 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Increased homocysteine (Hcy) levels have been associated with a higher risk of cardiovascular and neurodegenerative diseases. Passive DNA demethylation has been suggested as one of the mechanisms implicated in the development of these conditions, and most studies have investigated this relationship in older adult populations. Therefore, this study aimed to evaluate the relationship between corporal composition and biochemical and haematological indicators with plasma homocysteine levels and genome-wide methylation (Alu, LINE-1, and SAT2) in a population of healthy young adults (median age, 18 years). We showed that the prevalence of hyperhomocysteinemia was significantly higher in men (18.5%) than in women (6.6%) (P = 0.034). Increased Hcy level was substantially associated with higher levels of body mass index and visceral fat in females, whereas in males, it was significantly associated with reduced red cell distribution width and high-density lipoprotein (HDL) cholesterol (HDL-C) levels and increased low-density lipoprotein/HDL ratio. Hypomethylation of Alu was significantly associated with reduced levels of HDL-C (<40.0 mg dL–1), whereas hypomethylation of LINE-1 and SAT2 was significantly associated with higher levels of skeletal muscle (<39.3%) in males. These results highlight the participation of hormonal factors in regulating Hcy metabolism, primarily in the female population, whereas changes in DNA methylation observed in males might be associated with the consumption of a protein diet with high levels of methionine, independent of increased Hcy levels.
Introduction
Homocysteine (Hcy) is a sulphur-containing amino acid, and its metabolism primarily depends on the methionine cycle and a minor proportion of the folic acid pathway. Increased plasma Hcy levels are primarily related to alimentary habits and/or vitamin B deficiency [Citation1]. Polymorphisms of genes implicated in the folate pathway have been identified as a determining factor in Hcy recycling through the methionine cycle. For example, genetic variations of the methylenetetrahydrofolate reductase gene (MTHFR: A1298G and C677T) have been found to be highly associated with the reduced conversion of Hcy into methionine, enhancing the risk of developing hyperhomocysteinemia (HHcy) [Citation1–3]. Although polymorphisms in genes related to methionine metabolism (e.g., methionine synthase and methionine synthase reductase) have also been associated with increased Hcy levels, epidemiological evidence is less conclusive [Citation4,Citation5]. Moreover, chronic consumption of drugs that modify folate metabolism (e.g., methotrexate and valproic acid) as well as liver and kidney malfunction also result in increased plasma Hcy levels [Citation1].
Hyperhomocysteinemia is recognized as a predictive risk factor for developing several cardiovascular (e.g., coronary artery disease and stroke), neurological, and dermatological diseases [Citation1,Citation6]. The pathological potential of HHcy is primarily related to its proatherogenic behaviour. An increase in Hcy concentration above 15 μmol L–1 in plasma is associated with the generation of a pro-oxidative environment, favouring the formation of reactive oxidative species and inhibiting the synthesis of elements with redox potential. These changes have been associated with a higher risk of endothelial vascular damage and altered platelet function, enhancing blood clot formation risk [Citation1]. Moreover, it has been proposed that the deleterious effects of HHcy may be exacerbated by the presence of alterations in lipid metabolism (dyslipidemia) as well as by the systemic inflammatory state observed in some degenerative diseases (e.g., obesity and multiple sclerosis) [Citation7].
Epidemiological evidence indicates that variations in Hcy levels may also affect the disposition of methyl-donor molecules (S-adenosylmethionine), thus affecting the rate of DNA, RNA, and protein methylation. In this regard, most studies have been performed using DNA extracted from blood cells [Citation8–10]; however, some studies using DNA extracted from liver and breast tissue have obtained data that support the hypothesis that increased plasma Hcy levels promote passive demethylation of genomic DNA [Citation11]. In fact, it has been observed that in HHcy-induced changes in Arthrobacter luteus (Alu) and long interspersed nuclear element-1 (LINE-1) sequence methylation, similarities with the alterations observed in in vitro models of diet-induced liver carcinogenesis were present [Citation12]. These associations could indicate that epigenetic modifications induced by Hcy changes in response to high-fat diets correspond to the initial stages of cellular transformation. However, most epidemiological studies that have evaluated the relationship between Hcy and DNA methylation have been conducted in adult populations with a mean age of 40 years. Since the degree of HHcy severity increases according to age in the majority of the population [Citation13], the effect of this association in young populations has been poorly understood. In this study, we aimed to examine the associations between biochemical and haematological markers, plasma Hcy levels, and the percentage of genome-wide methylation in a population of healthy young adults. Given that growing evidence suggests that obesity may be related to alterations in Hcy levels and its incidence has significantly increased in the young population, we also conducted an anthropometric evaluation through bioimpedance analysis.
Materials and methods
Study population
This cross-sectional study was conducted in the Academic Division of Comalcalco (Universidad Juarez Autonoma de Tabasco), Tabasco, Mexico. The recruitment of the study population (convenience sampling) was performed during clinical diagnosis at admission, from August to September 2017. All new students were invited to participate in the study, and the purpose and procedures of the protocol were explained (blood sample extraction and anthropometric evaluations). Students indicating interest in participating in the study provided written informed consent before sampling. The consumption of any drug was recorded during the interview, and patients who reported recent (1 month) or current consumption of vitamin supplements as well as pregnant women were excluded from the study.
This study was designed according to the International Standards for Responsible Research Publication (COPE), registered (UJAT-364/DI2015), and approved by the Institutional Review Board of the Juarez Autonomous University of Tabasco.
Anthropometric measurements and body composition assessment
Weight and height were measured with the participants wearing light clothing and no shoes; measurements were performed using a weighing station with a stadiometer (Basculas Nuevo Leon, Mexico). The body mass index (BMI) was calculated as kg m–2, and data were classified according to the World Health Organization criteria (https://www.who.int/). The body fat (%), visceral fat (%), and skeletal muscle mass (%) were determined by hand-to-foot bioelectrical impedance analysis according to the manufacturer´s instructions (HBF-514 C; Omrom Healthcare, Osaka, JP). The accuracy of the anthropometric measurements was 0.4 kg and 0.5 cm for weight and height, respectively, 3.0% for visceral fat, and 3.5% for skeletal muscle and body fat. The data were categorized according to the criteria described by Gallagher et al. [Citation14].
Measurement of the haematologic and metabolic parameters
For the haematological and metabolic characterization of the participants, blood samples were collected after an overnight fasting period of 8 h. Haematology tests were performed on ethylenediaminetetraacetic acid (EDTA)–treated blood using an automatic haematology analyser (BC-2800; Mindray, Shenzhen, China), whereas the levels of cholesterol, triglycerides, high-density lipoprotein (HDL) cholesterol (HDL-C), low-density lipoprotein (LDL) cholesterol (LDL-C), and Hcy were determined via enzymatic assays (Randox, Crumlin, UK) using an automated clinical chemistry analyser (Selectra PRO XS; ELITechGroup, Puteaux, France). A Hcy level higher than 15.0 μmol L–1 was considered as HHcy. Very-low-density lipoprotein (VLDL) cholesterol levels were calculated according to the equation previously reported by Martin et al. [2013, Citation15]. The atherogenic index of plasma (AIP) was calculated using a previously validated equation [Citation16]:
The AIP values were categorized according to the following criteria: low-risk (<0.1), medium-risk (0.1–0.24), and high-risk (>0.24) [Citation16].
Peripheral blood mononuclear cell (PBMC) isolation and DNA extraction
The PBMCs were extracted using Lymphoprep density gradient medium according to the manufacturer’s instructions (Axis-Shield Diagnostics, Dundee, UK). The PBMCs were isolated from EDTA-treated blood within 2 h after sample collection and stored at −80°C until DNA isolation was performed. Thereafter, genomic DNA was isolated using a Wizard Genomic DNA Purification Kit according to the manufacturer´s instructions (Promega, Madison, WI, USA). The quality and quantity of the extracted DNA were evaluated using an Epoch Microplate Spectrophotometer (Biotek, Winooski, VT, USA). Finally, the integrity of the extracted DNA was evaluated by amplifying β-globin using real-time PCR.
DNA methylation analysis
Aliquots of 500 ng DNA were treated with sodium bisulphite using an EZ DNA Methylation-Gold Kit according to the manufacturer´s instructions (Zymo Research, Irvine, CA, USA). The methylation status of the Alu, LINE-1, and SAT2 sequences was analysed by real-time PCR, according to the conditions previously reported by Weisenberger et al. [2005, Citation17]. The percentage of methylated DNA was determined by preparing a 10-fold standard dilution curve using human methylated genomic DNA (Thermo Fisher Scientific, Waltham, MA, USA). The methylation levels of Alu, LINE-1, and SAT2 were normalized according to input DNA (ALU-CT) levels and were expressed as a percentage of methylated reference. The indicated values represent the mean values and standard deviations of assays performed in triplicate.
Statistical analysis
Statistical analyses were performed using SPSS software (v.22; SPSS Inc., Chicago, IL, USA) and GraphPad Prism version 8.0 for Mac (GraphPad Software, San Diego, CA, USA). The Mann–Whitney U-test was used for quantitative variables. Descriptive statistics were presented as mean ± standard deviations for normally distributed values and medians (25th and 75th percentile) for skewed variables. Spearman’s correlation coefficients were used to describe the correlations between plasma Hcy levels and the anthropometric, biochemical, and haematological variables. Values of ALU, LINE-1 and SAT2 methylation were compared using the Student’s t-test. Values of global DNA methylation were transformed using the natural log function for linear regression analysis. The distribution of Hcy concentrations according to the clinical classification of anthropometric parameters was evaluated using the Kruskal–Wallis test. Statistical significance was set at P < 0.05.
Results
Characteristics of study participants
A total of 348 individuals participated in this study; 240 (68.9%) were women, and 108 (31.0%) were men. shows the distribution of the anthropometric, biochemical, and haematological indicators by sex. According to the corporal composition, no significant differences were observed in the BMI between women and men (P = 0.500). However, females had higher percentages of body fat than males, whereas the median values of visceral fat and skeletal muscle were significantly higher in males than those of females. The haematological evaluation showed that females had significantly lower values for variables related to the number and content of red blood cells (erythrocytes, haematocrit, haemoglobin, and mean corpuscular haemoglobin) compared with those of males. In contrast, the leukocyte and platelet counts were significantly higher in females than in males. According to the biochemical evaluation, most lipid metabolism indicators showed no significant differences between females and males; only Hcy and HDL-C levels showed statistically significant differences ().
Table 1. Anthropometric, biochemical, and haematological indicators of the studied population according to gender.
Anthropometric, haematological, and lipid alterations associated with hyperhomocysteinemia
shows Spearman’s correlation coefficients between Hcy and the anthropometric, haematological, and lipid indicators. Among the anthropometric indicators, body fat and skeletal muscle were significantly correlated with Hcy in the female group. Of the lipid indicators, the LDL-C and LDL/HDL ratio positively correlated with Hcy in the female group, whereas in males, the HDL-C and AIP were significantly correlated with Hcy.
Table 2. Anthropometric, haematological, and lipid indicators associated with the homocysteine (Hcy) level.
According to the clinical criteria of HHcy (>15.0 μmol L–1), the overall prevalence in the studied population was 10.3% (36/348), which was considerably more frequent in males (18.5%) than in the female group (6.6%; chi-squared test, P = 0.034). Furthermore, the occurrence of HHcy in the female population was significantly associated with a higher BMI and visceral fat levels, whereas in males, HHcy was significantly associated with reduced red cell distribution width (RDW-CV) and HDL-C levels, and higher LDL/HDL ratios ().
Table 3. Levels of anthropometric, haematological, and lipid indicators according to the hyperhomocysteinemia (HHcy) criteria.
Anthropometric, haematological, and lipid indicators associated with DNA methylation
According to the mean values of Alu, LINE-1, and SAT2 methylation, no statistically significant differences were observed between females and males (Alu, P = 0.326; LINE-1, P = 0.279; SAT2, P = 0.185; ). Nevertheless, linear correlation analysis showed a higher level of correlation between LINE-1 and SAT2 methylation (females, r = 0.88; males, r = 0.94) compared with Alu-LINE1 and/or ALU-SAT2 methylation, which were statistically significant in both women and men ().
Table 4. Anthropometric, haematological, and biochemical indicators associated with the methylation levels of Alu, LINE-1, and SAT2.
Figure 1. The linear associations among Alu, LINE-1, and SAT2 methylations were evaluated according to gender: (a–c) females and (d–f) males. Alu, LINE-1, and SAT2 methylation percentages were transformed using the natural log function for linear regression.
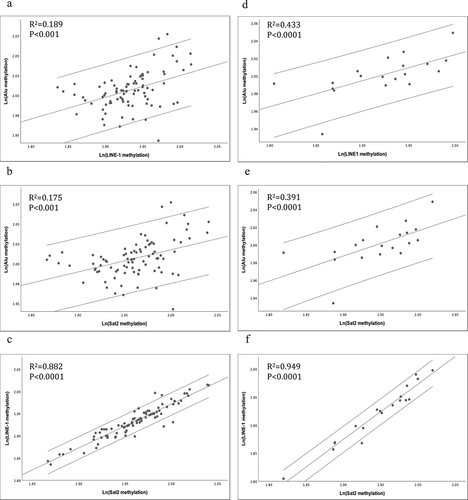
shows Spearman’s correlation coefficients between DNA methylation and the anthropometric, haematological, and lipid indicators. The bivariate analysis of Alu methylation showed significant differences according to gender; Alu methylation was positively correlated with skeletal muscle and HDL-C in females and males, respectively. However, LINE-1 and SAT2 methylation showed the same associations according to gender; in females, LINE-1 and SAT2 methylation was negatively correlated with haematocrit and mean corpuscular volume, whereas in males, their methylation was positively correlated with Hcy level, BMI, and body and visceral fat and negatively correlated with skeletal muscle. We also analysed Alu, LINE-1, and SAT2 methylation stratified with previously associated variables according to the clinical criteria (). Hypomethylation of Alu was significantly associated with reduced HDL-C levels (<40.0 mg dL–1) in males, whereas LINE-1 and SAT2 hypomethylation was significantly correlated with the increased percentage of skeletal muscle (<39.3%) in males.
Table 5. Levels of Alu, LINE-1, and SAT2 methylation according to the anthropometric, haematological, and biochemical categories.
Discussion
Plasma Hcy concentration varied according to sex, age, and race/ethnicity in the studied population [Citation13,Citation18,Citation19]. The present study investigated Hcy levels in a population of apparently healthy young adults, showing plasma Hcy concentrations similar to those previously reported for the 20–30-year-old age range. Plasma Hcy levels were markedly higher in men than in women, consistent with data obtained from Asian populations [Citation13,Citation20,Citation21] but different from findings reported in African populations [Citation18]. This gender difference has been associated with frequent intake of meat products during the growth period since males typically consume more animal proteins to increase muscular mass [Citation22]. Regarding differences with other populations, we should consider the higher prevalence of the MTHFR C677T allele reported in Mexican populations (0.46) relative to African populations (0.09), which might be associated with the higher levels of Hcy observed in the studied population [Citation3,Citation23]. These results suggest that genetic background could enhance the deleterious effect of the non-healthy alimentation. However, no study has been performed to characterize the prevalence of MTHFR polymorphisms in the population under study.
Epidemiological evidence suggests that increased Hcy levels are frequently detected in patients with metabolic diseases (diabetes mellitus and obesity), indicating that alimentary habits play a fundamental role in Hcy metabolism [Citation1,Citation3]. Chronic consumption of high-fat diets has been associated with increased Hcy levels due to lipid accumulation in hepatic tissue [Citation24]. In addition, individuals with non-alcoholic hepatic steatosis frequently show increased Hcy levels, which has been frequently associated with alterations in methionine metabolism [Citation11]. Given that BMI has been used as the main anthropometric indicator to identify alterations in normal body fat composition, most studies have employed this parameter to investigate its association with Hcy [Citation7,Citation25,Citation26]. Other studies have also complemented this association by employing parameters obtained by bioimpedance analysis (body fat, visceral fat, and skeletal muscle), with higher diagnostic accuracy for evaluating corporal composition showing significant correlations [Citation7,Citation27]. According to our results, the relationship between Hcy and anthropometric indicators showed significant associations only in the female population. Although females with HHcy tend to have higher levels of corporal fat, the occurrence of HHcy was only significantly associated with peripheral obesity higher than BMI > 30.0 kg m–2 and central obesity higher than 6.0% (visceral fat). These findings suggest that visceral fat may be a useful indicator to timeously detect HHcy development. Conversely, higher BMI and/or total fat levels are required to determine significant associations with pathological Hcy levels. However, further studies considering hormonal factors are required to clarify this association since several epidemiological studies and biological models have suggested the potential effect of oestrogen and leptin on the regulation of Hcy metabolism [Citation7,Citation28].
The correlation of HHcy with lipid indicators found in this study support the hypothesis that alterations in lipid may enhance the atherogenic properties of HHcy, even in apparently healthy young individuals. This observation was particularly evident in males, where HHcy was significantly correlated with a higher AIP, generated by the combination of low HDL and high TG levels. Indeed, some studies have suggested that individuals with increased Hcy and LDL-C levels present a higher risk of developing atherosclerosis and coronary artery diseases during periods of chronic systemic inflammation [Citation29,Citation30]. These findings highlight the importance of a properly balanced diet for Hcy metabolism. Accordingly, the excessive intake of a regular diet composed primarily of saturated fatty acids, cholesterol, and saccharose, increases the risk of developing alterations in components of the LDL/HDL ratio [Citation31,Citation32]. In contrast, increased methionine primarily from animal protein (e.g., meat, fish, dairy, and eggs) may negatively regulate Hcy methylation. This effect is particularly evident in vegetarian individuals whose dietary components (e.g., seeds, nuts, and legumes) provide lower methionine amounts [Citation31].
Modifications in the consumption, absorption, and metabolism of B vitamins are closely related to changes in plasma Hcy levels [Citation10,Citation33]. Depending on the cause of HHcy, supplementation with these vitamins has shown promising results as a strategy to reduce Hcy level and the risk of developing cardiovascular disease [Citation1,Citation34]. This correlation has encouraged the search for surrogate markers with a high predictive value for elevated Hcy, primarily in subjects with underlying metabolic alterations linked to cardiovascular disease. Given that folate deficiency and vitamin B-12 have been widely associated with variations in the size and form of red blood cells, the correlation with red cell indicators has been investigated in different studies, obtaining discrepant results depending on the age range and clinical condition of the studied population [Citation33,Citation35,Citation36]. According to our results, the prevalence of HHcy was associated with a slightly lower RDW-CV in men. It is worth noting that all RDW-CV values for males were within the normal range (11–15%). These findings suggest that increased Hcy level in young adults as a consequence of vitamin B deficiency is not initially associated with increased RDW-CV. Instead, the concomitant deficiency of B vitamins in individuals with slightly elevated Hcy levels may enhance alterations of pathways that converge in Hcy metabolism. Indeed, analysis of data from longitudinal studies through self-learning algorithms suggests that subjects with elevated Hcy levels may have a higher risk of increased RDW-CV [Citation33].
The relationship between increased Hcy levels and passive DNA demethylation has been evaluated in several epidemiological studies. However, since Hcy levels increase with age, most studies have investigated this association in adults >40 years old [Citation10,Citation37,Citation38]. In contrast, the present study investigated the relationship between Hcy and global methylation in a young population with no history of diseases. According to our results, Alu, LINE-1, and SAT2 methylation levels did not significantly differ between females and males. However, higher LINE-1 and SAT2 methylation levels were observed in the male population with increased BMI and total and visceral fat, consistent with previously reported findings from older adults [Citation39]. Moreover, that this association has also been reported in postmenopausal women [Citation40] highlights the role of hormonal factors in DNA methylation through Hcy alteration. Conversely, hypomethylation of LINE-1 and SAT2 was only observed in individuals with changes in parameters associated with methionine metabolism (skeletal muscle), regardless of gender, suggesting that metabolic changes associated with increased methionine levels may differentially modify Alu, LINE-1, and SAT2 methylation levels, even when Hcy levels are within normal range. This hypothesis is also supported by the positive correlation observed between Alu hypomethylation and HDL-C reduction due to lipid alteration that has been observed in biological models fed on a high-methionine diet [Citation32]. This could also be a consequence of the proportion of LINE-1 (13%) and Alu sequence (25%) proportions present in the human genome [Citation41], which suggest a higher susceptibility of LINE-1 and SAT2 hypomethylation in response to changes in methionine and folate intake.
It is worth noting that global methylation was evaluated in DNA extracted from mononuclear cells, reducing the bias of evaluating DNA methylation from total white blood cells, which has been proven to modify methylation levels because of the cellular heterogeneity of the sample [Citation38,Citation42]. Indeed, the high degree of correlation observed between LINE-1 and SAT2 methylation regardless of gender supports this hypothesis since it has been previously reported that several factors may affect the correlation between these markers [Citation38]. In addition, although liquid chromatography (HPLC) is considered the gold standard for determining Hcy concentration, enzymatic assays have shown a high rate of correlation (r = 0.98) with HPLC [Citation8], with low levels of inter- and intra-variation (<5%), supporting its utility in monitoring Hcy at a lower cost.
The current study has some limitations. The cross-sectional nature of the study and lack of data on the prevalence of MTHFR polymorphisms in the studied population limits the interpretation of the causal relationship between Hcy and the studied variables. In addition, no nutritional data on lifestyle factors were obtained during the recruitment of the study population, limiting the scope of our study. Nevertheless, our findings highlight the need for longitudinal studies evaluating the potential effect of chronic consumption of high-fat or high-methionine diets on global DNA methylation in relation to alterations in Hcy metabolism.
In conclusion, our study results suggest that the association between increased plasma Hcy level and the higher corporal fat percentage found in the female population highlights the participation of hormonal factors in the regulation of Hcy metabolism. However, because majority of the studied population comprised women, additional studies with a larger male cohort are necessary to replicate these findings. Furthermore, the association between anthropometric and haematological parameters and the modification of Alu, LINE-1, and SAT2 methylation highlights the usefulness of longitudinal studies to understand the possible regulatory effects exerted by Hcy on global methylation at a young age. Finally, it is worth mentioning that the association of increased Hcy level with a higher level of lipids supports the hypothesis that dyslipidemia plays a fundamental role in the atherogenic behaviour of Hcy, even in young individuals.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Komorniak N, Szczuko M, Kowalewski B, et al. Nutritional deficiencies, bariatric surgery, and serum homocysteine level: review of current literature. Obes Surg. 2019;29(11):3735–3742.
- Osadnik T, Pawlas N, Lejawa M, et al. Genetic and environmental factors associated with homocysteine concentrations in a population of healthy young adults. Analysis of the MAGNETIC study. Nutr Metab Cardiovasc Dis. 2020;30(6):939–947.
- Lee HS, In S, Park T. The homocysteine and metabolic syndrome: a mendelian randomization study. Nutrients. 2021;13(7: 2440).
- Palep-Singh M, Picton HM, Yates ZR, et al. Plasma homocysteine concentrations and the single nucleotide polymorphisms in the methionine synthase gene (MTR 2756A>G): associations with the polycystic ovary syndrome An observational study. Eur J Obstet Gynecol Reprod Biol. 2008;138(2):180–186.
- Gaughan DJ, Kluijtmans LA, Barbaux S, et al. The methionine synthase reductase (MTRR) A66G polymorphism is a novel genetic determinant of plasma homocysteine concentrations. Atherosclerosis. 2001;157(2):451–456.
- Wang WM, Jin HZ. Homocysteine: a potential common route for cardiovascular risk and DNA methylation in psoriasis. Chin Med J (Engl). 2017;130(16):1980–1986.
- Wang Y, Jiang Y, Wang N, et al. Central but not general obesity is positively associated with the risk of hyperhomocysteinemia in middle-aged women. Nutrients. 2019;11(7):1614.
- Fryer AA, Nafee TM, Ismail KM, et al. LINE-1 DNA methylation is inversely correlated with cord plasma homocysteine in man: a preliminary study. Epigenetics. 2009;4(6):394–398.
- Vats S, Sundquist K, Wang X, et al. Associations of global DNA methylation and homocysteine levels with abdominal aortic aneurysm: a cohort study from a population-based screening program in Sweden. Int J Cardiol. 2020;321:137–142.
- Kok DE, Dhonukshe-Rutten RA, Lute C, et al. The effects of long-term daily folic acid and vitamin B12 supplementation on genome-wide DNA methylation in elderly subjects. Clin Epigenetics. 2015;7(1):121.
- Lai Z, Chen J, Ding C, et al. Association of hepatic global DNA methylation and serum one-carbon metabolites with histological severity in patients with NAFLD. Obesity (Silver Spring). 2020;28(1):197–205.
- Asada K, Kotake Y, Asada R, et al. LINE-1 hypomethylation in a choline-deficiency-induced liver cancer in rats: dependence on feeding period. J Biomed Biotechnol. 2006;2006(1):17142.
- Xu R, Huang F, Wang Y, et al. Gender- and age-related differences in homocysteine concentration: a cross-sectional study of the general population of China. Sci Rep. 2020;10(1):17401.
- Gallagher D, Heymsfield SB, Heo M, et al. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000;72(3):694–701.
- Martin SS, Blaha MJ, Elshazly MB, et al. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA. 2013;310(19):2061–2068.
- Wu TT, Gao Y, Zheng YY, et al. Atherogenic index of plasma (AIP): a novel predictive indicator for the coronary artery disease in postmenopausal women. Lipids Health Dis. 2018;17(1):197.
- Weisenberger DJ, et al. Analysis of repetitive element DNA methylation by MethyLight. Nucleic Acids Res. 2005;33(21):6823–6836.
- Onyemelukwe OU, Maiha BB. Hyperhomocysteinemia and folate levels in normal healthy Nigerians living in Zaria: subanalysis of ABU homocysteine cross-sectional survey. Ann Afr Med. 2019;18(3):143–152.
- Davila-Rodriguez MI, Torres-de La Cruz VM, Novelo-Huerta HI, et al. Total homocysteine levels in healthy children from the Monterrey metropolitan area, Mexico. Prague medical Report. 2010;111(2):135–141.
- Chen PJ, Lu YC, Wang PM, et al. Factors associated with hyperhomocysteinemia in relatively healthy Taiwanese adults: a retrospective medical record study. Medicine (Baltimore). 2021;100(3):e23829.
- Malik A, Trilok-Kumar G. Status of Vitamin B12 among healthy adult and elderly population in India: a review. J Nutr Sci Vitaminol (Tokyo). 2020;66(Supplement):S361–S8.
- Ruby MB. Vegetarianism. A blossoming field of study. Appetite. 2012;58(1):141–150.
- Contreras-Cubas C, Sanchez-Hernandez BE, Garcia-Ortiz H, et al. Heterogenous distribution of MTHFR gene variants among mestizos and diverse amerindian groups from Mexico. PLoS One. 2016;11(9):e0163248.
- Yun KU, Ryu CS, Oh JM, et al. Plasma homocysteine level and hepatic sulfur amino acid metabolism in mice fed a high-fat diet. Eur J Nutr. 2013;52(1):127–134.
- Nakazato M, Maeda T, Takamura N, et al. Relation of body mass index to blood folate and total homocysteine concentrations in Japanese adults. Eur J Nutr. 2011;50(7):581–585.
- Huang Y, Wu K, Li H, et al. Homocysteine level, body mass index and clinical correlates in Chinese Han patients with schizophrenia. Sci Rep. 2020;10(1):16119.
- Al-Bayyari N, Hamadneh J, Hailat R, et al. Total homocysteine is positively correlated with body mass index, waist-to-hip ratio, and fat mass among overweight reproductive women: a cross-sectional study. Nutr Res. 2017;48:9–15.
- Kafkas S, Dost T, Ozkayran H, et al. Effect of estrogen therapy on adipocytokines in ovariectomized-aged rats. J Obstet Gynaecol Res. 2012;38(1):231–238.
- Chen S, Luan H, He J, et al. The relationships of serum homocysteine levels and traditional lipid indicators with disease activity and coronary artery involvement in Takayasu arteritis. Immunol Res. 2020;68(6):405–413.
- Vignini A, Nanetti L, Bacchetti T, et al. Modification induced by homocysteine and low-density lipoprotein on human aortic endothelial cells: an in vitro study. J Clin Endocrinol Metab. 2004;89(9):4558–4561.
- Kowalska K, Brodowski J, Pokorska-Niewiada K, et al. The change in the content of nutrients in diets eliminating products of animal origin in comparison to a regular diet from the area of Middle-Eastern Europe. Nutrients. 2020;12(10):2986.
- Velez-Carrasco W, Merkel M, Twiss CO, et al. Dietary methionine effects on plasma homocysteine and HDL metabolism in mice. J Nutr Biochem. 2008;19(6):362–370.
- Beydoun MA, Beydoun HA, MacIver PH, et al. Biochemical and hematological correlates of elevated homocysteine in national surveys and a longitudinal study of urban adults. Nutrients. 2020;12(4):950.
- Yang HT, Lee M, Hong KS, et al. Efficacy of folic acid supplementation in cardiovascular disease prevention: an updated meta-analysis of randomized controlled trials. Eur J Intern Med. 2012;23(8):745–754.
- He LM, Gao CY, Wang Y, et al. Red cell distribution width and homocysteine act as independent risk factors for cardiovascular events in newly diagnostic essential hypertension. Oncotarget. 2017;8(60):102590–102599.
- Margalit I, Cohen E, Goldberg E, et al. Reconsidering the relation between serum homocysteine and red blood cell distribution width: a cross-sectional study of a large cohort. Biomarkers. 2018;23(5):483–486.
- Zhu ZZ, Hou L, Bollati V, et al. Predictors of global methylation levels in blood DNA of healthy subjects: a combined analysis. Int J Epidemiol. 2012;41(1):126–139.
- Wu HC, Delgado-Cruzata L, Flom JD, et al. Global methylation profiles in DNA from different blood cell types. Epigenetics. 2011;6(1):76–85.
- Perng W, Villamor E, Shroff MR, et al. Dietary intake, plasma homocysteine, and repetitive element DNA methylation in the Multi-Ethnic Study of Atherosclerosis (Mesa). Nutr Metab Cardiovasc Dis. 2014;24(6):614–622.
- Boyne DJ, Friedenreich CM, McIntyre JB, et al. Associations between adiposity and repetitive element DNA methylation in healthy postmenopausal women. Epigenomics. 2017;9(10):1267–1277.
- Luo Y, Lu X, Xie H. Dynamic Alu methylation during normal development, aging, and tumorigenesis. Biomed Res Int. 2014;2014:784706.
- Delgado-Cruzata L, Vin-Raviv N, Tehranifar P, et al. Correlations in global DNA methylation measures in peripheral blood mononuclear cells and granulocytes. Epigenetics. 2014;9(11):1504–1510.
