ABSTRACT
Keratinocyte senescence contributes to skin ageing and epidermal dysfunction. According to the existing knowledge, the transcription factor ΔNp63α plays pivotal roles in differentiation and proliferation of keratinocytes. It is traditionally accepted that ΔNp63α exerts its functions via binding to promoter regions to activate or repress gene transcription. However, accumulating evidence demonstrates that ΔNp63α can bind to elements away from promoter regions of its target genes, mediating epigenetic regulation. On the other hand, several epigenetic alterations, including DNA methylation, histone modification and variation, chromatin remodelling, as well as enhancer-promoter looping, are found to be related to cell senescence. To systematically elucidate how ΔNp63α affects keratinocyte senescence via epigenetic regulation, we comprehensively compiled the literatures on the roles of ΔNp63α in keratinocyte senescence, epigenetics in cellular senescence, and the relation between ΔNp63α-mediated epigenetic regulation and keratinocyte senescence. Based on the published data, we conclude that ΔNp63α mediates epigenetic regulation via multiple mechanisms: recruiting epigenetic enzymes to modify DNA or histones, coordinating chromatin remodelling complexes (CRCs) or regulating their expression, and mediating enhancer-promoter looping. Consequently, the expression of genes related to cell cycle is modulated, and proliferation of keratinocytes and renewal of stem cells are maintained, by ΔNp63α. During skin inflammaging, the decline of ΔNp63α may lead to epigenetic dysregulation, resultantly deteriorating keratinocyte senescence.
Introduction
The largest organ of the human body, skin, acts as a protective barrier on our surface. It is also responsible for maintaining homoeostasis of various substances as well as sensory perception and temperature regulation [Citation1,Citation2]. Keratinocyte is the predominant cell type in the epidermis, which is the outermost layer of the skin. Senescence of keratinocytes is an important part of skin ageing, which may impair the functions of skin. Numerous studies show that epigenetic alterations are involved in skin ageing and cell senescence [Citation3–5]. p53 family proteins are key transcription factors controlling cell cycle [Citation6]. p63 belongs to p53 gene family and encodes multiple protein isoforms, among which ΔNp63α is the predominant species expressed in the skin tissue [Citation7]. As a key transcription factor in keratinocyte differentiation and renewal, ΔNp63α plays key roles to maintain proliferation of keratinocytes and to prevent them from undergoing senescence. It is traditionally accepted that ΔNp63α exerts proliferative functions via binding to promoter regions of various cell cycle arresting genes (e.g., Pten, p16INK4a and p21WAF1/CIP1) and consequently downregulating their expression [Citation7–9]. Recently, ΔNp63α has been found to act as a pioneer transcription factor to regulate gene expression via binding to regions other than promoters [Citation10,Citation11]. In this review, we focus on the epigenetic regulation of ΔNp63α in preventing keratinocyte senescence.
ΔNp63α and senescence of keratinocytes
The skin consists of three layers, the epidermis, dermis, and hypodermis, each of which is separate but functionally interdependent. The outermost layer, epidermis, is predominantly composed of keratinocytes, which provide hardness and moisture-lock properties to the skin [Citation1]. Keratinocytes are continuously proliferating to renew the outer skin barrier. They migrate upwards and differentiate into cells that comprise the epidermis [Citation12–14].
Skin undergoes intrinsic or chronological ageing, as a consequence of internal and external changes due to passage of time [Citation15]. Owing to extensive contact with the outside environment, epidermal tissues (especially keratinocytes) are continuously exposed to diverse external stimuli, which can cause inflammaging [Citation16–18]. Alongside inflammaging of the skin, the chronic low-level pro-inflammatory cytokines, in concert with the external stimuli, may induce and deteriorate senescence of keratinocytes and other skin-resident stromal cells. As a continuously renewing epithelium, the epidermis relies heavily on the proliferative potential of keratinocytes to work as a functional barrier of our body [Citation19]. Senescence of keratinocytes may lead to decline of their physiological functionality, which accelerates the ageing process of skin tissues [Citation13,Citation20]. In particular, these senescent keratinocytes produce a complex secretome, which is termed senescence-associated secretory phenotype (SASP) and leads to some detrimental effects, such as paracrine senescence, immune evasion, inflammation, and tumorigenesis (depicted as ) [Citation21].
Figure 1. Pathways through which ΔNp63α regulates keratinocyte senescence and ΔNp63α is regulated. (a) ΔNp63α regulates transcription of diverse genes involved in cell cycle. Consequently, keratinocyte senescence and its detrimental outcomes are prevented. (b) ΔNp63α transactivates multiple genes to activate Hh, Notch, and Wnt signaling pathways. In turn, stem cell self-renewal is maintained. (c) Various stresses can stimulate p38 MAPK or PI3K/Akt pathways, resulting in downregulation of ΔNp63α at the mRNA or protein level.
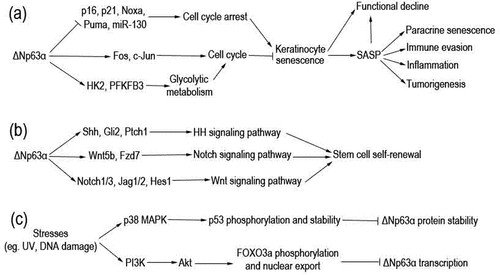
As an indispensable transcription factor in keratinocytes, ΔNp63α plays a master role in maintaining proliferation and preventing senescence of these cells [Citation19]. ΔNp63α is encoded by p63 gene, which belongs to the p53 transcription factor gene family and produces multiple protein isoforms [Citation7,Citation22]. Among them, ΔNp63α is the predominant isoform in somatic cells, especially in keratinocytes [Citation23–25]. As depicted in , ΔNp63α directly binds to p63-responsive elements (p63REs) in promoter regions of its target genes to mediate either transcriptional activation or inhibition in different scenarios [Citation7,Citation26]. These p63REs can also be recognized by other p53 family members, including p53, TAp63 and p73 proteins. Competitive association as well as heteromerization of ΔNp63α can also downregulate these target genes of p53 family transcription factors [Citation7,Citation8,Citation19]. Data from our group and other labs demonstrate that ΔNp63α downregulates a batch of cell-cycle arrest-related genes including p21WAF1/CIP1, p16INK4a, miR-130, MM1, Noxa and Puma, maintaining cell proliferation and survival (depicted as ) [Citation27–32]. On the other hand, there is also evidence that ΔNp63α maintains keratinocyte proliferative capacity via upregulating cell cycle genes such as Fos and c-Jun [Citation33,Citation34], as well as genes involved in glycolytic metabolism, such as hexokinase 2 (HK2) and 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 (PFKFB3) (depicted as ) [Citation35,Citation36].
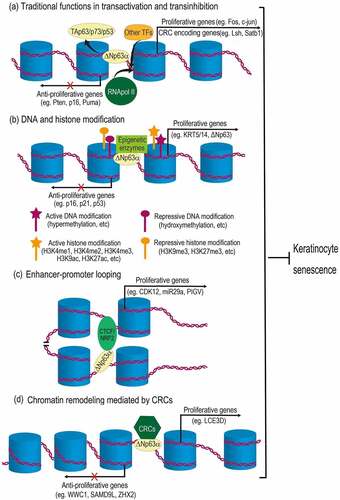
Figure 2. Mechanisms of ΔNp63α-mediated epigenetic regulation in preventing keratinocyte senescence. (a) As a traditional transcription factor, ΔNp63α binds to promoter regions, antagonizing its homologues (TAp63, p73 or p53), as well as recruiting other transcription factors (TFs) and RNA polymerase II (RNApol II), regulating genes related to cell cycle and chromatin remodeling complex (CRC). (b) ΔNp63α recruits diverse epigenetic enzymes (e.g., DNMT3A, KMT2D, HDAC1/2), leading to either active or repressive modification of DNA and histones. (c) ΔNp63α cooperates with CTCF or NRF2 to mediate enhancer-promoter looping. (d) ΔNp63α can also recruit CRCs (e.g., BAF, ACTL6A, SRCAP) to change chromatin configurations, which may either increase or decrease DNA accessibility for transcription machinery. Resultantly, proliferative genes are upregulated, while anti-proliferative genes are downregulated. Therefore, proliferation of keratinocytes is maintained and senescence is prevented.
Epidermal stem cells are important for replacing the cells lost during keratinocyte differentiation and senescence. It is documented that ΔNp63α keeps the regenerative capacity of stratified epithelial structure via maintaining epithelial stemness [Citation37]. ΔNp63α is essential for the asymmetric division of epithelial stem cells to maintain the stem cell pool [Citation38], which can be exhausted via symmetric division into transit-amplifying cells in the absence of p63 gene, resulting in epithelial defects [Citation39–41]. ΔNp63α may contribute to the stemness phenotype via controlling multiple signalling pathways (depicted as ): (1) transactivating Shh, Gli2 and Ptch1 to activate the Hedgehog (Hh) pathway [Citation42,Citation43]; (2) stimulating the Notch pathway via positively regulating the expression of a number of components of this pathway, including Notch1, Notch3, Jag1, Jag2, and Hes1 [Citation39,Citation44–47]; (3) activating Wnt signalling pathway through the direct upregulation of transcription of the Fzd7 receptor as well as the Wnt5a ligand [Citation48]. Via modulating these signalling pathways in different scenarios, ΔNp63α governs the balance between self-renewing and transit-amplifying cells [Citation37,Citation49].
The essential roles of ΔNp63α in antagonizing keratinocyte senescence are supported by data from mouse models and clinical studies. Keyes WM et al. found that accelerated skin ageing is induced in mice conditionally deleted with p63 in the epidermis, where ΔNp63α is the predominant isoform [Citation50]. Melino G group demonstrated that the expression of p63 in skin keratinocytes of elderly people is significantly lower than that of youths, and downregulation of ΔNp63α accelerates skin ageing [Citation19]. In one of our recent studies, we found that ΔNp63α is downregulated in either mouse skin or human keratinocytes during photoaging induced by ultraviolet B (UVB) [Citation51].
Downregulation of ΔNp63α during skin ageing may be due to the activation of Akt/Foxo3a, p38 MAPK or other signalling pathways mediated by ROS and DNA damage, according to the experimental results of our laboratory and other research groups (depicted as ) [Citation31,Citation51–53]. Ultraviolet radiation (UVR) can activate p38 MAPK pathway, which in turn phosphates and stabilizes p53 in keratinocytes; consequently, p53 binds to ΔNp63α and direct a caspase-1-mediated degradation of the latter protein [Citation52]. In our previous studies, we demonstrated that the treatment with DNA damage drugs down-regulates ΔNp63α at the mRNA level, which is independent of p53 [Citation31]. Recently, it is reported that UVB radiation can activate PI3K/Akt pathway [Citation54], which may increase FOXO3a phosphorylation and nuclear export, resulting in downregulation of ΔNp63α transcription [Citation53].
Epigenetics in cell senescence
Epigenetics is a class of mechanisms generating heritable phenotypic changes independently of DNA sequence changes. Mechanisms of epigenetics include DNA and histone modifications, chromatin remodelling, as well as other processes such as enhancer-promoter looping. Via these mechanisms, environment can regulate gene expression to produce visible phenotypes [Citation55,Citation56]. As a state of cell cycle arrest is regulated by a network of molecules, cell senescence is driven by diverse epigenetic changes [Citation5,Citation57].
DNA methylation
The templates of transcription, DNA, randomly contains cytosines, which can be potentially methylated by DNA methyltransferases (DNMTs), i.e., DNMT1, DNMT3A and DNMT3B. 5-methyl cytosine (5mC) is enriched in CpG dinucleotides and directly affects the ability of transcription factors and other DNA binding proteins to access DNA. Therefore, hypermethylation of CpGs in gene promoters generally results in silencing or low expression of downstream genes, while hydroxymethylation upregulates gene transcription [Citation58,Citation59].
It has been reported that senescence-associated DNA methylation alterations accumulate during cell senescence and consequently cause cell cycle arrest [Citation57]. Mislocalization or repression of DNMT1 contributes to senescence-associated DNA hypomethylation [Citation60,Citation61]. On the other hand, senescence-associated heterochromatin foci (SAHF) may recruit DNA methyltransferases (DNMTs) to focal sites to induce senescence-associated focal hypermethylation [Citation62,Citation63]. These DNA methylation alterations promote replicative senescence via multiple mechanisms [Citation64], including upregulating p16INK4a and p21WAF1/CIP1 [Citation65], downregulating telomerase reverse transcriptase (TERT) expression and telomerase activity [Citation66], as well as recruiting polycomb repressive complex 2 (PRC2) [Citation67].
Histone modification
In eukaryotic chromatin, DNA strands are packaged into nucleosomes by histones including H2A, H2B, H3, and H4 [Citation68]. Diverse modifications, including different types of acetylation and methylation at specific residues of histones, can change chromatin figuration and regulate gene expression via affecting DNA accessibility for the transcription machinery [Citation69].
It is well documented that histone-associated epigenetic processes occur during senescence. These processes include modification, variation and depletion of histones [Citation57]. It was reported that telomere shortening triggers histone depletion and gives rise to an open chromatin configuration, consequently enhancing RNA polymerase II elongation rates as well as resulting in pre-mRNA splicing defects in senescence [Citation70,Citation71]. In replicative senescence and some types of stress-induced premature senescence (SIPS), histone modification changes have been detected, which include global decreases in H3 lysine 4 trimethylation (H3K4me3), H3 lysine 9 trimethylation (H3K9me3), H3 lysine 27 trimethylation (H3K27me3) and H4 lysine 16 acetylation (H4K16Ac), as well as comprehensive increases in H3 lysine 9 acetylation (H3K9Ac) and H4 lysine 20 trimethylation (H4K20me3) [Citation72–74]. These alterations mediated by specific enzymes can affect its affinity to DNA strains and condensation state of nucleosomes, eventually modulating the expression and effects of cell cycle-related proteins including p14ARF [Citation75,Citation76], p15INK4b [Citation75,Citation76], p16INK4a [Citation75–77], p21WAF1/CIP1 [Citation78,Citation79], and Rb [Citation77,Citation80], as well as inflammaging-related cytokines such as IL-6 and IL-8 [Citation78,Citation79]. In turn, they lead to telomere shortening or dysfunction [Citation81,Citation82], proliferation suppression [Citation83,Citation84], and senescence-associated secretory phenotype (SASP) [Citation78,Citation79,Citation85].
Structural transformation of chromatin mediated by chromatin remodeling complexes
Chromatin remodelling complexes (CRCs), also named as remodellers, are needed to fully package the genome, to specialize chromatin regions, and to provide regulated DNA accessibility in packaged regions. Four classes of CRCs are found in eukaryotes [Citation86,Citation87]: switching defective/sucrose non-fermenting (SWI/SNF) family; imitation switch (ISWI) family; chromodomain, helicase, DNA binding (CHD) family; inositol requiring 80 (INO80) family. CRCs utilize ATP hydrolysis to alter histone-DNA contacts and modulate sliding of histone octamers across DNA, association between them, as well as the position of nucleosomes [Citation88,Citation89]. In replicative senescence, these CRC-mediated structural transformations of chromatin can induce cell cycle arrest via upregulating expression of p16INK4a, p21WAF1/CIP1, and p53 [Citation90–93].
Enhancer-promoter looping
Chromatin structure in mammals can also regulate transcription by modulating three-dimensional interactions between enhancers and promoters, which can be facilitated by chromatin loops [Citation94]. CCCTC-binding factor (CTCF) is a transcription factor which works together with the cohesion complex to drive the formation of chromatin loops [Citation95]. Recently, Olan I et al reported that enhancer-promoter interactions are extensively altered during cell senescence. These rewired enhancer-promoter interactions may modulate the expression of genes related to cell senescence, such as IL1B [Citation96].
ΔNp63α-mediated epigenetic regulation and keratinocyte senescence
As a traditional transcription factor, ΔNp63α is well established to bind to canonical p53 DNA binding sites in promoter regions of its target genes and thus compete with TAp63/p73/p53, resulting in transinhibition (). ΔNp63α-promoter association can also activate gene expression via recruiting other transcription factors (TFs) and RNA polymerase II (RNApol II) () [Citation7]. Recently, accumulating data demonstrate that ΔNp63α directly binds to DNA regions other than promoters and consequently regulates expression of genes related to keratinocyte differentiation and proliferation [Citation10,Citation97–99]. This indicates that, like its homologue p53, ΔNp63α may also function as a pioneer transcription factor to modulate chromatin structure and to regulate diverse biological processes, including keratinocyte senescence [Citation8,Citation11,Citation100].
ΔNp63α regulates DNA modification to promote keratinocyte proliferation
Using epigenome profiling of differentiating human primary epidermal keratinocytes, Kouwenhoven EN et al. found that ΔNp63α binds to a batch of enhancers [Citation10]. Rinaldi L et al found that via binding to the enhancers, ΔNp63α recruits DNA methyltransferase 3A (DNMT3A) to the centre of the enhancers. Then, TET2 mediates the sequential hydroxymethylation of cytosine there. This can activate the expression of diverse genes, such as KRT5, KRT14 and ΔNp63 itself. These genes are related to differentiation and proliferation of epidermal cells [Citation58,Citation101]. These data indicate that ΔNp63α may promote keratinocyte proliferation and antagonize senescence via facilitating cytosine hydroxymethylation at the enhancers of proliferative genes and upregulating their expression (depicted as ). It remains to be further investigated whether ΔNp63α regulates other types of DNA modification via recruiting other enzymes.
ΔNp63α regulates histone modifications to prevent keratinocyte senescence
Recent investigations suggest that ΔNp63α affects histone acetylation and nucleosome repositioning and changes DNA accessibility to regulate gene expression (depicted as ). Among the ΔNp63α-bound enhancers identified by Kouwenhoven EN et al, a half is active as defined by histone modification H3K27ac, which is an active enhancer mark. These data indicate that ΔNp63α upregulates nearby genes (e.g., KRT5, KRT10 and TGM1) related to keratinocyte proliferation and differentiation via promoting histone acetylation [Citation10]. In another study, Hamdan FH et al identified numerous ΔNp63α-occupied distal elements away from the transcription start sites of genes in pancreatic cancer cells. Many of these elements are active enhancers intersected with H3K27ac mark and locate in open chromatin regions identified by assay for transposase-accessible chromatin sequencing (ATAC-seq). This distal pattern of occupancy implies the function of ΔNp63α in enhancer activation to promote cell proliferation [Citation102]. In line with both reports, Yu X et al found that ΔNp63α can bind to inaccessible chromatin regions, which is characterized by unmodified histones; after ΔNp63α binding, histone modifications occur in these regions [Citation11]. These data indicate that ΔNp63α induces proliferative genes via increasing chromatin accessibility and this effect may be exerted in various situations.
ΔNp63α-induced histone modifications may be not limited to increasing chromatin accessibility. Data from Yu X et al show that histone modifications induced by ΔNp63α include not only transcriptional active histone modifications (H3K9ac, H3K27ac, H3K4me1, H3K4me2, H3K4me3), but also repressive ones (H3K27me3 and H3K9me3) [Citation11]. Consistent with the repression effect of p63 binding, Pattison JM et al found that p63 induces trimethylation of histone H3 lysine 27 (H3K27me3) and closes chromatin accessibility in keratinocytes [Citation98].
These modifications may be due to ΔNp63α-mediated recruitment of epigenetic enzymes. Lin-Shiao E et al found that the histone methyltransferase KMT2D interacts with ΔNp63α on chromatin and at a broad array of enhancers, resulting in histone modifications, including H3 lysine 4 monomethylation (H3K4me1) and H3 lysine 27 acetylation (H3K27ac), in keratinocytes. This may maintain transcription of hundreds of genes (e.g., KRT5, KLF4 and ZNF750), which are essential for p63 to keep proliferative capacity of keratinocytes through positively regulating genes critical for epithelial development, differentiation, and adherence [Citation97]. Ramsey MR et al reported that ΔNp63α recruits histone deacetylases HDAC1 and HDAC2 to erase the acetylation of histones. This histone deacetylation increases histone-DNA affinity and mediates transcriptional repression of genes (e.g., Puma) responsible for cell proliferation and tumorigenesis [Citation103,Citation104].
ΔNp63α coordinates with chromatin remodeling complexes or regulates their expression to prevent keratinocyte senescence
ΔNp63α can also coordinate chromatin remodelling complexes (CRCs) to orchestrate the remodelling (depicted as ). BRG1/BRM-associated factor (BAF) belongs to the SWI/SNF CRC family [Citation105]. Using assay for transposase-accessible chromatin with high throughput sequencing (ATAC-seq), Bao X et al revealed that BAF and p63 mutually recruit each other to maintain 14,853 open chromatin regions and cooperatively position nucleosomes, resultantly recruiting transcriptional machinery and controlling transcription during epidermal differentiation. Chromatin remodelling coordinately mediated by p63 and BAF upregulates multiple genes related to epidermal differentiation and cell proliferation, including LCE3D, KRT1 and KRT10 [Citation106].
The interaction and cooperation between ΔNp63α and CRCs in chromatin remodelling is also found in tumorigenesis. ΔNp63α may interact with actin-like protein 6A (ACTL6A), which is a subunit of SWI/SNF CRC and co-amplified with ΔNp63α in head and neck squamous cell carcinoma (HNSCC); this collaboration leads to a downregulation of tumour suppressors such as WWC1 and GPRC5A [Citation107]. In addition, ΔNp63α can participate in the formation of another CRC called SRCAP (SNF2-related CBP Activator Protein), which belongs to the INO80 family and facilitates the substitution of histone H2A to its variant H2A.Z in nucleosomes; as a result, anti-proliferative genes (e.g., SAMD9L, ZHX2, and IGFBP3) are repressed at transcription level [Citation108–110]. It remains obscure whether ΔNp63α modulates keratinocyte senescence via these mechanisms.
Apart from the direct interactions with CRCs, ΔNp63α can also modulate chromatin remodelling via regulating gene expression of multiple remodellers or related proteins (depicted as &d). Keyes WM et al previously reported that ΔNp63α drives proliferation of skin stem cells via transactivating lymphoid-specific helicase (LSH) [Citation111], which belongs to the SNF2 family of chromatin-remodelling ATPase and promotes cell proliferation in multiple scenarios [Citation112,Citation113]. Intriguingly, Mardaryev AN et al found that ΔNp63α can bind to intron 1 of BRG1 gene and upregulate its transcription in epidermal progenitor cell during skin development [Citation114]. As mentioned above, BRG1 is the core subunit of SWI/SNF-like BAF remodeller, which directly binds to p63 protein and cooperatively modulates DNA accessibility during epidermal differentiation [Citation106]. These reports indicate that ΔNp63α may promote BAF-mediated chromatin remodelling from two dimensions: transactivating BRG1 gene and coordinating with the BAF complex. In addition, Fessing MY et al found that ΔNp63α controls expression of genes encoding several regulators of the higher-order chromatin structure and ATP-dependent chromatin remodelling [Citation115]. Among which, special AT-rich binding protein 1 (SATB1) acts as a docking site for several chromatin remodelling enzymes to regulate gene expression [Citation116]. According to Fessing MY’s data, ΔNp63α directly binds to the promoter of SATB1 gene and upregulates its expression in keratinocytes, hence controlling chromatin remodelling during development of the epidermis [Citation115].
ΔNp63α prevents keratinocyte senescence via mediating enhancer-promoter looping
Several recent studies suggest that ΔNp63α promotes keratinocyte proliferation via mediating enhancer-promoter looping in cooperation with other transcription factors (depicted as ). Qu J et al found that ΔNp63α and CTCF are cooperatively involved in chromatin looping to regulate epidermal genes [Citation117]. CTCF is found to consistently bind to promoters of epidermal genes (e.g., PIGV and KRT5) [Citation118]. This consistent association of CTCF acts as a barrier for gene expression under most scenarios [Citation94]. However, the p63-CTCF cooperation assists the looping of p63-bound enhancers to gene promoters and turns them on during keratinocyte differentiation and proliferation [Citation117]. It is reported that CTCF mediates chromatin remodelling via physically interacting with BRD9, which is a component of non-canonical BAF complex [Citation119]. It remains unclear whether this SWI/SNF chromatin remodelling complex is involved in p63-CTCF cooperation in enhancer-promoter looping. According to a recent study conducted by Kurinna S et al, p63 can also mediate enhancer-promoter looping in cooperation with another transcription factor, nuclear factor erythroid 2-related factor 2 (NRF2). This can activate genes including CDK12 and miR29a and promotes keratinocyte proliferation in the epidermis [Citation99].
Discussion
Keratinocytes compose the epidermis and keep exuberant proliferating capacity to renew this skin layer [Citation19]. Senescence of keratinocytes heavily impairs skin functionality and seriously deteriorates the skin ageing process [Citation13,Citation20]. As a crucial transcription factor in epidermal differentiation and cell proliferation, ΔNp63α has been reported to play key roles in delaying keratinocyte senescence [Citation19]. Generally, ΔNp63α is assumed to specifically bind to elements in the promoter regions of its target genes to activate (e.g., Fos, c-Jun, HK2, and KRT14) or repress (e.g., Pten, p16INK4a, p21WAF1/CIP1 and Puma) their expression (depicted as ) [Citation7,Citation9]. As a consequence, ΔNp63α promotes epidermal differentiation and keratinocyte proliferation.
Since accumulating data demonstrate that ΔNp63α binds to regions away from promoters (e.g., enhancers and introns) [Citation10,Citation11,Citation120], it may mediate epigenetic regulation to prevent keratinocyte senescence via multiple mechanisms (depicted as ~d): (1) ΔNp63α recruits epigenetic enzymes (e.g., DNMT3A, KMT2D and HDAC1/2) to modify DNA or histones, resulting in either increasing or decreasing DNA accessibility for transcription machinery () [Citation10,Citation58,Citation102]; (2) ΔNp63α can also mediate enhancer-promoter looping in cooperation with other transcription factors (TFs), such as CTCF and NRF2 () [Citation99,Citation117]; (3) ΔNp63α physically interacts with and coordinates chromatin remodelling complexes (e.g., BAF, ACTL6A and SRCAP; depicted as ) [Citation106–108,Citation111,Citation114,Citation115]; (4) ΔNp63α transactivates genes related to chromatin remodelling and indirectly promotes the remodelling (). Via these mechanisms, ΔNp63α downregulates anti-proliferative genes (e.g., p16INK4a, p21WAF1/CIP1, Puma, p53, WWC1, SAMD9L, and ZHX2), as well as upregulates proliferative genes (e.g., Fos, c-Jun, HK2, PFKFB3, KRT5, KRT14, ΔNp63, CDK12 and miR29a). Hence, ΔNp63α promotes proliferation of keratinocytes and prevents them from senescence (depicted in ). It is worth noting that some genes (e.g., KRT5, KRT14, p16INK4a and p21WAF1/CIP1) are commonly regulated by the abovementioned mechanisms, indicating that these ΔNp63α-mediated epigenetic regulation mechanisms may be intertwined. During skin inflammaging, the decline of ΔNp63α induced by various factors may lead to dysregulation of chromatin remodelling, consequently deteriorating senescence of keratinocytes. It is worthful to study how these epigenetic changes differentially regulate proliferative or anti-proliferative genes. The genes regulated by ΔNp63α during keratinocyte senescence are also remained to be systematically investigated.
It has been reported that some chemicals (e.g., resveratrol, aspirin and metformin) exert anti-senescence effects in multiple cell types via regulating epigenetic alterations [Citation5,Citation57,Citation121–125]. More to the point, epigenetic drugs (e.g., DNMT inhibitor RG108 and histone methyltransferase Smyd3 inhibitor EPZ031686) can mitigate senescence phenotype in vitro and in vivo [Citation60,Citation126]. Given the roles of ΔNp63α-mediated chromatin remodelling against keratinocyte senescence, it is possible to delay skin ageing via therapeutic interventions with epigenetic drugs.
Article highlights
Knowledges of ΔNp63α and keratinocyte senescence were introduced
General epigenetic alterations in cellular senescence were summarized
Mechanisms of ΔNp63α-mediated epigenetic regulation in preventing keratinocyte senescence, including DNA and histone modification, enhancer-promoter looping, directly and indirectly promoting chromatin remodelling, were reviewed.
Author contributions
LK drafted the manuscript. CL revised the manuscript. Both authors read and approved the submitted version.
Disclosure statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability statement
Not applicable.
Additional information
Funding
References
- Gerasymchuk M, Cherkasova V, Kovalchuk O, et al. The Role of microRNAs in Organismal and Skin Aging. Int J Mol Sci. 2020;21(15):5281.
- Chen B, Yang J, Song Y, et al. Skin Immunosenescence and Type 2 Inflammation: a Mini-Review With an Inflammaging Perspective. Front Cell Dev Biol. 2022;10:835675.
- Li J, Jiang TX, Hughes MW, et al. Progressive alopecia reveals decreasing stem cell activation probability during aging of mice with epidermal deletion of DNA methyltransferase 1. J Invest Dermatol. 2012;132:2681–12.
- Xie HF, Liu YZ, Du R, et al. miR-377 induces senescence in human skin fibroblasts by targeting DNA methyltransferase 1. Cell Death Dis. 2017;8:e2663.
- Zhu X, Chen Z, Shen W, et al. Inflammation, epigenetics, and metabolism converge to cell senescence and ageing: the regulation and intervention. Signal Transduct Target Ther. 2021;6:245.
- Engeland K. Cell cycle regulation: p53-p21-RB signaling. Cell Death Differ. 2022;29:946–960.
- Chen Y, Peng Y, Fan S, et al. A double dealing tale of p63: an oncogene or a tumor suppressor. Cell Mol Life Sci. 2018;75:965–973.
- Woodstock DL, Sammons MA, Fischer M. p63 and p53: collaborative Partners or Dueling Rivals? Front Cell Dev Biol. 2021;9:701986.
- Leonard MK, Kommagani R, Payal V, et al. DeltaNp63alpha regulates keratinocyte proliferation by controlling PTEN expression and localization. Cell Death Differ. 2011;18:1924–1933.
- Kouwenhoven EN, Oti M, Niehues H, et al. Transcription factor p63 bookmarks and regulates dynamic enhancers during epidermal differentiation. EMBO Rep. 2015;16(7):863–878.
- Yu X, Singh PK, Tabrejee S, et al. DeltaNp63 is a pioneer factor that binds inaccessible chromatin and elicits chromatin remodeling. Epigenetics Chromatin. 2021;14:20.
- Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10:207–217.
- Gruber F, Kremslehner C, Eckhart L, et al. Cell aging and cellular senescence in skin aging - Recent advances in fibroblast and keratinocyte biology. Exp Gerontol. 2020;130:110780.
- Tigges J, Krutmann J, Fritsche E, et al. The hallmarks of fibroblast ageing. Mech Ageing Dev. 2014;138:26–44.
- Tobin DJ. Introduction to skin aging. J Tissue Viability. 2017;26:37–46.
- Baumann L. Skin ageing and its treatment. J Pathol. 2007;211:241–251.
- Rinnerthaler M, Bischof J, Streubel MK, et al. Oxidative stress in aging human skin. Biomolecules. 2015;5:545–589.
- Sanches Silveira JE, Myaki Pedroso DM. UV light and skin aging. Rev Environ Health. 2014;29:243–254.
- Rivetti D, Val Cervo P, Lena AM, et al. p63-microRNA feedback in keratinocyte senescence. Proc Natl Acad Sci U S A. 2012;109:1133–1138.
- Pilkington SM, Bulfone-Paus S, Griffiths CEM, et al. Inflammaging and the Skin. J Invest Dermatol. 2021;141:1087–1095.
- Birch J, Gil J. Senescence and the SASP: many therapeutic avenues. Genes Dev. 2020;34:1565–1576.
- Amelio I, Grespi F, Annicchiarico-Petruzzelli M, et al. p63 The Guardian of human reproduction. Cell Cycle. 2012;11:4545–4551.
- Mangiulli M, Valletti A, Caratozzolo MF, et al. Identification and functional characterization of two new transcriptional variants of the human p63 gene. Nucleic Acids Res. 2009;37:6092–6104.
- Osada M, Ohba M, Kawahara C, et al. Cloning and functional analysis of human p51, which structurally and functionally resembles p53. Nat Med. 1998;4:839–843.
- Trink B, Okami K, Wu L, et al. A new human p53 homologue. Nat Med. 1998;4:747–748.
- Chen H, Hu K, Xie Y, et al. CDK1 Promotes Epithelial-Mesenchymal Transition and Migration of Head and Neck Squamous Carcinoma Cells by Repressing Np63alpha-Mediated Transcriptional Regulation. Int J Mol Sci. 2022;23:7385.
- Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522.
- Li C, Chang DL, Yang Z, et al. Pin1 modulates p63alpha protein stability in regulation of cell survival, proliferation and tumor formation. Cell Death Dis. 2013;4:e943.
- Westfall MD, Mays DJ, Sniezek JC, et al. The Delta Np63 alpha phosphoprotein binds the p21 and 14-3-3 sigma promoters in vivo and has transcriptional repressor activity that is reduced by Hay-Wells syndrome-derived mutations. Mol Cell Biol. 2003;23:2264–2276.
- Chen Y, Li Y, Peng Y, et al. DeltaNp63alpha down-regulates c-Myc modulator MM1 via E3 ligase HERC3 in the regulation of cell senescence. Cell Death Differ. 2018;25:2118–2129.
- Li X, Chen J, Yi Y, et al. DNA damage down-regulates DeltaNp63alpha and induces apoptosis independent of wild type p53. Biochem Biophys Res Commun. 2012;423:338–343.
- Yang A, Kaghad M, Wang Y, et al. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–316.
- Della Gatta G, Bansal M, Ambesi-Impiombato A, et al. Direct targets of the TRP63 transcription factor revealed by a combination of gene expression profiling and reverse engineering. Genome Res. 2008;18:939–948.
- Soares E, Zhou H. Master regulatory role of p63 in epidermal development and disease. Cell Mol Life Sci. 2018;75:1179–1190.
- Hamanaka RB, Mutlu GM. PFKFB3, a Direct Target of p63, Is Required for Proliferation and Inhibits Differentiation in Epidermal Keratinocytes. J Invest Dermatol. 2017;137:1267–1276.
- Viticchie G, Agostini M, Lena AM, et al. p63 supports aerobic respiration through hexokinase II. Proc Natl Acad Sci U S A. 2015;112:11577–11582.
- Melino G, Memmi EM, Pelicci PG, et al. Maintaining epithelial stemness with p63. Sci Signal. 2015;8:re9.
- Senoo M, Pinto F, Crum CP, et al. p63 Is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129:523–536.
- Romano RA, Smalley K, Magraw C, et al. DeltaNp63 knockout mice reveal its indispensable role as a master regulator of epithelial development and differentiation. Development. 2012;139:772–782.
- Mills AA, Zheng B, Wang XJ, et al. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713.
- Yang A, Schweitzer R, Sun D, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–718.
- Li N, Singh S, Cherukuri P, et al. Reciprocal intraepithelial interactions between TP63 and hedgehog signaling regulate quiescence and activation of progenitor elaboration by mammary stem cells. Stem Cells. 2008;26:1253–1264.
- Memmi EM, Sanarico AG, Giacobbe A, et al. p63 Sustains self-renewal of mammary cancer stem cells through regulation of Sonic Hedgehog signaling. Proc Natl Acad Sci U S A. 2015;112:3499–3504.
- Candi E, Rufini A, Terrinoni A, et al. DeltaNp63 regulates thymic development through enhanced expression of FgfR2 and Jag2. Proc Natl Acad Sci U S A. 2007;104:11999–12004.
- Du Z, Li J, Wang L, et al. Overexpression of DeltaNp63alpha induces a stem cell phenotype in MCF7 breast carcinoma cell line through the Notch pathway. Cancer Sci. 2010;101:2417–2424.
- Kent S, Hutchinson J, Balboni A, et al. DeltaNp63alpha promotes cellular quiescence via induction and activation of Notch3. Cell Cycle. 2011;10:3111–3118.
- Terrinoni A, Serra V, Bruno E, et al. Role of p63 and the Notch pathway in cochlea development and sensorineural deafness. Proc Natl Acad Sci U S A. 2013;110:7300–7305.
- Chakrabarti R, Wei Y, Hwang J, et al. DeltaNp63 promotes stem cell activity in mammary gland development and basal-like breast cancer by enhancing Fzd7 expression and Wnt signalling. Nat Cell Biol. 2014;16:1004-1013.
- Nguyen BC, Lefort K, Mandinova Aet al. Tommasi di Vignano, A, Kitajewski, J, Chiorino, G, Roop, DR, Missero, C and Dotto, GP (2006) Cross-regulation between Notch and p63 in keratinocyte commitment to differentiation. Genes Dev. 2006;20:1028–1042.
- Keyes WM, Wu Y, Vogel H, et al. p63 deficiency activates a program of cellular senescence and leads to accelerated aging. Genes Dev. 2005;19:1986–1999.
- Zheng X, Chen L, Jin S, et al. Ultraviolet B irradiation up-regulates MM1 and induces photoageing of the epidermis. Photodermatol Photoimmunol Photomed. 2021;37:395–403.
- Hildesheim J, Belova GI, Tyner SD, et al. Gadd45a regulates matrix metalloproteinases by suppressing DeltaNp63alpha and beta-catenin via p38 MAP kinase and APC complex activation. Oncogene. 2004;23:1829–1837.
- Hu L, Liang S, Chen H, et al. DeltaNp63alpha is a common inhibitory target in oncogenic PI3K/Ras/Her2-induced cell motility and tumor metastasis. Proc Natl Acad Sci U S A. 2017;114:E3964–E3973.
- Bang E, Kim DH, Chung HY. Protease-activated receptor 2 induces ROS-mediated inflammation through Akt-mediated NF-kappaB and FoxO6 modulation during skin photoaging. Redox Biol. 2021;44:102022.
- Peixoto P, Cartron PF, Serandour AA, et al. From 1957 to Nowadays: a Brief History of Epigenetics. Int J Mol Sci. 2020;21:7571.
- Zhang L, Lu Q, Chang C. Epigenetics in Health and Disease. Adv Exp Med Biol. 2020;1253:3–55.
- Cheng LQ, Zhang ZQ, Chen HZ, et al. Epigenetic regulation in cell senescence. J Mol Med (Berl). 2017;95:1257–1268.
- Rinaldi L, Datta D, Serrat J, et al. Dnmt3a and Dnmt3b Associate with Enhancers to Regulate Human Epidermal Stem Cell Homeostasis. Cell Stem Cell. 2016;19:491–501.
- Unnikrishnan A, Freeman WM, Jackson J, et al. The role of DNA methylation in epigenetics of aging. Pharmacol Ther. 2019;195:172–185.
- Oh YS, Jeong SG, Cho GW. Anti-senescence effects of DNA methyltransferase inhibitor RG108 in human bone marrow mesenchymal stromal cells. Biotechnol Appl Biochem. 2015;62:583–590.
- Cruickshanks HA, McBryan T, Nelson DM, et al. Senescent cells harbour features of the cancer epigenome. Nat Cell Biol. 2013;15:1495–1506.
- Narita M, Nunez S, Heard E, et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–716.
- Smallwood A, Esteve PO, Pradhan S, et al. Functional cooperation between HP1 and DNMT1 mediates gene silencing. Genes Dev. 2007;21:1169–1178.
- Koch CM, Joussen S, Schellenberg A, et al. Monitoring of cellular senescence by DNA-methylation at specific CpG sites. Aging Cell. 2012;11:366–369.
- Tsai CC, Su PF, Huang YF, et al. Oct4 and Nanog directly regulate Dnmt1 to maintain self-renewal and undifferentiated state in mesenchymal stem cells. Mol Cell. 2012;47:169–182.
- Schoeftner S, Blasco MA. A ‘higher order’ of telomere regulation: telomere heterochromatin and telomeric RNAs. EMBO J. 2009;28:2323–2336.
- Qian H, Xu X. Reduction in DNA methyltransferases and alteration of DNA methylation pattern associate with mouse skin ageing. Exp Dermatol. 2014;23:357–359.
- Kornberg RD. Chromatin structure: a repeating unit of histones and DNA. Science. 1974;184:868–871.
- Hao SL, Ni FD, Yang WX. The dynamics and regulation of chromatin remodeling during spermiogenesis. Gene. 2019;706:201–210.
- Oesterreich FC, Herzel L, Straube K, et al. Splicing of Nascent RNA Coincides with Intron Exit from RNA Polymerase II. Cell. 2016;165:372–381.
- Platt JM, Ryvkin P, Wanat JJ, et al. Rap1 relocalization contributes to the chromatin-mediated gene expression profile and pace of cell senescence. Genes Dev. 2013;27:1406–1420.
- Nelson DM, Jaber-Hijazi F, Cole JJ, et al. Mapping H4K20me3 onto the chromatin landscape of senescent cells indicates a function in control of cell senescence and tumor suppression through preservation of genetic and epigenetic stability. Genome Biol. 2016;17:158.
- Sanders YY, Liu H, Zhang X, et al. Histone modifications in senescence-associated resistance to apoptosis by oxidative stress. Redox Biol. 2013;1:8–16.
- Takahashi A, Imai Y, Yamakoshi K, et al. DNA damage signaling triggers degradation of histone methyltransferases through APC/C(Cdh1) in senescent cells. Mol Cell. 2012;45:123–131.
- Jie B, Weilong C, Ming C, et al. Enhancer of zeste homolog 2 depletion induces cellular senescence via histone demethylation along the INK4/ARF locus. Int J Biochem Cell Biol. 2015;65:104–112.
- Salminen A, Kaarniranta K, Hiltunen M, et al. Histone demethylase Jumonji D3 (JMJD3/KDM6B) at the nexus of epigenetic regulation of inflammation and the aging process. J Mol Med (Berl). 2014;92:1035–1043.
- Macha MA, Rachagani S, Pai P, et al. MUC4 regulates cellular senescence in head and neck squamous cell carcinoma through p16/Rb pathway. Oncogene. 2015;34:1698–1708.
- Chen HZ, Wang F, Gao P, et al. Age-Associated Sirtuin 1 Reduction in Vascular Smooth Muscle Links Vascular Senescence and Inflammation to Abdominal Aortic Aneurysm. Circ Res. 2016;119:1076–1088.
- Hayakawa T, Iwai M, Aoki S, et al. SIRT1 suppresses the senescence-associated secretory phenotype through epigenetic gene regulation. PLoS One. 2015;10:e0116480.
- Duarte LF, Young AR, Wang Z, et al. Histone H3.3 and its proteolytically processed form drive a cellular senescence programme. Nat Commun. 2014;5:5210.
- Bernadotte A, Mikhelson VM, Spivak IM. Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging (Albany NY). 2016;8:3–11.
- Wu S, Ge Y, Huang L, et al. BRG1, the ATPase subunit of SWI/SNF chromatin remodeling complex, interacts with HDAC2 to modulate telomerase expression in human cancer cells. Cell Cycle. 2014;13:2869–2878.
- Faralli H, Wang C, Nakka K, et al. UTX demethylase activity is required for satellite cell-mediated muscle regeneration. J Clin Invest. 2016;126:1555–1565.
- Zhang, J, He, P, Xi, Y, Geng, M, Chen, Y and Ding, J. Down-regulation of G9a triggers DNA damage response and inhibits colorectal cancer cells proliferation. Oncotarget. 2015;6:2917–2927.
- Capell BC, Drake AM, Zhu J, et al. MLL1 is essential for the senescence-associated secretory phenotype. Genes Dev. 2016;30:321–336.
- Masliah-Planchon J, Bieche I, Guinebretiere JM, et al. SWI/SNF chromatin remodeling and human malignancies. Annu Rev Pathol. 2015;10:145–171.
- Clapier CR, Cairns BR. The biology of chromatin remodeling complexes. Annu Rev Biochem. 2009;78:273–304.
- Clapier CR, Cairns BR. Regulation of ISWI involves inhibitory modules antagonized by nucleosomal epitopes. Nature. 2012;492:280–284.
- Clapier CR, Iwasa J, Cairns BR, et al. Mechanisms of action and regulation of ATP-dependent chromatin-remodelling complexes. Nat Rev Mol Cell Biol. 2017;18:407–422.
- Alsayegh KN, Gadepalli VS, Iyer S, et al. Knockdown of CDK2AP1 in primary human fibroblasts induces p53 dependent senescence. PLoS One. 2015;10:e0120782.
- Conomos D, Reddel RR, Pickett HA. NuRD-ZNF827 recruitment to telomeres creates a molecular scaffold for homologous recombination. Nat Struct Mol Biol. 2014;21:760–770.
- Min JN, Tian Y, Xiao Y, et al. The mINO80 chromatin remodeling complex is required for efficient telomere replication and maintenance of genome stability. Cell Res. 2013;23:1396–1413.
- Tordella L, Khan S, Hohmeyer A, et al. SWI/SNF regulates a transcriptional program that induces senescence to prevent liver cancer. Genes Dev. 2016;30:2187–2198.
- Zuin J, Roth G, Zhan Y, et al. Nonlinear control of transcription through enhancer-promoter interactions. Nature. 2022;604:571–577.
- Kubo N, Ishii H, Xiong X, et al. Promoter-proximal CTCF binding promotes distal enhancer-dependent gene activation. Nat Struct Mol Biol. 2021;28:152–161.
- Olan I, Parry AJ, Schoenfelder S, et al. Transcription-dependent cohesin repositioning rewires chromatin loops in cellular senescence. Nat Commun. 2020;11:6049.
- Lin-Shiao E, Lan Y, Coradin M, et al. KMT2D regulates p63 target enhancers to coordinate epithelial homeostasis. Genes Dev. 2018;32:181–193.
- Pattison JM, Melo SP, Piekos SN, et al. Retinoic acid and BMP4 cooperate with p63 to alter chromatin dynamics during surface epithelial commitment. Nat Genet. 2018;50:1658–1665.
- Kurinna S, Seltmann K, Bachmann AL, et al. Interaction of the NRF2 and p63 transcription factors promotes keratinocyte proliferation in the epidermis. Nucleic Acids Res. 2021;49:3748–3763.
- Yi M, Tan Y, Wang L, et al. TP63 links chromatin remodeling and enhancer reprogramming to epidermal differentiation and squamous cell carcinoma development. Cell Mol Life Sci. 2020;77(21):4325–4346.
- Alam H, Sehgal L, Kundu ST, et al. Novel function of keratins 5 and 14 in proliferation and differentiation of stratified epithelial cells. Mol Biol Cell. 2011;22(21):4068–4078.
- Hamdan FH, Johnsen SA. DeltaNp63-dependent super enhancers define molecular identity in pancreatic cancer by an interconnected transcription factor network. Proc Natl Acad Sci U S A. 2018;115(52):E12343–E12352.
- Li Y, Seto E. HDACs and HDAC Inhibitors in Cancer Development and Therapy. Cold Spring Harb Perspect Med. 2016;6(10):a02683.
- Ramsey MR, He L, Forster N, et al. Physical association of HDAC1 and HDAC2 with p63 mediates transcriptional repression and tumor maintenance in squamous cell carcinoma. Cancer Res. 2011;71(13):4373–4379.
- He S, Wu Z, Tian Y, et al. Structure of nucleosome-bound human BAF complex. Science. 2020;367(6480):875–881.
- Bao X, Rubin AJ, Qu K, et al. A novel ATAC-seq approach reveals lineage-specific reinforcement of the open chromatin landscape via cooperation between BAF and p63. Genome Biol. 2015;16(1):284.
- Saladi SV, Ross K, Karaayvaz M, et al. ACTL6A Is Co-Amplified with p63 in Squamous Cell Carcinoma to Drive YAP Activation, Regenerative Proliferation, and Poor Prognosis. Cancer Cell. 2017;31:35–49.
- Gallant-Behm CL, Ramsey MR, Bensard CL, et al. DeltaNp63alpha represses anti-proliferative genes via H2A.Z deposition. Genes Dev. 2012;26:2325–2336.
- Marques M, Laflamme L, Gervais AL, et al. Reconciling the positive and negative roles of histone H2A.Z in gene transcription. Epigenetics. 2010;5:267–272.
- Ye B, Yang L, Qian G, et al. The chromatin remodeler SRCAP promotes self-renewal of intestinal stem cells. EMBO J. 2020;39:e103786.
- Keyes WM, Pecoraro M, Aranda V, et al. DeltaNp63alpha is an oncogene that targets chromatin remodeler Lsh to drive skin stem cell proliferation and tumorigenesis. Cell Stem Cell. 2011;8:164–176.
- Xiao D, Huang J, Pan Y, et al. Chromatin Remodeling Factor LSH is Upregulated by the LRP6-GSK3beta-E2F1 Axis Linking Reversely with Survival in Gliomas. Theranostics. 2017;7:132–143.
- He X, Yan B, Liu S, et al. Chromatin Remodeling Factor LSH Drives Cancer Progression by Suppressing the Activity of Fumarate Hydratase. Cancer Res. 2016;76:5743–5755.
- Mardaryev, AN, Gdula, MR, Yarker, JL, Emelianov, VU, Poterlowicz, K, Sharov, AA, Sharova, TY, Scarpa, JA, Joffe, B, Solovei, I, Chambon, P, Botchkarev, VA and Fessing, MY. p63 and Brg1 control developmentally regulated higher-order chromatin remodelling at the epidermal differentiation complex locus in epidermal progenitor cells. Development. 2014;141:101–111.
- Fessing, MY, Mardaryev, AN, Gdula, MR, Sharov, AA, Sharova, TY, Rapisarda, V, Gordon, KB, Smorodchenko, AD, Poterlowicz, K, Ferone, G, Kohwi, Y, Missero, C, Kohwi-Shigematsu, T and Botchkarev, VA. p63 regulates Satb1 to control tissue-specific chromatin remodeling during development of the epidermis. J Cell Biol. 2011;194:825–839.
- Pavan Kumar P, Purbey PK, Sinha CK, et al. Phosphorylation of SATB1, a global gene regulator, acts as a molecular switch regulating its transcriptional activity in vivo. Mol Cell. 2006;22:231–243.
- Qu J, Yi G, Zhou H. p63 cooperates with CTCF to modulate chromatin architecture in skin keratinocytes. Epigenetics Chromatin. 2019;12:31.
- Hilgendorf KI, Johnson CT, Mezger A, et al. Omega-3 Fatty Acids Activate Ciliary FFAR4 to Control Adipogenesis. Cell. 2019;179(1289–1305):e1221.
- Alpsoy A, Utturkar SM, Carter BC, et al. BRD9 Is a Critical Regulator of Androgen Receptor Signaling and Prostate Cancer Progression. Cancer Res. 2021;81:820–833.
- McDade SS, Patel D, McCance DJ. p63 maintains keratinocyte proliferative capacity through regulation of Skp2-p130 levels. J Cell Sci. 2011;124:1635–1643.
- Bridgeman SC, Ellison GC, Melton PE, et al. Epigenetic effects of metformin: from molecular mechanisms to clinical implications. Diabetes Obes Metab. 2018;20:1553–1562.
- Ido Y, Duranton A, Lan F, et al. Resveratrol prevents oxidative stress-induced senescence and proliferative dysfunction by activating the AMPK-FOXO3 cascade in cultured primary human keratinocytes. PLoS One. 2015;10:e0115341.
- Pietrocola F, Castoldi F, Markaki M, et al. Aspirin Recapitulates Features of Caloric Restriction. Cell Rep. 2018;22:2395–2407.
- Wang T, Tsui B, Kreisberg JF, et al. Epigenetic aging signatures in mice livers are slowed by dwarfism, calorie restriction and rapamycin treatment. Genome Biol. 2017;18:57.
- Zhang W, Qu J, Liu GH, et al. The ageing epigenome and its rejuvenation. Nat Rev Mol Cell Biol. 2020;21:137–150.
- Yang D, Wei G, Long F, et al. Histone methyltransferase Smyd3 is a new regulator for vascular senescence. Aging Cell. 2020;19:e13212.
