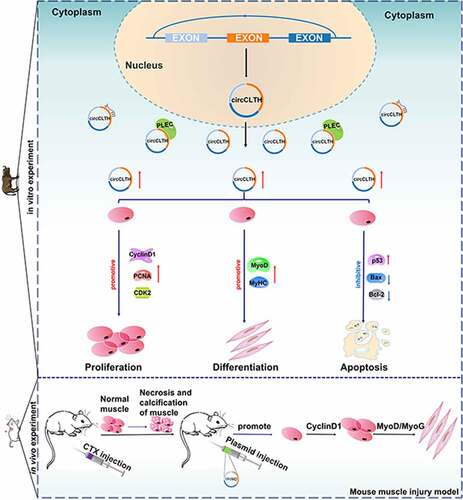
ABSTRACT
Buffalo holds an excellent potential for beef production, and circRNA plays an important role in regulating myogenesis. However, the regulatory mechanism of circRNAs during buffalo skeletal muscle development has not been fully explored. In this study, circRNA expression profiles during the proliferation and differentiation stages of buffalo myoblasts were analysed by RNA-seq. Here, a total of 3,142 circRNAs candidates were identified, and 110 of them were found to be differentially expressed in the proliferation and differentiation stages of buffalo myoblast libraries. We focused on a 347 nt circRNA subsequently named circCLTH. It consists of three exons and is expressed specifically in muscle tissues. It is a highly conserved non-coding RNA with about 95% homology to both the human and the mouse circRNAs. The results of cell experiments and RNA pull-down assays indicated that circCLTH may capture PLEC protein, promote the proliferation and differentiation of myoblasts as well as inhibit apoptosis. Overexpression of circCLTH in vivo suggests that circCLTH is involved in the stimulation of skeletal muscle regeneration. In conclusion, we identified a novel noncoding regulator, circCLTH, that promotes proliferation and differentiation of myoblasts and skeletal muscles.
Highlights
A new highly conserved circRNA was identified during muscle development
CircCLTH promotes proliferation and differentiation of myoblasts
CircCLTH promoted muscle damage repair in mice
CircCLTH may target the PLEC protein
GRAPHICAL ABSTRACT
KEYWORDS:
Introduction
Swamp Buffalo is recognized by the World Food and Agriculture Organization as the largest domestic animal to feed the world’s population. The buffalo was originally employed for animal power in farming, and now it has turned into one of the sources of meat and milk for human consumption [Citation1,Citation2]. Buffalo meat have significantly lower amounts of fat and cholesterol when compared to common beef products, which can be beneficial to the cardiovascular system and is of greater health value to humans [Citation3]. Under the same feeding and management conditions, our existing study shows the quality of buffalo beef is not different from ordinary beef in appearance, but significant differences are found in its shear force and observations of its muscle fibre structure [Citation4].
Myoblast cells are multipotent mesodermal cells derived from somatic nodes, and they belong to the myoblast line of skeletal muscle. The embryonic development process of myoblast cells is initiated by the terminal differentiation of mesenchymal stem cells into myoblast cells. Then they form primary muscle tubes which subsequently develop muscle fibres that then become hypertrophic [Citation5,Citation6]. Skeletal muscle satellite cells can participate in the damage of fibres which represent a state of dynamic balance in the body. This can lead to trauma or stimulation in the form of growth signals leading to symmetrical and asymmetrical cell divisions. The offspring cells can fuse with each other to restore the integrity of the muscle fibres [Citation7,Citation8]. In addition, these stages are usually accompanied by cell apoptosis which serve to balance and control the process of muscle development [Citation9,Citation10].
CircRNAs are stable in tissues and cells and are a set of transcripts characterized by closed continuous loops. Stable tissues and cells can be considered being closed and continuous. Developments in bioinformatics and RNA sequencing technology have facilitated the discovery of circRNAs in different species. Thousands of circRNAs that have been identified in the muscles of humans, mice and pigs and these have been shown to play an important role in muscle function [Citation11,Citation12]. CircRNA participates in biological processes such as muscle development and cell death via different mechanisms. CircRNAs can be used as microRNA sponges. CircFUT10, circFGFR4 and circLMO7 can be used as endogenous RNAs (ceRNAs) to regulate differentiation of bovine myoblasts [Citation12–14]. Also, circRNAs act as miRNA sponges to participate in apoptosis of myocardial cells during myocardial infarction. In addition, they can regulate RNA-protein interactions and selective splicing, and they can also be used as molecular scaffolds for the formation of protein-DNA complexes [Citation15–18].
In this study, RNA-seq was used to identify circRNA expression in buffalo myoblast cells during proliferation and differentiation. We focused on a 347 nt circRNA derived from a phosphatidylinositol-bound clathrin assembly lymphoid-myeloid leukaemia gene (PICALM), which is differentially expressed during the proliferation and differentiation of myoblasts, and it consists of three exons. PICALM encodes the clathrin assembly protein and was originally identified as a component of the leukaemia oncogene. This protein recruits clathrin and adaptor protein complex 2 (AP2) to form the membrane capsule fossae and clathrin vesicle assembly sites, and also participates in AP2-dependent clathrin-mediated endocytosis at neuromuscular junctions [Citation19]. The circRNA, was subsequently named circCLTH according to its function. CircCLTH was predicted to have a strong targeting relationship with many miRNAs known to be associated with muscle development and RNA hybrid. After a comprehensive investigation of buffalo myoblasts, we found that circCLTH could promote the proliferation and differentiation of buffalo myoblasts, but it inhibited their apoptosis, and it was able to stimulate the regeneration of skeletal muscle in C57BL/6 mice. The purpose of our research was to characterize the role of circCLTH in regulating muscle fibre development. These results provide reliable genetic evidence to aid in the understanding of bovine muscle development. In addition, this study provides a novel molecular basis for a role of circRNAs in the production of optimal meat quality in farmed animals.
Materials and methods
Sample preparation
The study design and animal care protocols were approved by the Animal Care Committee of the School of Animal Science and Technology, Guangxi University (GXU2022240), and they complied with the National Institutes of Health guidelines for the care and use of laboratory animals. The samples were obtained from six animals from the Buffalo Research Institute in Guangxi Province, China. Heart, liver, spleen, lung, kidney, brain, leg muscle and longissimus dorsi muscle tissues were collected from 3-month-old (n = 3) and 3-year-old adult male (n = 3, feeding and growing conditions are similar) buffaloes and were rapidly frozen in liquid nitrogen. Muscle tissues of longissimus muscle of 3 male buffaloes and male cattle (3 years old) were also collected and fixed with tissue fixation solution. Among them, the myoblasts used in this study were primary cells isolated from the muscle tissue of longissimus dorsi muscle of the above 3-month-old male buffaloes. The buffalo myoblasts at the proliferating and differentiating stages were stored in Trizol (Vazyme Biotech Co., Ltd, Nanjing, China) for RNA-seq sequencing, and differentiation experiments were performed in basal medium containing 2% horse serum for 6 days.
RNA-seq library construction and sequencing analysis
The total RNA quality of the RNA extract was evaluated by using a Qubit®3.0 Flurometer (Life Technologies, CA, USA). Ribo-Zero™ Gold Kits (Illumina, CA, USA) were used to remove ribosomal RNA (rRNA) (RNase R added before) and fragmentation of RNA into short fragments was made by adding Fragmentation Buffer into the reaction system. Then, the first cDNA strand was synthesized by using random hexamers, and the second cDNA strand was synthesized by adding buffer solution containing dNTPs, RNase H and DNA Polymerase I (TaKaRa, Dalian, China). The cDNA was purified using a QiaQuick PCR kit after elution with EB buffer solution followed by terminal repair, base A and sequence joining. The target fragments were recovered by agarose gel electrophoresis. The two strands of cDNA were digested by using UNG enzyme, and PCR amplification was performed. The constructed library was sequenced by using kits from Illumina. Finally, PE150 was used for sequencing.
Treatment of raw sequencing data
The raw reads obtained by Illumina sequencing were removed after the joint contaminated base number was set to greater than 5 bp. The reads with quality value Q ≤ 19 accounted for more than 50% of the total bases obtained. Reads containing more than 5% N were removed. High quality clean reads were obtained by removing all rRNA matched reads. The subsequent analyses were based on clean reads. After a basic call to the bcl2fastq2 (version number: V2.20) software, these were converted to sequential reads. Then, the BWA-MEM algorithm in BWA was used to compare the reads with the buffalo genome (Bubalus bubalis UOA_WB_1).
Identification and analysis of circRNAs
The circRNAs were identified with CIRI2 (version number: V2.0.6) software. DEGseq2 was used to analyse the differential expression of circRNA, and the treatment and the reference groups were compared. The ratio of multiples > 1.5 and p < 0.05 were selected as the significantly differentially expressed circRNAs, and the number of circRNAs that were up and down was obtained. The cluster analysis of differentially expressed circRNA in this experiment was completed by using R software (version number: V3.1.1).
Functional analysis and pathway analysis of circRNAs
Gene ontology (GO) analysis (http://www.geneontology.org) was used to characterize circRNA host genes. This was divided into three aspects: biological agents, cell composition and molecular function and the process highlights the molecular functions of some genes while reducing the complexity of presentation. Kyoto Encyclopaedia of Genes and Genomes (KEGG; http://www.kegg.jp) was used for the analysis and presentation of host genes of circRNAs and their associated biological pathways.
Cell culture of buffalo myoblasts
Isolation and culture of buffalo myoblasts were carried out according to existing methods. The procedure used was as follows: the longissimus dorsi muscle tissues of the 3 months old buffalo foetuses were collected and cut into fragments after the fascia fat was removed. The muscle tissue was washed with PBS and digested with 300 U of collagenase I (Gibco, Waltham, MA, USA, Waltham, MA, USA) in a water bath for about 1 h at 37°C. After digestion, the mixture was centrifuged at 600 g for 5 min, the supernatant was discarded and trypsin containing 0.25% EDTA of equal volume was added for further digestion for 20 min at 37°C. After this step a viscous liquid formed and DMEM medium (Gibco, Waltham, MA, USA) containing 10% FBS (Gibco, Waltham, MA, USA) was added to terminate the digestion, and the mixture was filtered successively through 100, 70 and 40 mesh filters in turn. After a further centrifugation at 600 g for 5 min, the supernatant was discarded and washed with PBS for 2–3 times. After another centrifugation step, the cells were re-suspended with medium containing 20% foetal bovine serum and 1% penicillin and streptomycin. Finally, the cells were inoculated in cell culture dishes and then they were cultured in an incubator with 5% CO2 for 2 h at 37°C. After that, the supernatant was transferred to a new dish to obtain purified myoblasts. Myoblast differentiation was induced with 2% horse serum medium (Gibco, Waltham, MA, USA), and the buffalo myoblasts were replaced with fresh medium every day, and the cells were induced for 6 days for cell differentiation experiments. The differentiated cells were used for later assessed of differentiation.
Reverse transcription synthesis cDNA and real-time qPCR detection
RNA was extracted with Trizol (Takara, Dalian, China) and Evo M-MLV Reverse Transcription Kit II (Accurate Biotechnology Co., Ltd., Hunan, China) was used to remove genomic DNA which were then reverse transcribed. The Bio-Rad CF96 system (Bio-Rad, California, USA) and ChamQ Universal SYBR qPCR Master Mix (Vazyme Biotech Co., Ltd, Nanjing, China) were used for real-time quantitative fluorescence PCR (qPCR, n = 3). Two units of RNase R were added to the RNA, incubated at 37°C for 20 min, purified by RNeasy MinElute cleaning kit RNeasy MinElute cleaning kit (Qiagen, Hilden, Germany) and then Marker L (Sangon Biotech, Shanghai, China) was used to measure the sizes of the bands after electrophoresis. The primers used are listed in Table S10.
Plasmid construction
The mature sequence of circCLTH was cloned by PCR (). Kpn I (NEB, Beijing, China) and Not I (NEB, Beijing, China) were double digested and T4 ligase (NEB, Beijing, China) was added and incubated overnight to link the sequence to the pCD2.1-ciR vector (Geneseed, Guangzhou, China). Ligation products were transfected into Trans5α chemoreceptor Escherichia coli (Transgene, Beijing, China). Cloned sequences were confirmed by sequencing to construct the pCD2.1-circCLTH. The primer sequences used are listed in Table S11.
Table 1. Summary of reads mapping to the Buffalo reference.
Table 2. Results from the assembly of circRNAs.
Table 3. MiRNA library.
Table 4. The sequence of circCLTH (Buffalo).
Cell counting kit-8 analysis
The proliferation state of buffalo myoblasts was assessed by using a cell counting kit-8 (Beyotime Biotechnology, Shanghai, China). The cells were transfected into 96-well plates and transfected with ExFect Transfection Reagent T101-01 (Vazyme Biotech Co., Ltd, Nanjing, China) for 16 h − 18 h when the cell density reached 60%. 10 μL CCK solution was added to each well, and incubated for 3 h. The optical density (OD) of each well was measured at 450 nm with Microplate Reader (TECAN, Männedorf, Switzerland).
5-Ethynyl-2′deoxyuridine EdU analysis
A Cell-Light™ EdU Apollo ®567 in vitro imaging kit (RiboBio, Guangzhou, China) was used to study the proliferation status of buffalo myoblasts. The cells were seeded in 6-well plates at a density of 6 × 105 cells per well and then they were cultured overnight at 5% CO2 and 37°C. After that they were treated in the dark for 8 h with 10 μM EdU. The cells were fixed in PBS containing 4% paraformaldehyde for 20 min at room temperature and treated with 2 mg/mL glycine and 0.5% Triton X-100 for 15–20 min at room temperature, respectively. After dyeing with 1× Apollo® staining reaction solution for 30 min according to the instructions, they were washed with PBS twice for 10 minutes each time. After incubation for 30 min in Hoechst 33,342, the cells were washed twice with PBS, and finally placed under an EVOS microscope for photography (Thermo, Boston, UAS).
Flow cytometry analysis
Flow cytometry (Becton Dickinson, Facscalibur, USA) in combination with a cell cycle detection kit (MultiSciences, Hangzhou, China) was used for cell cycle detection. The detailed procedures used strictly followed the manufacturer’s instructions. After cell incubation, detection was performed by Invitrogen Tune NxT acoustic Focused flow cytometry (Thermo, Boston, UAS), and data analysis was performed with FlowJo10.
Western blotting and cell immunofluorescence analysis
Protein was extracted using RIPA buffer (Beyotime Biotechnology, Shanghai, China) containing 1% PMSF (SolarBio, Beijing, China). BCA kit (Beyotime Biotechnology, Shanghai, China) and this was used to determine the protein concentration. The isolated proteins were transferred to polyvinylidene fluoride membranes after 10% SDS (Sangon Biotechnology Co., Ltd, Shanghai, China) polyacrylamide gel electrophoresis. The membranes were incubated overnight with primary antibodies to CyclinD1 (Wanlei, Shenyang, China), PCNA (Wanlei, Shenyang, China), Bax (Wanlei, Shenyang, China), P53 (Wanlei, Shenyang, China), Bcl-2 (Wanlei, Shenyang, China), MyHC (Genetex, Southern California, USA) and β-actin (Abcam, Cambridge, UK) at 4°C. The membranes were then incubated with the corresponding secondary antibodies at room temperature for 1 h. After washing, the membranes were exposed to ECL Plus (SolarBio, Beijing, China) and the Chemidoc XRS+ system (Bio Rad, California, USA) was used for imaging the resultant bands.
For immunofluorescence, cells were fixed with paraformaldehyde for 20 min at 4°C, and then washed 3 times with cold PBS over a 15 min period. They were then washed with 0.5% Triton X-100 for 10 min followed by 3 times with PBS over a 30 min period. The cells were incubated with 5% bovine serum albumin (BSA) in a closed chamber for 30 min, and then they were washed with PBS for 3 times each for 5 min. Cells were incubated overnight at 4°C with MyHC antibody (Genetex, Texas, USA) and MyoD antibody (Abcam, Cambridge, UK) on a shaker and washed three times in cold PBS the next day. Fluorescent-labelled secondary antibody was added and the cells were further incubated at room temperature in the dark for 2 h and then washed with cold PBS for 3–4 times under low-light conditions. The nuclei were stained in a darkened environment with DAPI (2-(4-Amidinophenyl)-6-indolecarbamidine dihydrochloride, Cell Signalling) for 10 min, washed with cold PBS for 2–3 times and then observed under a fluorescence microscope (Nikon, Tokyo, Japan).
Fluorescence in situ hybridization
Fluorescence in situ hybridization (FISH) (Servicebio Technology Co., Ltd., Wuhan, China) was conducted as follows: buffalo myoblast cells were cultured to 70% confluence and then treated with in situ hybridization fixation solution for 30 min as instructed. The prepared premix was incubated with the cells for 1 h at 37°C. The fixed cells were then incubated overnight with a mixed solution containing circCLTH probes. After the cells were washed with PBS 2–3 times, DAPI solution was added to the cell surface in a darkened environment for 8 min. Finally, the cells were observed using a fluorescent microscope (Nikon, Tokyo, Japan).
RNA pull-down assay and mass spectrometric determination
A total of 1 × 107 buffalo myoblasts were harvested for RNA pull-down analysis. The circCLTH probe was synthesized by Sangon Biotechnology Co., Ltd. The probe sequences are shown in . Pierce™ Magnetic RNA-Protein Pull-Down Kit was used to extract RNA conjugates. The mass spectrometry detection was completed by Sangon Bioengineering Co., Ltd. The mass spectrometry analysis was performed using the TripleTOF 5600 LC/MS system of SCIEX Company, and the mass spectrometry data generated by TripleTOF 5600 was searched by ProteinPilot (version number: V4.5), and the database search algorithm used was Paragon. The database used for the search was the bovine and target sequence proteome reference database in UniProt. In order to facilitate statistical analysis and reduce false-positive results caused by the identification of low-abundance proteins, the data with a spectrum number of 0 will be artificially filled with 1. The ratio of the number of spectra (ratio) and the average number of spectra (Mean SP) of each protein in different samples were calculated where x = log2 ratio, y = log2 Mean SP. Based on the quantitative difference and abundance of each protein in different samples, the differential protein screening boundary line y = c/(x–x0) was set. According to the screening criteria, proteins with spectrum differences more than 1.5 times (the number of spectra approaching + ∞) to 3 times (the number of spectra approaching 1) were marked as differential proteins, and the difference in the number of spectra was more than 2 times (the spectrum number is close to 1). Proteins with the number approaching + ∞) to 4 times (the number of spectra approaching 1) were marked as significantly different proteins.
Table 5. Probe sequences for RNA pull down.
Animal studies, frozen sections and H & E staining
C57BL/6 N mice were obtained from the Experimental Animal Center of Guangxi Medical University (Nanning, China). The study design and animal care protocols were approved by the Animal Care Committee of the School of Animal Science and Technology, Guangxi University (GXU2022240), and we complied with the National Institutes of Health guidelines for the care and use of laboratory animals. Mice were prepared by injecting cardiotoxin (CTX; 50 uL of 10 uM CTX solution) into the tibialis anterior muscle of 5-week-old male mice of similar body weight to construct a skeletal muscle injury model. Mice were divided into experimental and control groups. In each experimental group, 50 uL of mixture (which was prepared by mixing the overexpressed plasmid, pCD2.1-circCLTH, with in vivo transfection reagent Entranster TM and glucose) was injected into the left tibialis anterior muscle of 5 mice. The CTX muscle was injected at 12 h, 48 h and 96 h after the injury was successfully constructed. The mice of the control group were injected with plasmid-free glucose solution. After 144 h of CTX treatment, the tibialis anterior muscle of mice was collected with Trizol for RNA extraction, and the gene expression was quantified by qPCR measurements (n = 3). Fresh tissues were also treated with 4% formaldehyde fixation solution for 24 h and the tissues were dehydrated with 10%, 20% and 30%. The tissues were cut into 0.5 cm3 and then embedded with OCT (optimal cutting temperature compound, Sakura, Torrance, CA, USA) frozen section embedding agent (Sakura Tissue-Tek OCT Compound 4583) in a frozen slicer for 10 min for tissue embedding and tissue 5 μm sections were cut. OCT was removed with water at room temperature for H and E staining, and then haematoxylin staining solution was added to stain for 1 min. After rinsing with water, eosin staining solution was added to stain for 10s, and then rinsed again. After H and E staining, the slices were fixed with neutral resin, sealed with clean cover glass, and observed under a fluorescence microscope (Nikon, Tokyo, Japan).
Immunohistochemistry
Fresh tissues were immersed and stored in 4% paraformaldehyde for 24 h for fixation. These were then cut into 0.5 cm3 cubes with surgical shears following gradient dehydration with 10%, 20% and 30% sucrose solutions. The clipped tissues were placed in the middle of a 17 × 17 × 5 mm embedding box (CITOTEST, Jiangsu, China), CTX was added, and these were then transferred to a frozen slicer for 10 min at −25°C. The tissues were cut into 5 μm sections and immersed in PBS for 10 min to remove the CTX solution. The sections were perturbed for 20 min at room temperature with PBS solution containing 0.5% Triton X-100, then covered for 1 h with PBS containing 10% goat serum (Absin, Shanghai, China). The sections were then incubated with MyHC antibody (Genetex, Texas, USA) overnight at 4°C. After washing, they were incubated with fluorescently labelled secondary antibody (Abmart, Shanghai, China) in the dark for 1 h at 37°C. The samples were incubated with 0.1% DAPI solution at 25°C for 15 min, washed with PBS, and observed with fluorescence microscope (Laika, Vizlar, Germany). Image J software was used to analyse and count the average fluorescence intensity of proteins (n = 6).
Statistical analysis
The data in the Tables and Figures are shown as means ± SEMs. The p values were calculated by the two-tailed Student t test. p < 0.05 was considered statistically significant and p < 0.01 was considered extremely significant. The original sequence data files discussed in these experiments are stored in the National Center for Biotechnology Information (NCBI) and the SRA ID is PRJNA639027 (https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA639027).
Results
Identification of circRNAs related to the development of Buffalo myoblasts
To investigate the potential regulatory mechanism of circRNAs in buffalo skeletal muscle cell development, we analysed circRNAs expression during proliferation and differentiation of buffalo skeletal muscle cells by removing ribosomal RNA RNA-seq. After rRNA removal, 233 million and 235 million unique reads were obtained from the transcriptome of buffalo skeletal muscle cells at proliferating and differentiating stages. The results of RNA-seq showed that 100, 061, 338 to 103, 546, 858 and 96, 266, 686 to 101, 952, 364 clean reads were obtained from 6 libraries of proliferating and differentiated myoblast cells, respectively. When the sequences were compared with the buffalo genome, 100, 039, 479 to 103, 527, 375 and 96, 227, 208 to 101, 936, 644 could be mapped to the genome, respectively, with a comparison rate of over 99% (). 3, 142 circRNAs were identified in subsequent analyses ( and S1).
The numbers of circRNAs in the proliferation and differentiation libraries were counted to further identify the circRNAs that have potential functions during these processes of buffalo myoblasts. There were 1, 118 and 1, 063 circRNAs specifically expressed during proliferation and differentiation (Table S2), respectively, and 961 circRNAs were expressed during both phases (). The sources of circRNAs identified were widely distributed on the whole chromosome (). In addition, the number of sequenced reads was positively correlated with chromosome length, indicating that the number of circRNAs formed in the chromosome would increase with the chromosomal length. The circRNAs derived from exons and introns accounted for 96.93% and 1.33% of the total circRNAs, respectively, and the remaining few circRNAs were derived from inter-gene regions ()).
Figure 1. Identification of circRNAs related to the development of Buffalo myoblasts. (a) The Venn diagrams of different circRNAs found at two growth stages (proliferation and differentiation) of Buffalo myoblasts. (b) A Circos plot showing the distribution of circRNAs in different chromosomes. (c and d) The origin of circRNAs from the Buffalo genome that are described in this study. The data represent means ± SEM of at least three independent experiments. (e) A volcano map of the differentially expressed circRNAs. (f) Clustering of the differential circRNAs. Changes in expression are indicated by changes in colour with blue and yellow representing low and high expression respectively.
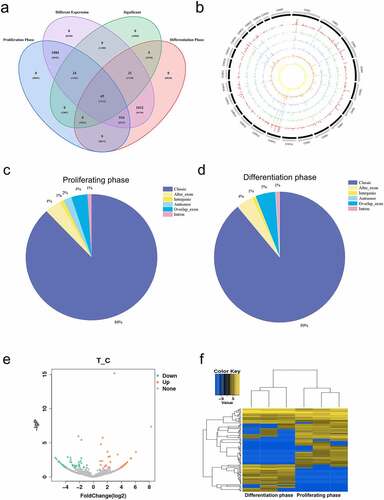
Differential expression of circRNAs was analysed by DEseq2 [Citation20]. The proliferating myoblasts and differentiated cells were compared, and the ratio > 1.5 and p < 0.05 were selected as significantly differentially expressed circRNAs. 110 circRNAs were selected among which 63 circRNAs were down-regulated and 47 were up-regulated (). All the differentially expressed circRNAs are listed in the attached table (Table S3). In order to further analyse their possible functions, a hierarchical clustering analysis was performed on the 110 circRNAs that were significantly differentially expressed (). After the hierarchical clustering analysis, the 6 sequencing libraries were clustered into two parts according to the proliferation and the differentiation phases, and the biological repetition within the two phases were found to be similar. This indicated that the differentially expressed circRNAs identified in this study reflected the differences in circRNAs transcripts of buffalo myoblasts during these two phases, and it also reflected the reliability of the identified circRNAs.
In order to analyse whether the expression level of circRNAs were consistent with that of the host genes. The differentially expressed circRNAs (n = 110) and mRNAs from circRNA host genes (n = 85) were directly compared. The expression patterns of many circRNAs were found to be inconsistent at different developmental stages. At these two different physiological stages, the expression patterns of many circRNAs were inconsistent with the changes of the host genes, and the differential expression of mRNA could not explain the difference in the expression of circRNAs. This strongly suggests that random by-products of classical splicing of mRNA precursors are not the primary source of circRNAs, and it is likely that circRNAs act independently of host gene mRNAs (Figure S1a).
Characterization of differentially expressed circRNAs
GO (Gene Ontology) and KEGG (Kyoto Encyclopaedia of Genes and Genomes) analyses revealed differentially expressed circRNAs and their associated signal transduction pathways. In order to determine the main biological functions of the source genes, the 110 circRNAs host genes with significant differences were analysed by GO clustering enrichment analysis. It was found that the circRNAs mother genes with significant differences were mainly involved in the cellular, metabolic and regulatory processes. These are linked to single-organism processes, in cell components enriched in organelles and molecular functions in relation to binding and catalytic activities. To predict the function of significantly enriched host genes, pathway analysis was performed based on the KEGG pathway database. Of the 66 pathways of enrichment, ubiquitin mediated proteolysis, endocytosis, fatty acid degradation, caffeine metabolism, drug metabolism-other Enzymes, fatty acid metabolism, PPAR signalling pathway, peroxisome, T cell receptor signalling pathway, lysine’s enrichment of degradation, ubiquinone and other terpenoid – quinone biosynthesis and fatty acid biosynthesis were the most significant (); Tables S4 and S5).
Figure 2. Characterization of circRNAs and identification of differentially expressed circRNAs. (a) GO statistics of differentially expressed genes. (b) KEGG signalling pathway was enriched and classified, and the more genes that were enriched, the longer was the column obtained. (c) The size distribution of the circRNAs including flanking introns lengths. (d) The number of circRNAs containing different number of exons. (e) The number of circRNAs produced from one gene. (f) The overall expression distribution of circRNAs during the proliferation and differentiation phases. The data represent means ± SEM of at least three independent experiments. ***p < 0.001, 0.001< **p < 0.01, 0.01< *p < 0.05.
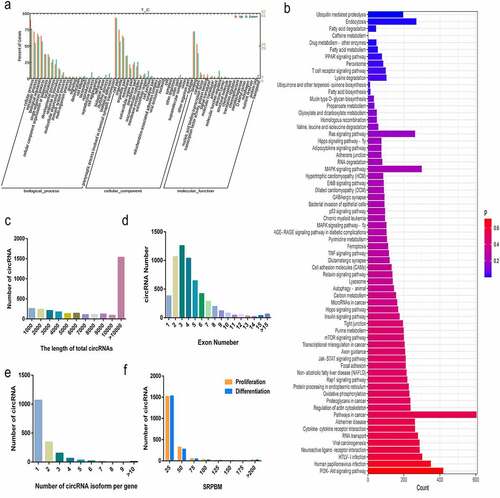
By analysing the circRNAs in buffalo myoblasts, it was found that most of the circRNAs identified were found to be > 10,000 nt in length, including the length of flanking introns. (; ). The minimum length was only 182 nt and the maximum length was 189, 473 nt (). Most of the circRNAs were composed of 2 to 6 exons (), and more than 90% of the parent genes could produce 1 to 3 circRNAs with some producing more than 4 circRNAs (). We used the normalization method of SRPBM (spliced reads per billion mapping) [Citation21] to quantify the expression of circRNAs, and found that the expression levels of these RNAs in buffalo myoblasts was maximal during the processes of proliferation and differentiation in 25 ~ 50 SRPBMs ().
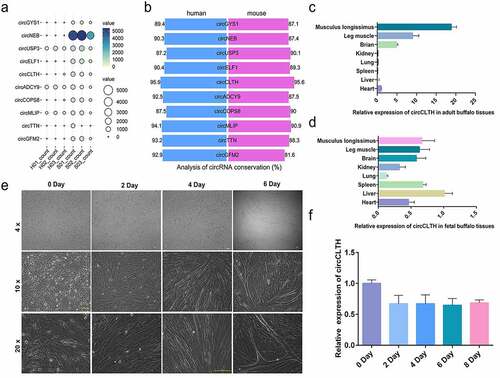
Expression characteristics of circCLTH
According to the RNA-seq data analysis of buffalo and cattle muscles [Citation22], 10 circRNAs with high expression in buffalo muscle tissues were screened (, Table S6). Among the 10 candidate circRNAs, circCLTH showed the highest conservation () and it was highly expressed in foetal and adult buffalo muscle tissues ()). It was also stably expressed during the proliferation and differentiation of buffalo myoblasts ()), which indicated that circCLTH may play a regulatory role in the muscle development of buffaloes. Therefore, in this study, we focused on circCLTH and the data showed its composition and secondary structure are highly conserved (Figure S1(b, c)).
Figure 3. Expression characteristics of circCLTH. (a) Expression bubble map of 10 circRNAs that were highly expressed in buffalo tissue. (b) Conservation analysis of 10 highly expressed circrnas in buffalo tissues, where blue represents the alignment rate on the human genome and red represents the alignment rate on the mouse genome. (c) CircCLTH was differentially expressed in different adult buffalo tissues. (d) CircCLTH was differentially expressed in different foetal buffalo tissues. (e) The Figures show that the primary myoblasts isolated were in a state of progressive differentiation after induction with 2% horse serum for 0, 2, 4 and 6 days, respectively. (f) CircCLTH was differentially expressed in buffalo myoblasts on different days of cell culture. The data represent means ± SEM of at least three independent experiments. ***p < 0.001, 0.001< **p < 0.01, 0.01< *p < 0.05.
Analysis of CircCLTH interactive network
RNAhybrid [Citation23] was used to predict the targeting relationships between circCLTH and some known bovine muscle development-related miRNAs (). We found that circCLTH had strong targeting relationships with several known muscle development-related miRNAs, and the minimum free energy of the two sequences was less than −20 (kcal/mol) as the standard to predict the muscle-related miRNAs targeted by circCLTH (, Tables S7 and S8).
Figure 4. Analysis of CircCLTH interactive network. (a) The miRNA-circRNA network constructed using miRNA binding site prediction results of differentially expressed circRNAs. The blue dots represent circRNAs, and the purple dots represent miRNAs. (b) The network including circCLTH interactions with predicted miRNAs associated with muscle development.
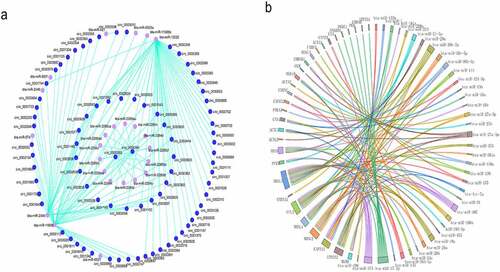
Several circRNAs (such as CDR1 and miR-7, SRY and miR-138) have been reported to have multiple binding sites for miRNAs [Citation24,Citation25]. After miRNAs are adsorbed, they cannot regulate their corresponding target genes. The role of miRNA is to act as a molecular sponge. We selected 110 differentially expressed circRNAs, combined with the miRNA library of muscle development, and used miRanda (3.3a) [Citation26] to predict the miRNA binding sites of the circRNAs. Then, a miRNA-circRNA network of buffalo myoblasts was constructed ().
Localization and Expression of circCLTH in Buffalo myoblasts
Convergent and divergent primers were designed and synthesized based on the structural characteristics of circRNAs. PCR amplification was then performed using buffalo cDNA and genomic DNA as templates. The results of agarose gel electrophoresis showed that both the divergent and convergent primers of circCLTH could be amplified with the cDNA samples, but the divergent primer of circCLTH could not be used to amplify from the genomic DNA (). The RNase R digestion assay further confirmed the circular structure of circCLTH ()). CircRNAs located in different locations of cells play different regulatory roles: circRNAs located in the cytoplasm can bind with microRNAs or proteins for post-transcriptional regulation, while circRNAs located in the nuclei can play regulatory roles by affecting gene transcription. CircCLTH is well localized in the cytoplasm (). Therefore, we hypothesized that circCLTH might regulate differentiation of myoblasts by binding to RNA or proteins.
Figure 5. Localization and expression of circCLTH in Buffalo myoblasts. (a) The results of using buffalo cDNA and genomic DNA as templates to conduct PCR amplification in the junction regions of circCLTH to verify their authenticity. (b and c) The results of digestion with RNase R showed that the expression levels of circCLTH were resistant to RNase R degradation. (d) The results of RNA-FISH subcellular localization showed that circCLTH was mainly located in the cytoplasm. Magnification 65 x. (e) Immunofluorescence results showed that myoblasts used were differentiated. (f) The overexpression plasmid of circCLTH was constructed.
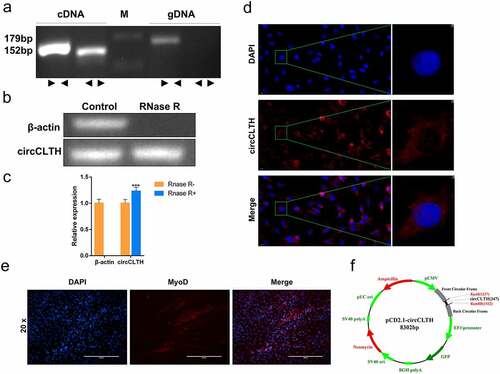
The isolated buffalo myoblasts were identified by using MyoD staining. This ensured that the buffalo myoblasts could be differentiated for subsequent differentiation experiments (). Also, the expression levels of the differential circRNAs (mean SRPBM = 28.63) were consistent with the host genes (mean FPKM = 39.66) (Tables S1 and S9). We constructed a pCD2.1-circCLTH overexpression vector in order to explore the role of circCLTH in buffalo muscle development ().
CircCLTH promoted proliferation and differentiation of Buffalo myoblasts and inhibited their apoptosis
In order to elucidate the effect of circCLTH on the proliferation and apoptosis of myoblasts, the overexpressed vector, pCD2.1- circCLTH ()) was transfected into buffalo myoblasts. QPCR and western blotting results showed that the expression of the proliferating gene, cyclin D1, was significantly increased at both the mRNA and protein levels after overexpression of pCD2.1-circCLTH for 24 h ()). The proportion of EdU-positive cells increased with overexpression of circCLTH, indicating that this circular RNA may enhance cell proliferation ()). Moreover, analysis of the cell cycle data showed that overexpression of circCLTH could increase the proportion of S and G2 phase myoblasts and reduce the number of G0/G1 phase cells ()).
Figure 6. The effects of circCLTH on myoblasts. (a) CircCLTH overexpression levels. (b) QRT-PCR to detect the mRNA expression of muscle proliferating genes, PCNA, CyclinD1 and CDK2. (c) Image J was used for data statistics of the EdU assay results. (d) The EdU assay was used to detect the effect of circCLTH on cell proliferation. (e) Flow cytometry was used to analyse the effect of circCLTH on the cell cycle. (f) The results from cell cycle analysis showed that the number of G2 cells increased significantly after the overexpression of circCLTH. (g) The expression of PCNA and CyclinD1 proteins were detected by Western blot. (h) MyHC protein expression was determined by western blotting. (i) The mRNA expression of MyoD and MyHC genes was measured by qPCR. (j and k) The effect of circCLTH transfection on MyHC gene expression in buffalo myoblasts during differentiation was assessed by immunofluorescence assay. (l)Apoptosis was detected following circCLTH overexpression level in cells. (m) QPCR was used to measure the mRNA expression of apoptosiss-associated genes, P53, Bax and Bcl-2. (n) The expression of P53, Bax and Bcl-2 proteins were detected by Western blot. The data represent means ± SEM of at least three independent experiments. ***p < 0.001, 0.001< **p < 0.01, 0.01< *p < 0.05.
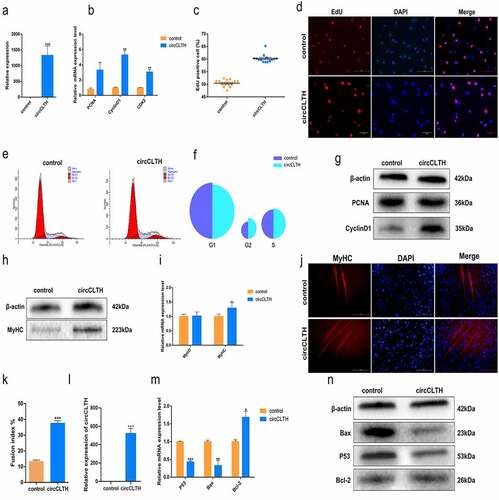
All the above experimental results indicated that circCLTH could promote the proliferation of buffalo myoblasts. To explore the regulatory effects of circCLTH on cell differentiation, we examined the expression of MyoD and MyHC in differentiated buffalo myoblast cells after overexpression with pCD2.1-circCLTH. It was found that overexpression of circCLTH promoted mRNA and protein expression of MyHC significantly ()). Immunofluorescence assay results showed that overexpression of circCLTH promoted the differentiation and fusion of myocytes into myotubules ()). These results suggest that circCLTH promoted myoblast differentiation.
It was reported that PICALM, the source gene of circCLTH, was associated with apoptosis. Red blood progenitor cells lacking PICALM were shown to absorb transferrin-bound iron in vivo through PICALM-independent pathways (such as via direct contact with surrounding cells) and promote red blood cell-apoptosis [Citation27]. Bcl-2, Bax and p53 are considered to be key regulators of apoptosis. In order to analyse whether circCLTH had an effect on the apoptosis of myoblasts, circCLTH was overexpressed and qPCR and western blotting experiments were designed to verify whether circCLTH affected the expression levels of Bcl-2, Bax and p53. The results showed that circCLTH could reduce the expression of p53 and Bax at both the mRNA and protein levels significantly, while the expression of Bcl-2 was increased ()). The results confirmed that circCLTH had an inhibitory effect on buffalo myoblast apoptosis.
The effect of CircCLTH on Buffalo myoblast regeneration
We compared circCLTH homologues in mice and buffalo and found 95% homology between the two species (). A mouse-muscle injury model was constructed to test the role of circCLTH in skeletal muscle development in individual animals (). The tibialis anterior muscle injury induced by CTX injection. After 48 h, haematoxylin eosin (H&E) staining showed that the tibialis anterior muscle was significantly damaged in CTX-treated mice compared with the control group, and this confirmed that the model was successfully established (). The expression of circCLTH was increased experimentally by transfection of the circCLTH overexpression plasmid in vivo ()). Quantitative results showed that the mRNA expression of MyoD, MyoG, PAX7 and other genes were significantly increased ()), indicating that circCLTH may have a positive effect on muscle injury and repair in mice.
Figure 7. CircCLTH promotes skeletal muscle regeneration. (a) Homology comparison of bovine and mouse circCLTH. (b) Schematic diagram of the construction protocol for a mouse muscle injury model. (c) Expression levels after 3 days of circCLTH plasmid injection for skeletal muscle injury. (d) QPCR to determine the mRNA levels of myogene-related genes 3 days after transfection of the tibialis anterior with circCLTH expression plasmid. (e) Expression levels after 7 days of circCLTH plasmid injection of skeletal muscle injury. (f) QPCR to determine the mRNA levels of myogenesis-related genes 7 days after transfection of the tibialis anterior with circCLTH expression plasmid. (g) H & E staining 48 h after CTX treatment showing that the muscle injury model was successfully constructed. (h) H & E-staining CTX-injected muscle after transfection of the circCLTH expression plasmid. (i) Average single muscle fibre/area statistics. (j) After circCLTH was overexpressed in muscle injury model for 7 days, MyHC protein expression was detected by immunofluorescence method. (k) Immunofluorescence fluorescence intensity was analysed, and photomicrographs were taken for each protein in each group for statistics purposes. The data represent means ± SEM of at least three independent experiments. ***p < 0.001, 0.001< **p < 0.01, 0.01< *p < 0.05.
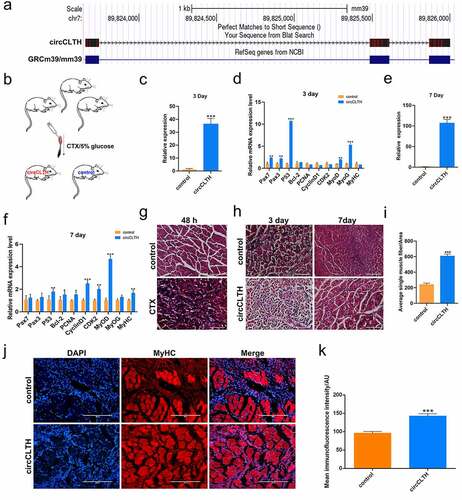
On the 7th day, the tibia anterior muscle of mice was stained with H & E. The muscle fibres in the circCLTH overexpression group were basically repaired and their morphology appeared to be recovered, while the calcified tissue damage caused by injection of cardiac toxin was still visible in the control group. Image Pro Plus software was used to analyse and statistically show that the average area of the single muscle fibres was significantly larger than that of the control group ()). Similarly, in order to illustrate the effect of circCLTH on regeneration of buffalo myoblasts, we performed immunofluorescence staining of MyHC on the injured mouse tissues in order to detect the difference in the number of new muscle fibres ()), and the results showed that the expression of MyHC protein was significantly increased after overexpression of circCLTH. The number of new muscle fibres was significantly higher when compared to control tissues.
CircCLTH interacts with proteins
RNA pull-down assays and mass spectrometry were used to detect the proteins that may bind to circCLTH. The mass deviations of the identified peptides in all samples were counted and it was found to be ± 20 ppm, indicating the accuracy of the mass spectrometric detection (). Most of the peptides were between 8 and 20 amino acid residues in length, which was in line with the law governing trypsin digestion and conducive to mass spectrometry detection. The cleavage sites of each peptide deletion are shown in . The length of each identified peptide is shown in . Most peptides had no missing cleavage sites, with a few of them having one or two missing cleavage sites, indicating that the enzyme digestion was sufficient and the sample preparation met the standard procedures. The results showed that circCLTH could capture TUBA1B, NPM1, PLEC as well as several other proteins (; ).
Figure 8. CircCLTH interacts with proteins. (a) The mass deviation of the peptides identified in the samples was in the range of ±20 PPM, indicating that the mass deviation of each peptide was less than 20 ppm, and the detection accuracy of the mass spectrometry was good. (b) Using mass spectrometry, unused ≥ 1.3 proteins were screened out, and the number of proteins identified in the sample. (c) The length of the identified peptide. (d) The circCLTH RNA pull down differential protein distribution. The data represent means ± SEM of at least three independent experiments. ***p < 0.001, 0.001< **p < 0.01, 0.01< *p < 0.05.
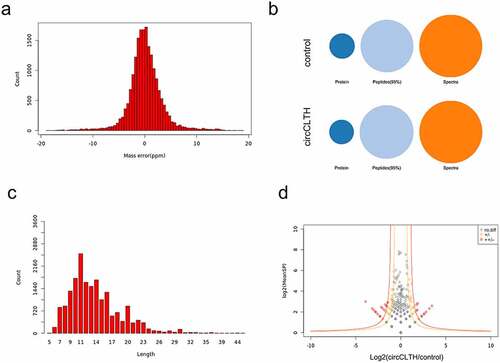
Table 6. Differential binding protein of circCLTH obtained by RNA pulldown.
Discussion
In this study, high-throughput RNA-seq analysis was used to identify 1,118 circRNAs in proliferating buffalo myoblasts and 1,063 circRNAs in differentiated buffalo myoblasts. Differentiated circRNAs were screened by comparing proliferating myoblasts and differentiated cells, and 63 circRNAs were found to be down-regulated and 47 circRNAs were up-regulated. These results suggest that the regulation of circRNAs expression may be one of the potential factors leading to the difference in beef quality. Here, we identified the transcription of a circRNA that is highly expressed in skeletal muscle, circCLTH, and we suggest that circCLTH may be involved in the regulation of muscle development. In recent years, it has been reported that circRNAs can participate and regulate gene expression in the growth and developmental stages of skeletal muscle [Citation28–31]. We also conducted functional studies in vivo and in vitro. The results of CCK-8 and EdU analysis showed that overexpression of circCLTH promoted the proliferation of buffalo myoblasts. It is noteworthy that overexpression of circCLTH can also induce the differentiation of buffalo myoblasts, with more obvious myotubes and increased the expression of differentiation-related proteins.
It has been reported that circRNAs usually have a variety of regulatory mechanisms, which perform different functions according to their localization in cells. CircRNAs usually act as molecular scaffolds in the nuclei to recruit transcription factors or transcriptase in order to mediate gene transcription. In the cytoplasm, circRNAs bind miRNAs competitively, thereby reducing their inhibitory effects on target genes [Citation24,Citation25]. For example, circMYBPC1 regulates myogenesis by binding to miR-23a to reduce its inhibition of MyHC [Citation32], circFGFR4 reduces its inhibition of SRF by binding to miR-107 [Citation13] and circFUT10 reduces its inhibition of Wnt by binding to miR-133a [Citation14]. In the nucleus, circRNAs can bind proteins, and multiple circRNAs can be sponges for proteins. For example, circMbl binds to the MBL protein and prevents its function [Citation33]. CircANRIL is associated with atherosclerotic cardiovascular disease and inhibits ribosome production in vascular smooth muscle cells by binding to factors essential for ribosomal 60S subunit assembly, resulting in atherosclerotic nucleolar stress and cell death [Citation33]. CircPABPN1 mostly locates in the cytoplasm and inhibits the binding of RBP HuR (also known as ELAVL1) to its linear homologous PABPN1 mRNA, resulting in reduced mRNA translation [Citation34]. CircCLTH mainly locates in the cytoplasm, suggesting that CircCLTH may play a regulatory role through competition with miRNAs.
After analysing the sequence of circCLTH, we listed the microRNAs that circCLTH might bind to, and this provided a direction for further research on the mechanism of circCLTH. CircCLTH is partially localized in the nucleus. RNA pull-down analysis, showed that circCLTH may bind to TUBA1B, NPM1 and plectin (PLEC) proteins so as to regulate the myogenesis of buffalo myoblasts. Among these, PLEC is a cell junction protein, widely expressed in skin and muscle, as well as in the plasma membranes of many other tissues and most types of cells. Studies have shown that PLEC can promote the differentiation and proliferation of C2C12 myoblast cells, but inhibition of apoptosis can also regulate the expression of atrophy-related genes (such as atrogin-1 and muRF-1) to avoid muscular atrophy [Citation35]. This is consistent with the regulation phenomenon that circCLTH promotes the proliferation and differentiation of buffalo myoblast cells and inhibits cell apoptosis. Therefore, we concluded that circCLTH may play its biological function by binding with PLEC protein.
In general, abnormal skeletal muscle development can lead to muscle diseases such as muscular dystrophy, myo-hypertrophy and various primary muscle diseases [Citation36]. It has been proposed that non-coding RNAs (ncRNAs) play an important role in muscle regeneration and disease. A series of circRNAs, including circ-ZNF609 [Citation37], circ-FoxO3 [Citation38], CircFndc3b [Citation39], CircMAP3K5 [Citation37] may be molecular markers for diagnosing diseases and analysis of various myoblast types, including skeletal, cardiac and vascular smooth muscles [Citation40–43]. CircEch1 was found to promote the differentiation of myoblasts, revealing its potential role in the treatment of muscle diseases [Citation22].
Studies have reported that in both in vitro and in vivo experiments, overexpression of hsa_circ_0023919 (circPICALM, sequence 2) can significantly inhibit the migration, invasion and wound healing capacity of bladder cancer cells, and hsa_circ_0023919 co-localizes with miR-1265 and acts as a sponge for this miRNA [Citation44]. The sequence length of hsa_circ_0023919 is 451 nt, which is 104 nt longer than hsa_circ_0023925 and the buffalo circCLTH sequence. The alignment rate in similar segments was found to be > 95%, and there was a 16 nt difference. This indicates that the circCLTH may be a potential therapeutic target for human bladder cancer. It is also worth noting that circCLTH has 95% homology between mice and cattle. Overexpression of circCLTH in buffalo myoblasts promotes muscle fusion, suggesting that circCLTH may play an important role in myogenesis.
In general, primary muscle diseases such as hypertrophy and atrophy are caused by skeletal muscle dysplasia. Therefore, we further studied the function of circCLTH in mice. The results of muscle injury experiment in mice showed that the expression of Pax7 in the tibialis anterior muscle was significantly increased after 3 days in circCLTH group, indicating that muscle satellite cells were activated. In addition, the expression of MyoD and MyoG was significantly increased, promoting the repair of muscle injury. The mRNA expression of PCNA, CDK2, cyclin D1, MyoD and MyHC in the overexpressed circCLTH group were significantly increased after 7 days. Immunofluorescence results also showed that MyHC protein was significantly increased. These results suggest that in vivo overexpression of circCLTH promoted muscle regeneration, revealing a potential role of circCLTH in muscle injury diseases.
In conclusion, this study mapped the expression of circRNAs in buffalo skeletal muscle cells. We identified a large number of new circRNAs during the proliferation and differentiation phases of buffalo myoblasts. Among them, 110 circRNAs were expressed significantly and differentially during the proliferation and differentiation of myoblasts. This laid the foundation for further research on the role of these circRNAs in the process of buffalo myogenesis. We focused on exploring the functions of a circRNA, circCLTH, which is a buffalo-specific high expressing circRNA. Our results showed that circCLTH can promote the proliferation and differentiation of buffalo myoblasts, inhibit cell apoptosis, and partially stimulate mouse skeletal muscle regeneration. This study has increased the current understanding of the developmental mechanisms involved in buffalo skeletal muscle. In addition, it has laid the molecular foundation for the breeding of Chinese buffaloes in order to provide a new potential source of meat for human consumption.
Author contributions
DS, HL and QL designed the study. HL, MC, MS performed the experiments and together with SRS drafted the manuscript. XL, KH, MC, YC and DZ helped to perform the experiments and analyzed the data. MJ, JS and OY helped to collect tissue samples. RS helped to draft the manuscript. All the authors read and approved the final manuscript. All authors contributed to the article and approved the submitted version.
Supplemental Material
Download Zip (4.9 MB)Acknowledgments
The authors thank the State Key Laboratory for Conservation and Utilization of Subtropical Agro-Bioresources of Guangxi University for providing the experimental platform and conditions. They also thank Guangxi Medical University for assistance with sample collection.
Disclosure statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability statement
Raw sequencing data that support the findings of this study were deposited in the NCBI Sequence Read Archive (SRA) under the Bioproject accession number PRJNA639027 (https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA639027). The details are available in Supplementary Tables. The raw data are provided with this paper.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15592294.2022.2117115
Additional information
Funding
References
- Hernández-Castellano L E, Nally J E, Lindahl J, Wanapat M, Alhidary I A, Fangueiro D, Grace D, Ratto M, Bambou J Christophe and de Almeida A M. (2019). Dairy science and health in the tropics: challenges and opportunities for the next decades. Trop Anim Health Prod, 51(5):1009–1017.
- Huang J, Zheng Q, Wang S, et al. High-Throughput RNA sequencing reveals -AS lncRNA Promotes adipogenic differentiation in Chinese buffalo (L). Genes (Basel). 2019;10(9):689.
- Di Stasio L, Brugiapaglia A. Current knowledge on river buffalo meat: a critical analysis. Animals (Basel). 2021;11(7):2111.
- Li H, Huang K, Wang P, et al. Comparison of long non-coding RNA expression profiles of cattle and buffalo differing in muscle characteristics. Front Genet. 2020;11:98.
- Buckingham M, Bajard L, Chang T, et al. The formation of skeletal muscle: from somite to limb. J Anat. 2003;202(1):59–68.
- Li L, Cheng X, Chen L, et al. Long Noncoding Ribonucleic Acid MSTRG.59589 Promotes Porcine Skeletal Muscle Satellite Cells Differentiation by Enhancing the Function of PALLD. Front Genet. 2019;10:1220.
- Buckingham M. Myogenic progenitor cells and skeletal myogenesis in vertebrates. Curr Opin Genet Dev. 2006;16(5):525–532.
- Skrivergaard S, Rasmussen MK, Therkildsen M, et al. Bovine satellite cells isolated after 2 and 5 days of tissue storage maintain the proliferative and myogenic capacity needed for cultured meat production. Int J Mol Sci. 2021;22(16):8376.
- Bailey P, Holowacz T, Lassar AB. The origin of skeletal muscle stem cells in the embryo and the adult. Curr Opin Cell Biol. 2001;13(6):679–689.
- Rybak-Wolf A, Stottmeister C, Glažar P, et al. Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol Cell. 2015;58(5):870–885.
- Legnini I, Di Timoteo G, Rossi F, et al. Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Mol Cell. 2017;66(1):22–37.e9.
- Wei X, Li H, Yang J, et al. Circular RNA profiling reveals an abundant circLMO7 that regulates myoblasts differentiation and survival by sponging miR-378a-3p. Cell Death Dis. 2017;8(10):e3153.
- Li H, Wei X, Yang J, et al. circFGFR4 promotes differentiation of myoblasts via binding miR-107 to relieve its inhibition of Wnt3a. Mol Ther Nucleic Acids. 2018;11:272–283.
- Li H, Yang J, Wei X, et al. CircFUT10 reduces proliferation and facilitates differentiation of myoblasts by sponging miR-133a. J Cell Physiol. 2018;233(6):4643–4651.
- Hansen TB, Jensen TI, Clausen BH, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495(7441):384–388.
- Li Z, Huang C, Bao C, et al. Corrigendum: exon-intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol. 2017;24(2):194.
- Memczak S, Jens M, Elefsinioti A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495(7441):333–338.
- Zhang Y, Zhang X-O, Chen T, et al. Circular intronic long noncoding RNAs. Mol Cell. 2013;51(6):792–806.
- Tian Y, Chang JC, Fan EY, et al. Adaptor complex AP2/PICALM, through interaction with LC3, targets Alzheimer’s APP-CTF for terminal degradation via autophagy. Proc Natl Acad Sci U S A. 2013;110(42):17071–17076.
- I LM, Wolfgang H, Simon A. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550.
- Li Y, Zheng Q, Bao C, et al. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 2015;25(8):981–984.
- Huang K, Chen M, Zhong D, et al. Circular RNA profiling reveals an abundant circEch1 that promotes myogenesis and differentiation of bovine skeletal muscle. J Agric Food Chem. 2021;69(1):592–601.
- Rehmsmeier M, Steffen P, Höchsmann M, et al. Fast and effective prediction of microRNA/target duplexes. RNA. 2004;10(10):1507-17.
- Hansen TB, Jensen TI, Clausen BH, et al. Natural RNA circles function as efficient microRNA sponges. Nature. International weekly journal of science. 2013;495(7441):384–388.
- Sebastian M, Marvin J, Antigoni E, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495(7441):333-8.
- John B, Enright AJ, Aravin A, et al. Human MicroRNA Targets. PLOS Biol. 2004;2(11):e363.
- Yuichi I, Manami M, Mithun P, et al. Role of the clathrin adaptor PICALM in normal hematopoiesis and polycythemia vera pathophysiology. Haematologica. 2015;100(4):439-51.
- Reut A-F, Markus M, Reddy PN, et al. circRNA biogenesis competes with pre-mRNA splicing. Mol Cell. 2014;56(1):55-56.
- Y W, M L, Y W, et al. A Zfp609 circular RNA regulates myoblast differentiation by sponging miR-194-5p. Int J Biol Macromol. 2019;121:1308–1313.
- X W, H L, J Y, et al. Circular RNA profiling reveals an abundant circLMO7 that regulates myoblasts differentiation and survival by sponging miR-378a-3p. Cell Death Dis. 2017;8(10):e3153. 10.1038/cddis.2017.541.
- Simona G, Beatrice C, Germana F, et al. Circular RNAs in muscle function and disease. Int J Mol Sci.2018 ;19(11):3454.
- Chen M, Wei X, Song M, et al. Circular RNA circMYBPC1 promotes skeletal muscle differentiation by targeting MyHC. Mol Ther Nucleic Acids. 2021;24:352–368.
- Ashwal-Fluss R, Meyer M, Pamudurti NR, et al. circRNA biogenesis competes with pre-mRNA splicing. Mol Cell. 2014;56(1):55–66.
- Li XX, Xiao L, Chung HK, et al. Interaction between HuR and circPABPN1 modulates autophagy in the intestinal epithelium by altering ATG16L1 translation. Mol Cell Biol. 2020;40(6). 10.1128/MCB.00492-19
- Huadong Y, Shunshun H, Can C, et al. Plectin regulates Wnt signaling mediated-skeletal muscle development by interacting with Dishevelled-2 and antagonizing autophagy Gene, (prepublish). 2021.
- Mukund K, Subramaniam S. Skeletal muscle: a review of molecular structure and function, in health and disease wiley interdisciplinary reviews: systems biology and medicine. 2020;12(1).
- Qian Y, Li Y, Li R, et al. circ-ZNF609: a potent circRNA in human cancers. J Cell Mol Med. 2021;25(22):10349–10361.
- Yan B, Zhang Y, Liang C, et al. Stem cell-derived exosomes prevent pyroptosis and repair ischemic muscle injury through a novel exosome/circHIPK3/ FOXO3a pathway. Theranostics. 2020;10(15):6728–6742.
- Garikipati VNS, Verma SK, Cheng Z, et al. Circular RNA CircFndc3b modulates cardiac repair after myocardial infarction via FUS/VEGF-A axis. Nat Commun. 2019;10(1):4317.
- Chen J-F, Mandel EM, Thomson JM, et al. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38(2):228–233.
- Kyun KH, Sun LY, Umasundari S, et al. Muscle-specific microRNA miR-206 promotes muscle differentiation. J Cell Biol. 2006;174(5):677-87.
- Rooij EV, Quiat D, Johnson BA, et al. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev cell. 2009;17(5):662–673.
- Yang D, Min WY, Ran ZW, et al., The role of microRNA-1 and microRNA-206 in the proliferation and differentiation of bovine skeletal muscle satellite cells.In vitro cellular & developmental biology. Animal. 2016;52(1):27-34.
- Yan D, Dong W, He Q, et al. Circular RNA circPICALM sponges miR-1265 to inhibit bladder cancer metastasis and influence FAK phosphorylation. EBioMedicine. 2019;48:316-331.
