In lung cancer, HAT1 was found closely associated with Fas. Restoration of HAT1 induced expression of Fas and activation of cell death pathway, driving lung cancer cells to apoptosis [Citation78]. In pancreatic ductal adenocarcinoma, HAT1 was also shown to have a role in BRD4-mediated cancer immunity response, where it is able to regulate programmed death-ligand 1 expression with consequent promotion of tumorigenesis [Citation80].
Recent studies reported a link between HAT1 and cell proliferation via modulation of gene transcription. The depletion of HAT1 in cancer cells, as reported for HCC [Citation77], pancreatic cancer [Citation80], and oesophageal carcinoma [Citation81], determines a block of cell growth and proliferation in vitro and in vivo [Citation77,Citation80] due to a cell cycle progression defect with consequent G2/M blockage [Citation81]. In all these studies HAT1 expression levels in cancer cells or tumour tissues were assessed without taking into account its subcellular localization or its isoforms. However, a previous report underscored the importance of HAT1 localization as it was found to alter dramatically in normal tissue, primary carcinoma, and cells that metastasize [Citation82]. Specifically, cytoplasmic levels of HAT1 increased in primary and metastatic tumours compared to human normal colonic mucosa; nuclear staining of HAT1 was comparable in normal and primary tumours, whereas it tended to decrease in metastases [Citation82].
HAT1 also seems to play an important role in other biological pathways related to cancer initiation as well as in glucose metabolism regulation, [Citation77,Citation83]. In HCC, HAT1 is upregulated and involved in regulating metabolism, especially in glycolysis promotion, and HAT1 knockdown sensitized HCC cells to cell death induced by cisplatin, highlighting its potential role in novel therapeutic strategies [Citation77]. Depletion of HAT1 or RbAp46 in the human mammary epithelial cell line immortalized with telomerase (hTert-HME1) showed diminished levels of histones H3 and H4, while H2A and H2B levels remained the same. In particular, the HAT1/RbAp46 complex bound a specific site located upstream of the start codon of histone H4 promoters regulating transcription of H4 genes, demonstrated to be also glucose and acetate sensitive. Acetate is the product of histone deacetylation, and it was investigated whether administration of exogenous acetate could interfere with HAT1 activity in cells depleted of this enzyme [Citation83]. The presence of acetate restored histone H3 and H4 levels, with no effect on acetylation of histone H4. Acetate was able to regenerate AcCoA via activity of Acyl-CoA short-chain synthetase family member 2 which functions when glucose levels are limited. Therefore, histone H4 deacetylation resulted in acetate formation which in turn led to AcCoA production, and this mechanism enhanced H4 protein levels.Interestingly, depletion of HAT1 in hTert-HME control and HAT1-depleted cells caused a decrease in histone H3 Lys9 acetylation affecting several pathways, including cell proliferation, cell cycle, DNA damage, RNA processing, nucleosome assembly, and kinetochore functioning. It was suggested that di-acetylated histone H4 was a source of acetyl groups which HAT1 could provide to other HATs, such as GCN5, one of the main enzymes responsible for H3 Lys9 acetylation, making chromatin more accessible [Citation83].
HAT1 modulation
HAT inhibitors are divided into three categories, bisubstrate inhibitors, natural products, and synthetic compounds, all able to hamper the catalytic activity of HAT enzymes [Citation84–86]. The majority of inhibitors target type A HATs, such as p300 and PCAF, which unlike type B HATs have well-described structures and cellular functions [Citation87–90] (). Consequently, there is a huge discrepancy between the number of molecules developed for type A and B HATs.
Table 2. Bisubstrate inhibitors of type A and B HATs
To identify HAT1 inhibitors, a recent study developed a set of different H4-CoA conjugates based on the premise that i) the main substrates of HAT1 are Lys 5 and Lys12 of histone H4 [Citation34,Citation58] and ii) peptide-CoA bisubstrates had already been synthesized and shown to be selective inhibitors of the type A HATs p300 and PCAF [Citation91–95]. Specifically, these peptides contained amino acids 1 to 20 of histone H4 (H4-20) linked to a CoA moiety at Lys5, Lys8, Lys12, or Lys16 [Citation95] (). Of all the peptide-CoA substrates tested, H4K12CoA bound HAT1 with nanomolar potency, indicating that it is a specific inhibitor (; ) [Citation95]. This high substrate specificity could partly be explained by its binding in the human crystal structure [Citation36], in which the proximity of the sulphur group of CoA to the amino group of Lys12 in the H4-20 peptide was comparable to the distance between AcCoA and Lys12 of histone H4 (3.3 Å and 4.5 Å, respectively) [Citation36,Citation95].
Table 3. HAT1 inhibitors and their sequences
Based on the above study, a set of HAT1 inhibitors was recently designed (). However, several issues (typical of bisubstrate inhibitors) still need to be addressed, such as delivery (cell permeability), toxicity, specificity, and synthetic availability [Citation86]. One possible approach may be to develop a construct containing cell-permeabilizing peptide sequences, thereby facilitating the entrance of these inhibitors through the cellular membrane, as previously done for a series of derivatives of Lys-CoA, a potent inhibitor of p300/CBP HAT activity [Citation85].
Although recently identified bisubstrate inhibitors have provided an insight into the enzymatic properties of HAT1, further molecular and functional studies will be required to design more specific and effective HAT1 inhibitors, which may have major implications in the clinic.
Conclusion
While HAT1 was initially thought only to be involved in acetylation of newly translated histone H4 at Lys5 and Lys12 in the cytoplasm, new studies have revealed the importance of this enzyme in nuclear function. Uncovering the non-canonical role of HAT1 first in DNA damage repair, then in chromatin assembly and chromatin maturation prompted in-depth investigations into the functional role of this histone acetyltransferase in several biological processes (). Further studies are needed to better characterize its two isoforms and their possible implications in pathogenesis. Since HAT1 plays an important role in cell proliferation, histone production, and glucose metabolism, it may prove to be a potential target for future cancer therapies. While efforts to identify molecules able to block its enzymatic activity are ongoing, developing compounds targeting one of its interactors could provide another entry point for HAT1 inhibition. It will therefore be important to assess in which protein complexes HAT1 resides. In sum, a greater molecular understanding of HAT1 could shed more light on its role, ultimately allowing for the design and development of novel effective inhibitors for therapeutic intervention.
Acknowledgments
We thank C. Fisher for English language editing.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Brownell JE, Allis CD. Special HATs for special occasions: linking histone acetylation to chromatin assembly and gene activation. Curr Opin Genet Dev. 1996;6:176–184.
- Biswas S, Rao CM. Epigenetic tools (The writers, the readers and the erasers) and their implications in cancer therapy. Eur J Pharmacol. 2018;837:8–24.
- Xu H, Wang Y, Lin S, et al. PTMD: A database of human disease-associated post-translational modifications. Genom Proteom Bioinform. 2018;16:244–251.
- Yang X-J. The diverse superfamily of lysine acetyltransferases and their roles in leukemia and other diseases. Nucleic Acids Res. 2004;32:959–976.
- Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis. 2010;31:27–36.
- Allfrey VG, Faulkner R, Mirsky AE. Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis. Proc Natl Acad Sci. 1964;51:786–794.
- Ehrenhofer-Murray AE. Chromatin dynamics at DNA replication, transcription and repair. Eur J Biochem. 2004;271:2335–2349.
- Thiagalingam S, Cheng KH, Lee HJ, et al. Histone deacetylases: unique players in shaping the epigenetic histone code. Ann N Y Acad Sci. 2003;983:84–100.
- Seto E, Yoshida M. Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harb Perspect Biol. 2014;6:a018713–a018713.
- Josling GA, Selvarajah SA, Petter M, et al. The role of bromodomain proteins in regulating gene expression. Genes (Basel). 2012;3:320–343.
- Tessarz P, Kouzarides T. Histone core modifications regulating nucleosome structure and dynamics. Nat Rev Mol Cell Biol. 2014;15:703–708.
- Murr R, Loizou JI, Yang Y-G, et al. Histone acetylation by Trrap–Tip60 modulates loading of repair proteins and repair of DNA double-strand breaks. Nat Cell Biol. 2006;8:91–99.
- Gong F, Miller KM. Mammalian DNA repair: HATs and HDACs make their mark through histone acetylation. Mutat Res Mol Mech Mutagen. 2013;750:23–30.
- Ruiz-Carrillo A, Wangh L, Allfrey V. Processing of newly synthesized histone molecules. Science. 1975;190:117–128.
- Jackson V, Shires A, Tanphaichitr N, et al. Modifications to histones immediately after synthesis. J Mol Biol. 1976;104:471–483.
- Annunziato AT, Hansen JC. Role of histone acetylation in the assembly and modulation of chromatin structures. Gene Expr. 2001;9:37–61.
- Krude T. Chromatin: nucleosome assembly during DNA replication. Curr Biol. 1995;5:1232–1234.
- Verreault A. De novo nucleosome assembly: new pieces in an old puzzle. Genes Dev. 2000;14:1430–1438.
- Campos EI, Reinberg D. Histones: annotating chromatin. Annu Rev Genet. 2009;43:559–599.
- Sobel RE, Cook RG, Perry CA, et al. Conservation of deposition-related acetylation sites in newly synthesized histones H3 and H4. Proc Natl Acad Sci. 1995;92:1237–1241.
- Kuo MH, Brownell JE., Sobel RE, et al. Transcription-linked acetylation by Gcn5p of histones H3 and H4 at specific lysines. Nature. 1996;383:269–272.
- Parthun MR. Histone acetyltransferase 1: more than just an enzyme? Biochim Biophys Acta - Genet Regul Mech. 2012;1819:256–263.
- Chicoine LG, Schulman IG, Richman R, et al. Nonrandom utilization of acetylation sites in histones isolated from Tetrahymena evidence for functionally distinct H4 acetylation sites. J Biol Chem. 1986;261.
- Benson LJ, Gu Y, Yakovleva T, et al. Modifications of H3 and H4 during chromatin replication, nucleosome assembly, and histone exchange. J Biol Chem. 2006;281:9287–9296.
- Cousens LS, Alberts BM. Accessibility of newly synthesized chromatin to histone acetylase. J Biol Chem. 1982;257.
- Loyola A, Bonaldi T, Roche D, et al. PTMs on H3 variants before chromatin assembly potentiate their final epigenetic state. Mol Cell. 2006;24:309–316.
- Sakamoto A, Terui Y, Uemura T, et al. Polyamines regulate gene expression by stimulating translation of histone acetyltransferase mRNAs. J Biol Chem. 2020;295:8736–8745.
- Di Martile M, Del Bufalo D, Trisciuoglio D. The multifaceted role of lysine acetylation in cancer: prognostic biomarker and therapeutic target. Oncotarget. 2016;7:55789–55810.
- Trisciuoglio D, Di Martile M, Del Bufalo D. Emerging role of histone acetyltransferase in stem cells and cancer. Stem Cells Int. 2018;2018:1–11.
- Avvakumov N, Côté J. The MYST family of histone acetyltransferases and their intimate links to cancer. Oncogene. 2007;26:5395–5407.
- Iyer NG, Özdag H, Caldas C. p300/CBP and cancer. Oncogene. 2004;23:4225–4231.
- McCullough CE, Marmorstein R. Molecular basis for histone acetyltransferase regulation by binding partners, associated domains, and autoacetylation. ACS Chem Biol. 2016;11:632–642.
- Kleff S, Andrulis ED, Anderson CW, et al. Identification of a gene encoding a yeast histone H4 acetyltransferase. J Biol Chem. 1995;270:24674–24677.
- Parthun MR, Widom J, Gottschling DE. The major cytoplasmic histone acetyltransferase in yeast: links to chromatin replication and histone metabolism. Cell. 1996;87:85–94.
- Verreault A, Kaufman PD, Kobayashi R, et al. Nucleosomal DNA regulates the core-histone-binding subunit of the human Hat1 acetyltransferase. Curr Biol. 1998;8:96–108.
- Wu H, Moshkina N, Min J, et al. Structural basis for substrate specificity and catalysis of human histone acetyltransferase 1. Proc Natl Acad Sci U S A. 2012;109:8925–8930.
- Murzina NV, Pei XY, Zhang W, et al. Structural basis for the recognition of histone H4 by the histone-chaperone RbAp46. Structure. 2008;16:1077–1085.
- Lusser A, Eberharter A, Loidl A, et al. Analysis of the histone acetyltransferase B complex of maize embryos. Nucleic Acids Res. 1999;27:4427–4435.
- Imhof A, Wolffe AP. Purification and properties of the xenopus Hat1 acetyltransferase: association with the 14-3-3 proteins in the oocyte nucleus. Biochemistry. 1999;38:13085–13093.
- Lebel EA, Boukamp P, Tafrov ST. Irradiation with heavy-ion particles changes the cellular distribution of human histone acetyltransferase HAT1. Mol Cell Biochem. 2010;339:271–284.
- Ruiz-García AB, Sendra R, Galiana M, et al. Hat1 and Hat2 proteins are components of a yeast nuclear histone acetyltransferase enzyme specific for free histone H4. J Biol Chem. 1998;273:12599–12605.
- Ai X, Parthun MR. The nuclear Hat1p/Hat2p complex. Mol Cell. 2004;14:195–205.
- Poveda A, Pamblanco M, Tafrov S, et al. Hif1 is a component of yeast histone acetyltransferase B, a complex mainly localized in the nucleus. J Biol Chem. 2004;279:16033–16043.
- Mosammaparast N, Guo Y, Shabanowitz J, et al. Pathways mediating the nuclear import of histones H3 and H4 in yeast. J Biol Chem. 2002;277:862–868.
- Barman HK, Takami Y, Nishijima H, et al. Histone acetyltransferase-1 regulates integrity of cytosolic histone H3-H4 containing complex. Biochem Biophys Res Commun. 2008;373:624–630.
- Campos EI, Fillingham J, Li G, et al. The program for processing newly-synthesized histones H3.1 and H4 HHS public access author manuscript. Nat Struct Mol Biol. 2010;17:1343–1351.
- Ejlassi-Lassallette A, Mocquard E, Arnaud M-C, et al. H4 replication-dependent diacetylation and Hat1 promote S-phase chromatin assembly in vivo. Mol Biol Cell. 2011;22:245–255.
- Saade E, Mechold U, Kulyyassov A, et al. Analysis of interaction partners of H4 histone by a new proteomics approach. Proteomics. 2009;9:4934–4943.
- Saavedra F, Gurard-Levin ZA, Rojas-Villalobos C, et al. JMJD1B, a novel player in histone H3 and H4 processing to ensure genome stability. Epigene Chromat. 2020;13. DOI:10.1186/s13072-020-00331-1.
- Dunleavy EM, Pidoux AL, Monet M, et al. A NASP (N1/N2)-related protein, Sim3, binds CENP-A and is required for its deposition at fission yeast centromeres. Mol Cell. 2007;28:1029–1044.
- Fillingham J, Recht J, Silva AC, et al. Chaperone control of the activity and specificity of the histone H3 acetyltransferase Rtt109. Mol Cell Biol. 2008;28:4342–4353.
- Blackwell JS, Wilkinson ST, Mosammaparast N, et al. Mutational analysis of H3 and H4 N termini reveals distinct roles in nuclear import. J Biol Chem. 2007;282:20142–20150.
- Alvarez F, Muñoz F, Schilcher P, et al. Sequential establishment of marks on soluble histones H3 and H4. J Biol Chem. 2011;286:17714–17721.
- Agudelo Garcia PA, Lovejoy CM, Nagarajan P, et al. Histone acetyltransferase 1 is required for DNA replication fork function and stability. J Biol Chem. 2020;295:8363–8373.
- An S, Yoon J, Kim H, et al. Structure-based nuclear import mechanism of histones H3 and H4 mediated by Kap123. Elife. 2017;6. DOI:10.7554/eLife.30244
- Garcia PAA, Hoover ME, Zhang P, et al. Identification of multiple roles for histone acetyltransferase 1 in replication-coupled chromatin assembly. Nucleic Acids Res. 2017;45:9319–9335.
- Varga J, Korbai S, Neller A, et al. Hat1 acetylates histone H4 and modulates the transcriptional program in drosophila embryogenesis. Sci Rep. 2019;9:17973.
- Nagarajan P, Ge Z, Sirbu B, et al. Histone acetyl transferase 1 is essential for mammalian development, genome stability, and the processing of newly synthesized histones H3 and H4. PLoS Genet. 2013;9. DOI:10.1371/journal.pgen.1003518.
- Agudelo Garcia PA, Nagarajan P, Parthun MR. Hat1-dependent lysine acetylation targets diverse cellular functions. J Proteome Res. 2020;19:1663–1673.
- Suter B, Pogoutse O, Guo X, et al. Association with the origin recognition complex suggests a novel role for histone acetyltransferase Hat1p/Hat2p. BMC Biol. 2007;5:38.
- Yang X, Li L, Liang J, et al. Histone acetyltransferase 1 promotes homologous recombination in DNA repair by facilitating histone turnover. J Biol Chem. 2013;288:18271–18282.
- Braunstein M, Sobel RE, Allis CD, et al. Efficient transcriptional silencing in saccharomyces cerevisiae requires a heterochromatin histone acetylation pattern. Mol Cell Biol. 1996;16:4349–4356.
- Qin S, Parthun MR. Histone H3 and the histone acetyltransferase Hat1p contribute to DNA double-strand break repair. Mol Cell Biol. 2002;22:8353–8365.
- Benson LJ, Phillips JA, Gu Y, et al. Properties of the type B histone acetyltransferase Hat1. J Biol Chem. 2007;282:836–842.
- Tong K, Keller T, Hoffman CS, et al. Schizosaccharomyces pombe Hat1 (Kat1) is associated with Mis16 and is required for telomeric silencing. Eukaryot Cell. 2012;11:1095–1103.
- Barman HK, Takami Y, Ono T, et al. Histone acetyltransferase 1 is dispensable for replication-coupled chromatin assembly but contributes to recover DNA damages created following replication blockage in vertebrate cells. Biochem Biophys Res Commun. 2006;345:1547–1557.
- Kelly TJ, Qin S, Gottschling DE, et al. Type B histone acetyltransferase Hat1p participates in telomeric silencing. Mol Cell Biol. 2000;20:7051–7058.
- Mersfelder EL, Parthun MR. Involvement of Hat1p (Kat1p) catalytic activity and subcellular localization in telomeric silencing. J Biol Chem. 2008;283:29060–29068.
- Mukherjee SP, Behar M, Birnbaum HA, et al. Analysis of the RelA: CBP/p300 interaction reveals its involvement in NF-κB-driven transcription. PLoS Biol. 2013;11:e1001647.
- Sadler AJ, Suliman BA, Yu L, et al. The acetyltransferase HAT1 moderates the NF-κB response by regulating the transcription factor PLZF. Nat Commun. 2015;6:6795.
- Olsen JV, Blagoev B, Gnad F, et al. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127:635–648.
- Lin RJ, Nagy L, Inoue S, et al. Role of the histone deacetylase complex in acute promyelocytic leukaemia. Nature. 1998;391:811–814.
- Yan Q, Carmody RJ, Qu Z, et al. Nuclear factor-κB binding motifs specify Toll-like receptor-induced gene repression through an inducible repressosome. Proc Natl Acad Sci U S A. 2012;109:14140–14145.
- Yang G, Feng J, Liu Y, et al. HAT1 signaling confers to assembly and epigenetic regulation of HBV cccDNA minichromosome. Theranostics. 2019;9:7345–7358.
- Yang L, Lin C, Jin C, et al. LncRNA-dependent mechanisms of androgen-receptor-regulated gene activation programs. Nature. 2013;500:598–602.
- Espíndola MS, Soares LS, Galvão-Lima LJ, et al. Epigenetic alterations are associated with monocyte immune dysfunctions in HIV-1 infection. Sci Rep. 2018;8:1–14.
- Jin X, Tian S, Li P. Histone acetyltransferase 1 promotes cell proliferation and induces cisplatin resistance in hepatocellular carcinoma. Oncol Res. 2017;25:939–946.
- Han N, Shi L, Guo Q, et al. HAT1 induces lung cancer cell apoptosis via up regulating Fas. Oncotarget. 2017;8:89970–89977.
- Miao BP, Zhang RS, Yang G, et al. Histone acetyltransferase 1 up regulates Bcl2L12 expression in nasopharyngeal cancer cells. Arch Biochem Biophys. 2018;646:72–79.
- Fan P, Zhao J, Meng Z, et al. Overexpressed histone acetyltransferase 1 regulates cancer immunity by increasing programmed death-ligand 1 expression in pancreatic cancer. J Exp Clin Cancer Res. 2019;38:1–12.
- Xue L, Hou J, Wang Q, et al. RNAi screening identifies HAT1 as a potential drug target in esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2014;7:3898–3907.
- Seiden-Long IM, Brown KR, Shih W, et al. Transcriptional targets of hepatocyte growth factor signaling and Ki-ras oncogene activation in colorectal cancer. Oncogene. 2006;25:91–102.
- Gruber JJ, Geller B, Lipchik AM, et al. HAT1 coordinates histone production and acetylation via H4 promoter binding. Mol Cell. 2019;75:711–724.e5.
- Luan Y, Ngo L, Han Z, et al. Histone Acetyltransferases. In: Epigenetic technological applications Elsevier; 2015. p 291–317. DOI: 10.1016/B978-0-12-801080-8.00014-4
- Zheng Y, Balasubramanyam K, Cebrat M, et al. Synthesis and evaluation of a potent and selective cell-permeable p300 histone acetyltransferase inhibitor. J Am Chem Soc. 2005;127:17182–17183.
- Huang M, Huang J, Zheng Y, et al. Histone acetyltransferase inhibitors: an overview in synthesis, structure-activity relationship and molecular mechanism. Eur J Med Chem. 2019;178:259–286.
- Eliseeva ED, Valkov V, Jung M, et al. Characterization of novel inhibitors of histone acetyltransferases. Mol Cancer Ther. 2007;6:2391–2398.
- Manzo F, Tambaro FP, Mai A, et al. Histone acetyltransferase inhibitors and preclinical studies. Expert Opin Ther Pat. 2009;19:761–774.
- Dekker FJ, Haisma HJ. Histone acetyl transferases as emerging drug targets. Drug Discov Today. 2009;14:942–948.
- Brown JAL. Patent spotlight: small-molecule lysine acetyltransferase inhibitors (KATi). Pharm Pat Anal. 2020;9:17–28.
- Lau OD, Kundu TK, Soccio RE, et al. HATs off: selective synthetic inhibitors of the histone acetyltransferases p300 and PCAF. Mol Cell. 2000;5:589–595.
- Lau OD, Courtney AD, Vassilev A, et al. p300/CBP-associated factor histone acetyltransferase processing of a peptide substrate. J Biol Chem. 2000;275:21953–21959.
- Sagar V, Zheng W, Thompson PR, et al. Bisubstrate analogue structure–activity relationships for p300 histone acetyltransferase inhibitors. Bioorg Med Chem. 2004;12:3383–3390.
- Cebrat M, Kim CM, Thompson PR, et al. Synthesis and analysis of potential prodrugs of coenzyme A analogues for the inhibition of the histone acetyltransferase p300. Bioorg Med Chem. 2003;11:3307–3313.
- Ngo L, Brown T, Zheng YG. Bisubstrate inhibitors to target histone acetyltransferase 1. Chem Biol Drug Des. 2019;93:865–873.