ABSTRACT
Postmenopausal women with overweight or obesity have an increased risk of developing breast cancer but many of the mechanisms underlying this association remain to be elucidated. MicroRNAs (miRNAs), short non-coding single-stranded RNAs, regulate many physiological processes by controlling post-transcriptional regulation of mRNA. We measured circulating miRNA from 192 overweight/obese postmenopausal women (50–75 years) who were part of a randomized controlled trial, comparing independent and combined effects of a 12-month reduced-calorie weight-loss diet and exercise programme, versus control. RNA was extracted from stored plasma samples, and 23 a priori selected miRNA targets related to aetiology of breast cancer or obesity were measured using NanoString nCounter miRNA Expression assays. Changes from baseline to 12-months between controls and women in the diet/exercise weight loss arms were analysed using generalized estimating equations modification of linear regression, adjusted for confounders. We next examined changes in levels of circulating miRNA by amount of weight loss (0–10% versus ≥10%). Participants randomized to weight-loss interventions had statistically significantly greater reductions in miR-122 (−7.25%), compared to controls (+ 33.5%, P = 0.009), and miR-122 levels were statistically significantly correlated with weight loss (rho = 0.24; P = 0.001) Increasing weight loss was associated with greater reductions in miR-122 vs. controls (−11.7% (≥10% weight loss); +2.0% (0–10% weight loss) +33.5% (controls); Ptrend = 0.006), though this was not significant after correction for multiple testing (P = 0.05/23) Our study supports the effect of weight loss on regulation of miRNA.
Introduction
Overweight and obesity increase risk for postmenopausal breast cancer, and negatively impact breast cancer survival regardless of age at diagnosis. Obesity is associated with elevated blood levels of sex hormones, inflammation-related biomarkers, insulin resistance, leptin, oxidative stress, and angiogenesis-related biomarkers, all linked to increased breast cancer risk [Citation1]. However, the effect of weight loss on regulation of expression of these biomarkers has not been established.
Mature miRNAs are short (20–22 nucleotides) non-coding single-stranded RNAs that exert their biological effects by post-transcriptionally regulating protein-coding mRNAs. Mature miRNA are released into the circulation as exosomes, microvesicles or bound to lipoproteins, and remain stable in the circulation; miRNA can be transferred to neighbouring or distant cells in these secretory forms to modulate cell function [Citation2,Citation3]. miRNAs are involved in the development of obesity and metabolic disorders, and deregulation of miRNA has been linked to breast cancer development [Citation4].
While many studies have characterized the role of miRNA in obesity [Citation5], few studies tested the effects of weight loss on circulating miRNAs [Citation6–8], and to our knowledge, none in healthy overweight/obese postmenopausal women. None have examined the dose response of weight loss on miRNA. The current study is ancillary to the Nutrition and Exercise in Women (NEW) randomized controlled trial (RCT), which assessed the effects of a 12-month reduced-calorie dietary weight-loss programme, a moderate-to-vigorous exercise intervention, alone or in combination, on circulating biomarkers related to breast cancer risk [Citation9].
We tested the combined effects of dietary weight loss and exercise on circulating levels of 23 a priori selected miRNAs, shown to control expression of genes related to breast cancer risk, obesity, or obesity-related processes (Supplementary Table 1), previously demonstrated to be altered by weight loss in the NEW RCT [Citation10–14]. Prior to conducting this ancillary study, we pre- selected participants in the weight loss arms with the highest degree of weight loss, and compared them with controls (no intervention) who lost no weight; we then examined the effects of weight loss on circulating miRNA. We hypothesized that women who lost more than 10% of starting weight would have the largest changes in miRNA (see for hypothesized directions of changes in circulating miRNA).
Table 1. Selected miRNA targets.
Methods
This study is ancillary to the NEW study (www.clinicaltrials.gov NCT00470119, ) which tested the effects of a reduced calorie weight loss diet and/or exercise programme on breast cancer-related biomarkers and other outcomes [Citation10–13,Citation15]. It was carried out in the Fred Hutchinson Cancer Center (FHCC), Seattle, USA, and performed with the approval of the FHCC Institutional Review Board. Written informed consent was obtained from each participant.
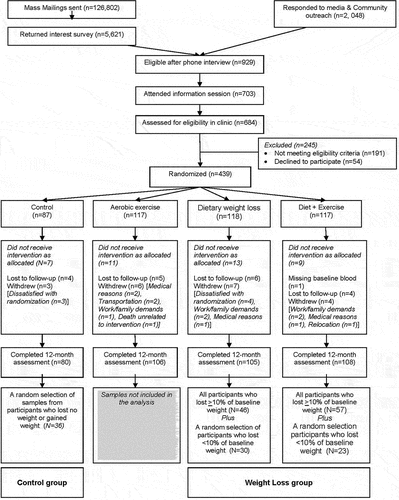
The study is described in detail elsewhere [Citation9]. Briefly, 438 postmenopausal, healthy overweight (BMI≥25 kg/m2), sedentary women aged 50–75 years, were recruited. Exclusion criteria included > 100 min/week of moderate physical activity; diagnosed diabetes or other serious medical condition(s); use of oestrogen, progesterone, or testosterone; >2 alcoholic drinks/day; smoking; participation in another structured weight-loss programme; and contraindications to participation (e.g., abnormal exercise tolerance test). Eligible women were randomized to one of the following: i) reduced-calorie dietary modification (N = 118); ii) moderate-to-vigorous intensity aerobic exercise (N = 117); iii) combined diet and exercise (N = 117); or iv) control (no intervention) (N = 87). Randomization was stratified by BMI (≥ or <30 kg/m2) and race/ethnicity. Investigators and laboratory staff were blinded to randomization arm.
Interventions
The dietary intervention was a modification of the Diabetes Prevention Program (DPP) and Look AHEAD (Action for Health in Diabetes) lifestyle behaviour change programmes with goals of: 1200–2000 kcal/day, <30% daily calories from fat, and 10% weight loss, by 6-months, and weight maintenance thereafter. Participants had at least two individual meetings with a dietician followed by weekly group meetings for 6-months; thereafter, they attended monthly, with biweekly phone/email contact. Participants kept daily food journals, weighed themselves weekly, and were weighed at nutrition sessions. Intervention adherence was defined by percent of in-person nutrition session attendance. The exercise intervention goal was 45 minutes of moderate-to-vigorous intensity exercise at a target heart rate of 70–85% observed maximum, 5 days/week by week 7. Participants attended three facility-based supervised sessions/week and exercised 2 days/week at home. They recorded exercise mode, duration, peak heart rate, and perceived exertion at each session. Women randomized to combined diet+exercise received both interventions. Controls were asked not to change their diet or exercise habits.
Participants
A subsample of 192 participants with complete data from both timepoints were chosen for this ancillary study (). A random sample of participants who lost no weight/gained weight were selected from the control arm ‘Controls’; those who lost any weight (<10% and ≥10% of baseline weight) were selected from the diet and diet+exercise arms. All participants who lost >10% weight were included, and a random sample of participants who had lost <10% were selected. The final ancillary study sample included: controls – 36 participants with no change/gained weight at 12-months, and the weight loss group – 53 participants who lost <10% of baseline weight and 103 participants who lost ≥10% of baseline weight
Blood Specimen Collection and Processing
Fasting (12 hours) venous blood samples (50 mL) were collected during clinic visits at baseline (pre-randomization) and at 12-months. Participants refrained from alcohol (48 hours), vigorous exercise or NSAID use (24 hours) prior to blood collection. Blood was processed (serum, plasma) within 1 hour, and stored at −70°C.
Selection of miRNA Targets. Twenty-three miRNA targets shown to regulate the expression of genes involved in pathways associated with breast cancer development, adipogenesis, inflammation, and angiogenesis were selected a priori from the literature and include let-7a-5p, let-7b-5p, miR-122-5p, miR-126-3p, miR-130a-3p, miR-132-3p, miR-143-3p, miR-146a-5p, miR-146b-3p, miR-148a-3p, miR-191-5p, miR-21-5p, miR-221-3p, miR-222-3p, miR-26a-5p, miR-26b-5p, miR-27a-3p, miR-27b-3p, miR-29b-3p, miR-29c-3p, miR-30a-5p, miR-30c-5p, and miR-99a-5p (Supplementary Table 1).
Assays
Total RNA was extracted from stored plasma using the Plasma/Serum Circulating and Exosomal RNA Purification Kit (Norgen Biotek Corp. Thorold, ON, Canada). RNA was transferred to a Millipore Amicon Ultra YM-3 columns, and centrifuged, and normalized to a concentration of 33ng/μl in a volume of 3 μl. To allow for downstream data normalization, a mixture of three synthetic small RNA ‘spike-ins,’ (ath-miR-159a, cel-miR-248, cel-miR-254 (Integrated DNA Technologies, Coralville, IA) was added (1000 attomoles/500 μl) to each plasma sample. miRNAs were analysed using a customized NanoString nCounter miRNA Expression assay (Nanostring Technologies Inc., Seattle, USA), which utilizes a digital colour-coded molecular ‘barcode’ and single-molecule imaging system to detect and count unique transcripts in a single reaction. Mature miRNAs were combined with miRNA Assay Controls and ligated to a species-specific miRNA Tag Reagent. Tagged miRNAs were subsequently hybridized with a custom nCounter Expression Assay Codeset overnight at 65°C. Unhybridized CodeSet was removed via an automated purification performed on an nCounter Prep Station, and resulting target:probe complexes were deposited and bound to the nCounter cartridge imaging surface. Reporter counts were tabulated for each sample by the nCounter Digital Analyser and output as raw data in .csv format.
Data normalization. The synthetic spike-ins were added after the RNA was extracted. The purpose of the spike-in is to monitor the efficiency of RNA recovery rates during purification as well as account for any variance in ligation efficiencies across samples. For these assays three spike-in probes were used for normalization: ath-miR 159a, cel-miR-248, and cel-miR-254. Negative ligation and positive ligations probes were used to guide background thresholding. Normalization was performed using the nSolver™ Analysis Software (Nanostring Technologies) where specific miRNA counts are normalized against the spike-in controls. Logarithmic transformation was applied to the miRNAs data to achieve approximate normality and variance stabilization.
Covariates
All study measures were obtained at baseline and 12-months by trained personnel. Height and weight were measured, and body mass index (BMI, kg/m [Citation2]) was calculated. Body composition was measured by DXA (Dual-energy X-ray absorptiometry) whole-body scanner (GE Lunar, Madison, WI). Cardiorespiratory fitness (VO2max) was assessed using a maximal graded treadmill test according to a modified branching protocol [Citation9]. Validated questionnaires collected information on demographics, medical history, dietary intake, supplement use and physical activity patterns.
Statistical analysis
We compared average changes from baseline to 12-months in the targeted miRNAs between (1) all weight loss groups combined versus controls; and (2) participants who lost <10% of baseline weight and who lost ≥10% of baseline weight, versus controls (dose-response). Generalized estimating equations (GEE) modification of linear regression was used to model the relationship between measurements of each of the targeted miRNAs and weight loss, and to account for the correlation within individual data over time. Models were adjusted for baseline age, baseline BMI (<30, ≥30 kg/m2) and race (non-Latina White, other). We compared weight loss as a continuous variable with miRNA levels using Pearson correlations. Statistical tests were two sided., and significance was set at P ≤ 0.002, using Bonferroni correction for 23 multiple comparisons. Statistical analyses were performed using SAS software (version 8.2, SAS Institute Inc., Cary, NC). Data are available on request from the authors.
Results
At baseline, participants were a mean 57.8 years, with a mean BMI of 30.6 kg/m2, and were predominantly non-Latina Whites ().
Table 2. Baseline characteristics of NEW and ancillary study participants.
Intervention Fidelity Data on intervention adherence, weight loss, and body composition changes in the parent RCT have been previously reported [Citation9]; Briefly, women in all intervention groups significantly reduced body fat percentage (all p < 0.001) compared to controls; percent of daily calories from fat decreased in the diet and diet+exercise arms (−6.7% and −8.0%, respectively). In both diet groups, women attended an average of 27 diet counselling sessions (86% of expected sessions). Mean weight changes were: −8.5% (p < 0.001; diet arm), −10.8% (p < 0.001; diet+exercise arm), and −0.8% among controls. In this ancillary study, mean weight changes were −12.6% in the weight-loss arms, and +3.3% in the control arm. These mean weight changes reflect the purposeful sampling of only women who had lost weight in the diet or diet+exercise arms, and only women who had not lost weight in the control arm.
Intervention effects Compared with controls who did not lose weight, women selected for this ancillary study from the weight loss groups had statistically significantly (hereon ‘significantly’) greater reductions in miR-122 at 12-months (−7.25% vs, +33.5%, P < 0.009, in the hypothesized direction; ). Participants who lost <10% and ≥10% of their baseline weight at 12-months, had statistically significant reductions in levels of circulating miR-122 (2.0% and −11.7% respectively; compared to controls (+33.5%; Ptrend2 = 0.006, ), although the results were no longer significant after Bonferroni correction for multiple testing (P = 0.002 for significance). When expressed as a continuous variable, BMI was statistically significantly associated with miR-122 (rho = 0.24, P = 0.0001; ). While levels of miR-30c were lower at 12 months in the weight-loss group compared to controls (0.18% vs. 52.74%, P = 0.04, ), this was not statistically significant after correction for multiple testing. Participants who lost <10% of their baseline weight had an 18.8% reduction in levels of miR-30c at 12 months compared to controls (+52.70%, P = 0.001); however, in participants who lost ≥10% of baseline weight, levels increased by +11.6% (P = 0.14, ).
Table 3. Change from baseline to 12 months in normalized miRNAs.
Table 4. Change from baseline to 12 months in normalized miRNAs (log-transformed) by weight loss category.
Table 5. Association (Pearson correlation) between change in log transformed (normalized) miRNAs and continuous weight change.
With the exception of miR-122 and miR-30c, none of the observed changes in circulating miRNA with weight loss reached statistical significance, although several showed greater effect of weight loss vs control in hypothesized directions (miR-126, miR-143, miR-146a, miR-148a, miR-26b, miR-27b, miR29c).
Discussion
This study compared the effects of weight loss on circulating levels of miRNA, in overweight/obese postmenopausal women. Participants with the highest degree of weight loss had statistically significant reductions in circulating levels of miR-122 compared to those who lost no weight.
Levels of miR-122 were significantly lower in women who lost weight in the intervention arms versus controls who did not lose weight (−7.25% vs. +33.49% change compared to baseline, P = 0.009), which was in the hypothesized direction. Participants with more weight loss had greater reductions in miR-122 compared to controls (Ptrend = 0.006). While this result was not significant after adjustment for multiple testing, the effect size of ±40.7% change in miR-122 comparing weight loss to controls, and the statistically significant correlation between BMI and miR-122 (P = 0.001), supports the role of weight loss in miR-122 expression. miR-122 is elevated in both children with obesity [Citation16], and in individuals with metabolic syndrome and type 2 diabetes [Citation17], has been suggested as a biomarker of non-alcoholic fatty liver disease (NAFLD) [Citation18], and is associated with regional adiposity [Citation19]. Recently, studies have demonstrated that overexpression of miR-122 reduces breast cancer cell proliferation in vitro, reduced tumour growth in vivo, and enhanced tumour cell mobility [Citation20,Citation21]. However, an intriguing study on the role of miR-122 in breast cancer metastasis demonstrated that breast-cancer cells secrete miR-122, which modified glucose uptake in metastatic niche tissues through downregulation of PKM2 and GLUT1. While miR-122 reduced primary tumour cell proliferation by restricting glucose uptake, it simultaneously reprogrammed the premetastatic niches to promote tumour cell colonization and metastatic formation. In vivo inhibition of miR-122 restored glucose uptake in distant organs, including brain and lungs, and decreased the incidence of metastasis [Citation22]. Uen et al suggested that the role of miR-122 may have different roles in the tumorigenic vs. metastatic process, and reported that liver-derived exosomes released under chronic conditions containing miR-122 enhanced breast cancer cell mobility [Citation21]. The principal risk factors for NAFLD are obesity, type-2 diabetes and hyperlipidaemia, and weight loss in the NEW study improved the metabolic profile of participants [Citation11] which may have improved hepatic profiles, reducing circulating levels of miR-122.
miR-30c was decreased in participants who lost weight, but the relationship was non-linear: participants who lost <10% of their baseline weight had a mean −18.8% reduction in miR-30c compared to controls (+52.70%; P = 0.001), and participants who lost ≥10% of baseline weight, had a 11.6% non-statistically significant increase (P = 0.14). Given miR-30's role in suppressing breast cancer cell growth and metastasis [Citation23–25],and associations with improved outcomes in breast cancer [Citation26], the reduction of miR-30c in participants who lost <10% of baseline weight was unexpected, although the ±11.6% increase in participants who lost ≥10% weight is in the expected direction, but was not statistically significant. To our knowledge there are no studies of the effects of weight loss on miR-30c expression. However, a study investigating differences in expression in miRNA in blood vs. adipose tissue in overweight vs. normal weight Korean women [Citation27], found that miR-30c was down-regulated in both blood and tissue in overweight vs. normal weight individuals. However, it also plays a role in adipocyte differentiation, and its inhibition blocks adipogenesis, which may explain its decrease in women who lost weight [Citation28,Citation29].
While there have been relatively few studies investigating the role of weight loss on circulating miRNAs, there is increasing evidence that weight loss can restore circulating levels of miRNAs in obese subjects towards normal, contributing to these improved metabolic outcomes [Citation5]. For example, circulating levels of 18 miRNAs that were dysregulated in women with a BMI> 30 kg/m2 were normalized after 4 weeks of a low caloric diet associated with weight loss [Citation5], also associated with improved blood glucose. The authors suggested that the effects of weight loss might be modulated via normalization of circulating miRNA. One study compared the expression of miRNAs in 80 obese women before and after weight loss (mean 7.2%) compared to 80 lean controls, and identified statistically significant alterations in the expression level of 21 miRNAs in obese women involved with cellular senescence, inhibition of angiogenesis, apoptosis, inhibition of cellular proliferation, promotion of inflammation and impaired glucose tolerance. After weight loss, 18 of these miRNAs reverted to an expression patterns seen in the lean controls [Citation30]. A small study (N = 22) on the effects of gastric-bypass induced weight loss found that levels of circulating miR-7, miR-15a, miR-34a, miR-106a, miR-122 and miR-221 were statistically significantly altered after weight loss, including a reduction in miR-122 [Citation6]. A recent study investigated 30 circulating miRNA in 155 morbidly obese patients before and after bariatric surgery; as in this study, miR-122 correlated with BMI, and was reduced at 3 months post-surgery where the average weight loss among patients was 20%. Similar to our findings, they saw no change in miR-21, miR-29, miR-130, miR-148a, miR-222, miR-221, mi-R-99, miR-143, miR-26 or let-7b with weight loss. Given the significant weight loss experienced by patients at 12 months (mean: 40%) the authors suggested these serum miRNAs are either not adipose tissue-derived or that adipose tissue-derived exosomes do not contribute significantly to circulating miRNA levels [Citation31]. A 16-week RCT compared diet and exercise weight loss interventions vs controls among 89 overweight men and women. Post-study participants were stratified by degree of weight loss, and miR-221, miR-223, and miR-140 were differentially expressed in the different weight loss groups [Citation7]. Finally an RCT examined the effects of dietary weight loss intervention vs. control on circulating levels of 22 miRNA in 85 overweight or obese participants. miR-122 decreased in the weight loss group compared to controls; in contrast to our findings miR-126a and miR-222 levels increased in response to weight loss [Citation32].
Circulating miRNAs not only enable communication between cells, but also provide insight into the pathological and physiological state of the originating cells. While miRNAs have pleiotropic regulatory functions and are tissue specific in their effects, and recognizing that it is impossible to ascertain whether reductions in miRNAs via weight loss have clinical benefits, the putative role in miR-122 in metastasis and its regulation via weight loss, may represent a future area of clinical research.
Strengths of the present study include: the randomized trial design, the inclusion of dietary weight loss and combined dietary weight-loss and exercise interventions, the enrichment with women with significant amounts of weight loss, and the sample size that was larger than previous studies. Limitations include measurement of miRNA only in blood rather than in target tissue and measurement of only 23 miRNAs, selected based on their roles in inflammation, adipogenesis, angiogenesis, and sex steroid hormone regulation. Future studies should encompass larger studies of miRNAs in RCTs, to further investigate the interplay of miRNA in controlling physiological mechanisms linking obesity and cancer risk.
Supplemental Material
Download MS Word (144.7 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author (CD) and PI of the parent grant (AMcT), upon reasonable request
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15592294.2022.2107841
Additional information
Funding
References
- Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, et al. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67(5):378–397.
- Valadi H, Ekstrom K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659.
- Vickers KC, Remaley AT. Lipid-based carriers of microRNAs and intercellular communication. Curr Opin Lipidol. 2012;23(2):91–97.
- Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6(11):857–866.
- Brandao BB, Lino M, Kahn CR. Extracellular miRNAs as mediators of obesity-associated disease. J Physiol. 2022;600(5):1155–1169.
- Nunez Lopez YO, Coen PM, Goodpaster BH, et al. Gastric bypass surgery with exercise alters plasma microRNAs that predict improvements in cardiometabolic risk. Int J Obes (Lond). 2017;41(7):1121–1130.
- Parr EB, Camera DM, Burke LM, et al. Circulating microRNA responses between ‘high’ and ‘low’ responders to a 16-wk diet and exercise weight loss intervention. PloS One. 2016;11(4):e0152545.
- Manning P, Munasinghe PE, Bellae Papannarao J, et al. Acute weight loss restores dysregulated circulating microRNAs in individuals who are obese. J Clin Endocrinol Metab. 2019;104(4):1239–1248.
- Foster-Schubert KE, Alfano CM, Duggan CR, et al. Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity. 2012;20(8):1628–1638. (Silver Spring,Md).
- Imayama I, Ulrich CM, Alfano CM, et al. Effects of a caloric restriction weight loss diet and exercise on inflammatory biomarkers in overweight/obese postmenopausal women: a randomized controlled trial. Cancer Res. 2012;72(9):2314–2326.
- Mason C, Foster-Schubert KE, Imayama I, et al. Dietary weight loss and exercise effects on insulin resistance in postmenopausal women. Am J Prev Med. 2011;41(4):366–375.
- Campbell KL, Foster-Schubert KE, Alfano CM, et al. Reduced-calorie dietary weight loss, exercise, and sex hormones in postmenopausal women: randomized controlled trial. J Clin Oncol. 2012;30(19):378–397.
- Duggan C, Tapsoba Jde D, Wang CY, et al. Dietary weight loss and exercise effects on serum biomarkers of angiogenesis in overweight postmenopausal women: a randomized controlled trial. Cancer Res. 2016;76(14):4226–4235.
- Imayama I, Ulrich CM, and Alfano CM, et al. Effects of a caloric restriction weight loss diet and exercise on inflammatory biomarkers in overweight/obese postmenopausal women: a randomized controlled trial. Cancer Res. 2012;72(9): 2314–2326.
- Campbell KL, Foster-Schubert KE, Makar KW, et al. Gene expression changes in adipose tissue with diet- and/or exercise-induced weight loss. Cancer Prev Res (Phila). 2013;6(3):217–231.
- González-Arce LM, Lara-Riegos JC, Pérez-Mendoza GJ, et al. High expression levels of circulating microRNA-122 and microRNA −222 are associated with obesity in children with Mayan ethnicity. Am J Hum Biol. 2021;33(6):e23540.
- Willeit P, Skroblin P, Moschen AR, et al. Circulating microRNA-122 is associated with the risk of new-onset metabolic syndrome and type 2 diabetes. Diabetes. 2017;66(2):347–357.
- Salvoza NC, Klinzing DC, Gopez-Cervantes J, et al. Association of Circulating Serum miR-34a and miR-122 with dyslipidemia among patients with non-alcoholic fatty liver disease. PloS One. 2016;11:e0153497–e.
- Shah R, Murthy V, Pacold M, et al. Extracellular RNAs are associated with insulin resistance and metabolic phenotypes. Diabetes Care. 2017;40(4):546–553.
- Wang B, Wang H, Yang Z. MiR-122 inhibits cell proliferation and tumorigenesis of breast cancer by targeting IGF1R. PloS One. 2012;7(10):e47053.
- Uen Y, Wang J-W, Wang C, et al. Mining of potential microRNAs with clinical correlation - regulation of syndecan-1 expression by miR-122-5p altered mobility of breast cancer cells and possible correlation with liver injury. Oncotarget. 2018;9(46):28165–28175.
- Fong MY, Zhou W, Liu L, et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat Cell Biol. 2015;17(2):183–194.
- Croset M, Pantano F, Kan CWS, et al. miRNA-30 family members inhibit breast cancer invasion, osteomimicry, and bone destruction by directly targeting multiple bone metastasis-associated genes. Cancer Res. 2018;78(18):5259–5273.
- Zhang N, Wang X, Huo Q, et al. MicroRNA-30a suppresses breast tumor growth and metastasis by targeting metadherin. Oncogene. 2014;33(24):3119–3128.
- Di Gennaro A, Damiano V, Brisotto G, et al. A p53/miR-30a/ZEB2 axis controls triple negative breast cancer aggressiveness. Cell Death Differ. 2018;25(12):2165–2180.
- Jamshidi M, Fagerholm R, Muranen TA, et al. High miR-30 expression associates with improved breast cancer patient survival and treatment outcome. Cancers (Basel). 2021;14(1):13.
- Kim NH, Ahn J, Choi YM, et al. Differential circulating and visceral fat microRNA expression of non-obese and obese subjects. Clin Nutr. 2020;39(3):910–916.
- Zaragosi LE, Wdziekonski B, Brigand KL, et al. Small RNA sequencing reveals miR-642a-3p as a novel adipocyte-specific microRNA and miR-30 as a key regulator of human adipogenesis. Genome Biol. 2011;12(7):R64.
- Karbiener M, Neuhold C, Opriessnig P, et al. MicroRNA-30c promotes human adipocyte differentiation and co-represses PAI- and ALK2. RNA Biol. 2011;8(5):850–860.
- Manning P, Munasinghe PE, and Bellae Papannarao J, et al. Acute weight loss restores dysregulated circulating microRNAs in obese individuals. J Clin Endocrinol Metab. 2018;104(4):1239–1248
- Sangiao-Alvarellos S, Theofilatos K, and Barwari T, et al. Metabolic recovery after weight loss surgery is reflected in serum microRNAs. BMJ Open Diabetes Res Care. 2020;8(2):e001441 . doi:10.1136/bmjdrc-2020-001441.
- Hess AL, Larsen LH, Udesen PB, et al. Levels of circulating miR-122 are associated with weight loss and metabolic syndrome. Obesity. 2020;28(3):493–501. (Silver Spring,Md)