ABSTRACT
Prenatal maternal stress has a negative impact on child health but the mechanisms through which maternal stress affects child health are unclear. Epigenetic variation, such as DNA methylation, is a likely mechanistic candidate as DNA methylation is sensitive to environmental insults and can regulate long-term changes in gene expression. We recruited 155 mother-newborn dyads in the Democratic Republic of Congo to investigate the effects of maternal stress on DNA methylation in mothers and newborns. We used four measures of maternal stress to capture a range of stressful experiences: general trauma, sexual trauma, war trauma, and chronic stress. We identified differentially methylated positions (DMPs) associated with general trauma, sexual trauma, and war trauma in both mothers and newborns. No DMPs were associated with chronic stress. Sexual trauma was positively associated with epigenetic age acceleration across several epigenetic clocks in mothers. General trauma and war trauma were positively associated with newborn epigenetic age acceleration using the extrinsic epigenetic age clock. We tested the top DMPs for enrichment of DNase I hypersensitive sites (DHS) and found no enrichment in mothers. In newborns, top DMPs associated with war trauma were enriched for DHS in embryonic and foetal cell types. Finally, one of the top DMPs associated with war trauma in newborns also predicted birthweight, completing the cycle from maternal stress to DNA methylation to newborn health outcome. Our results indicate that maternal stress is associated with site-specific changes in DNAm and epigenetic age acceleration in both mothers and newborns.
Introduction
Prenatal maternal stress is known to influence offspring health throughout childhood [Citation1] and even into adulthood [Citation2]. These effects are consistent with the Developmental Origins of Health and Disease Hypothesis (DOHaD), which posits that early life adversity is associated with poor adult health outcomes [Citation3]. For example, prenatal maternal stress is associated with newborn health outcomes like low birthweight [Citation4], which is in turn associated with adult disease and mortality [Citation5]. In childhood, prenatal maternal stress predicts poorer cognitive functioning [Citation6] and behavioural problems [Citation7]. These findings have resulted in a focus on early development as a critical time period in which stressors can influence health outcomes over the life course [Citation8].
The biological mechanisms for the long-lasting effects of prenatal maternal stress are unclear. One candidate mechanism for the biological embedding of prenatal maternal stress is epigenetic variation [Citation9], and in particular DNA methylation (DNAm) at cytosine-guanine (CpG) dinucleotide sites. DNAm helps regulate gene expression and plays an important role in human development and disease [Citation10] and is sensitive to social experiences, such as trauma [Citation11–13]. Several studies have reported associations between prenatal maternal stress and DNAm in epigenome-wide association studies (EWAS) [Citation14,Citation15]. These findings hint at the possibility that prenatal maternal stress may be biologically embedded through altered DNAm patterns, which then alter health outcomes.
DNAm may mediate the impact of maternal stress on health outcomes through site-specific changes, such as differentially methylated positions (DMPs), or more global measures, such as epigenetic age. Epigenetic age is an estimate of biological age based on age-associated CpG sites [Citation16]. If the estimated epigenetic age is greater than predicted based on chronological age, that individual is said to demonstrate accelerated epigenetic age. Psychosocial stress has consistently been found to associate with accelerated epigenetic ageing in adults and children [Citation17]. Lifetime stress [Citation18] and exposure to violence [Citation19] have been associated with accelerated epigenetic ageing in adults and children, respectively. Prenatal maternal stress has been found to predict epigenetic age in children [Citation20]. Given associations between epigenetic age acceleration and morbidity and mortality [Citation21], these results suggest that accelerated epigenetic ageing could be a mechanism linking early life stress with adult health outcomes.
Previous studies from our research group have reported associations between prenatal maternal stress and DNA methylation in a small sample of mothers and offspring in the Democratic Republic of Congo (DRC). Specifically, our studies have revealed associations with maternal stress in epigenome-wide methylation contexts [Citation22,Citation23], and at candidate genes involved in the stress response [Citation24,Citation25], brain development [Citation26], growth [Citation27], and the regulation of DNA methylation [Citation28]. In the current study, we tested for the effect of maternal stress on maternal and newborn DNAm in an expanded sample of mothers and newborns from the DRC. We hypothesized that prenatal maternal stress would associate with site-specific and global measures of DNA methylation. Specifically, we tested for associations of maternal stress on mother and newborn DNAm by investigating 1) individual CpG sites through EWAS, 2) global DNAm through principal component analysis (PCA) and general mean methylation (GMM), and 3) epigenetic age acceleration using a range of epigenetic clocks. We also tested for association of maternal stress and DNAm with birthweight as an exemplar health outcome.
Methods
Study sample
Participants were recruited at HEAL Africa Hospital in Goma, DRC from 2013 to 2017. Mothers were recruited from the maternity ward which served both the general population as well as victims of sexual violence. The inclusion criterion for the present study was singleton birth at HEAL Africa Hospital. After quality control procedures, 310 participants (n = 155 mothers and n = 155 newborns) were included in epigenetic age analyses. Four dyads were excluded in EWAS analyses (n = 151 mothers and n = 151 newborns) to improve protection against batch effects.
Participant recruitment began with the informed consent process. Upon arrival at HEAL Africa, mothers were asked by a staff midwife about their interest in participating in the study. If participants wanted to learn more about the study, they were given the option of remaining in the maternity ward or moving to a private room. Most participants elected to remain in the maternity ward because they felt comfortable there. An explanation of the study was given in Congolese Swahili. Mothers were asked if they had any questions about the study and were told they could withdraw from the study at any time. Oral informed consent was considered most culturally appropriate given high rates of illiteracy and a history of colonial exploitation. In cultures of the eastern DRC, pregnancy initiates a girl’s entry into adulthood. Young mothers typically move out of their parents’ homes and live outside their parents’ responsibilities. Consistent with cultural norms, all mothers were considered capable of consenting to enrolment in the study, even if they were under 18 years of age. Following informed consent, semi-structured interviews and surveys were conducted and blood samples were collected from mothers and babies within one day of delivery. Study procedures were approved by the Western Institutional Review Board, Olympia, WA (www.wirb.com, WIRB Project #20100993) and the University of Florida (Project #IRB202001503). The study was also approved by local ethics committees at the University of Goma and HEAL Africa Hospital.
Maternal stress measures
Interviews were conducted by two local female members of the research team in Congolese Swahili. The interviews followed a semi-structured life course format, starting from childhood and ending in the present. Next, the general trauma and sexual abuse parts of the Early Trauma Inventory-Self Report [Citation29] were administered.
Four different measures were used to capture experiences of maternal stress: general trauma, sexual trauma, war trauma, and chronic stress (Table S1). Both the general trauma and sexual trauma measures come from the validated Early Trauma Inventory-Self Report [Citation29]. General trauma was a sum of endorsed items such as witnessing violence and serious injuries in the family. One item loaded negatively (separation of parents) and was removed. The theoretical range for the general trauma scale was zero to ten. The sexual trauma measure was a sum of the sexual abuse subscale in the Early Trauma Inventory-Self Report [Citation29], which contains six items such as being forced to touch intimate parts and forced genital sex. The theoretical range for the sexual abuse scale was zero to six.
War trauma and chronic stress were both ethnographically derived measures of stress that were specific to the local cultural context of eastern DRC. The development of these two scales in a pilot sample from HEAL Africa Hospital has been previously described [Citation25]. Briefly, a combination of items relating to stress and trauma from previously described constructs, such as the Trauma History Questionnaire [Citation30] and the Hassles Scale [Citation31], were used to construct a list of sources of stress. More items were subsequently added to this list that were relevant stressors in the eastern Democratic Republic of Congo, such as war-related stressors. Factor analyses were then used to identify structure in the responses to these items, resulting in two measures: war trauma and chronic stress [Citation25]. In the present study, items related to sexual violence were removed from the war trauma measure because they were captured in the sexual trauma measure described above. The theoretical range for war trauma was zero to six. The chronic stress measure for this study was modified by combining ‘No help cleaning during pregnancy’ and ‘No help at home’ into a single item. Two questions that were used in earlier iterations of the chronic stress measure were not asked in the present study and were therefore not included in the measure (‘travelled alone to the hospital’ and ‘choice in birth location’). The theoretical range for chronic stress was zero to 16.
Anthropometric and demographic data
Anthropometric and demographic data were collected from mothers and newborns. For mothers, height, weight, age, delivery method (vaginal birth or caesarean section), alcohol use in pregnancy (no use of alcohol = 0, use of alcohol = 1), parity (primigravida = 0, multigravida = 1), and recruitment population were recorded. For newborns, sex, birthweight, and gestational age were recorded. Gestational age was estimated from DNAm data using a validated epigenetic clock of gestational age [Citation32]. Smoking was not included in the analyses because only one mother reported this exposure.
DNA methylation
DNA was extracted from venous blood using the QIAmp DNA Blood Mini Kit (Qiagen, Germantown, Maryland). The protocol was performed in two parts. Steps one through seven were performed at HEAL Africa Hospital to kill infectious agents and stabilize DNA prior to shipment to the University of Florida (UF). The remaining steps were performed at UF and samples were cleaned and concentrated as needed [Citation33]. Two hundred fifty grams of DNA were hybridized to the Infinium Methylation EPIC BeadChip (Illumina) at the Hussman Institute for Human Genomics, University of Miami. Data processing was performed in R version 4.2.0 [Citation34]. Quality control of raw methylation data was performed at UF using the R packages meffil [Citation35] and ewastools [Citation36]. Immune cell type proportions were estimated using the IDOL algorithm [Citation37,Citation38]. The SeSame package was used for non-linear dye-bias correction, p-value detection, and noob background correction [Citation39]. Batch correction was performed using ComBat [Citation40] in the sva package [Citation41]. Probes known to map to multiple regions of the genome were masked, as well as probes that could be affected by SNPs prevalent in the African superpopulation using a published probe annotation [Citation42]. X chromosome probes were retained in analyses of mothers but not newborns. Y chromosome probes were masked in all analyses. For mothers, 706,981 probes were analysed. For newborns, 691,867 probes were analysed.
Epigenome-wide association study of maternal stress
Eight EWAS were performed that regressed DNAm at each probe on one of the four maternal stress measures (general trauma, sexual trauma, war trauma, and chronic stress) within each generation (mothers or newborns). For EWAS in mothers, robust linear models controlled for maternal body mass index (BMI), age, parity, delivery mode, alcohol use in pregnancy, recruitment site, and the first principal component (PC) of cell type variation, which accounted for 89% of the variance in cell type proportions. For newborns, covariates in EWAS included maternal BMI, maternal age, parity, delivery mode, alcohol use in pregnancy, recruitment site, gestational age, and the first two principal PCs of cell type variation, which accounted for 90% of the variance in cell type proportions. Covariates were chosen based on prior literature suggesting associations with DNA methylation [Citation32,Citation43–48]
Epigenetic clock analyses
Six different epigenetic clocks were used in analyses of maternal stress in mothers and the first three were tested in newborns. 1) Horvath’s pan-tissue clock [Citation16] was trained on chronological age. 2) Intrinsic epigenetic age was similar to Horvath’s clock, but the effect of variation in immune cell type was removed [Citation49]. 3) Extrinsic epigenetic age was also similar to Horvath’s clock, but the contribution of variation in immune cell type proportion to estimated biological age was upweighted [Citation49]. 4) The telomere epigenetic clock was trained on telomere length and outperforms telomere length in predicting chronological age and mortality [Citation50]. 5) The PhenoAge epigenetic clock was trained on a composite score of nine clinical biomarkers associated with ageing and chronological age, and was strongly associated with morbidity and mortality [Citation51]. 6) The GrimAge epigenetic clock was trained on seven clinical biomarkers associated with ageing and smoking pack years. The GrimAge clock produced a measure of biological age strongly associated with morbidity and mortality [Citation52]. In mothers, maternal BMI, delivery mode, alcohol use in pregnancy, parity, and recruitment site in epigenetic age acceleration analyses were controlled. In newborns, the same covariates plus newborn sex and gestational age were controlled. We restricted epigenetic clocks used in analyses of newborns to clocks which included newborns in the original publications describing development of the respective epigenetic clocks [Citation16,Citation49]. Covariates were chosen based on prior literature suggesting associations with DNA methylation [Citation32,Citation43–48].
DNase I hypersensitivity analyses
eFORGE was used for enrichment analyses [Citation53]. Enrichment of DNase I hypersensitivity sites was tested in the top 125 differentially methylated positions (DMPs), and the top 125 hyper- and the top 125 hypo-methylated probes identified in the EWAS analyses. Recommendations for eFORGE suggest using between 100 and 1000 DMPs [Citation54]; 125 sites were chosen here to focus on the most significant findings while also ensuring that minimum number of sites were available for analysis after filtering input DMPs as part of the eFORGE analysis. Enrichment analyses were controlled for multiple testing within maternal stress measures and analysis method (top 125 DMPs, top 125 hyper methylated DMPs, top 125 hypo methylated DMPs) using the false discovery rate. Results were visualized using the complexHeatmap package [Citation55].
Statistical analyses
Additional statistical tests related to maternal stress and global measures of DNAm. The top ten PCs of a PCA to describe variation in DNAm and general mean methylation (GMM), which is the mean of DNAm across all probes for a given participant, were tested for associations with maternal stress. In both analyses, covariates included maternal BMI, age, delivery method, alcohol use in pregnancy, parity, recruitment site, and immune cell type proportion PCs.
Bonferroni correction for multiple testing within maternal stress measures was used in the EWAS and tests of DNAm PCs. Multiple testing correction was not used for tests of epigenetic age acceleration because of the exploratory nature of association tests between maternal stress and recently developed epigenetic clocks.
Finally, associations between maternal stress, DNA methylation, and birthweight were tested as follow-up to pilot study results [Citation22–25,Citation27,Citation28]. Linear regression models were used to test for relationships between maternal stress measures and birthweight. Regression models were fit to test for associations between Bonferroni-significant DMPs and birthweight. Both analyses controlled for maternal BMI, maternal age, delivery method, alcohol use in pregnancy, parity, recruitment site, gestational age, and newborn sex. Tests of relationships between DNAm and birthweight additionally controlled for immune cell type proportion. All analyses were completed in R [Citation34] (version 4.2.0).
Results
Sample characteristics
Demographic and maternal stress data for participants are described in . A higher number of newborns were recruited in the general maternity population (n = 90) compared to the sexual violence population (n = 65). Mothers recruited from the sexual violence population tended to be younger than mothers recruited as part of the general hospital population because younger women were more likely to be raped and were more likely to seek help through services offered by the hospital. In general, newborns recruited in the sexual violence population were born to younger, lower BMI, and more frequently primigravida (all p < 0.001) mothers. Of the four maternal stress measures, general trauma and war trauma were comparable across recruitment populations (both p > 0.05). Mothers recruited from the sexual violence population had higher scores for chronic stress (p < 0.001) and sexual violence (p < 0.003). In some cases, there are small differences in summary scores for covariate and maternal stress measures because blood samples were not collected for mother and newborn in some dyads, e.g., see Delivery mode. A complete list of items and endorsement frequencies in the stress measures for all four maternal stress measures is available in Table S1.
Table 1. Descriptive statistics of newborns and mothers included in the study.
Associations between maternal stress and DNA methylation
Epigenome-wide association studies (EWAS)
Fifteen DMPs were identified in mothers that associated with one of the maternal stress measures and met Bonferroni-corrected significance (p < 7.70 × 10−8; ). Four DMPs associated with general trauma (), nine with sexual trauma (), and two with war trauma (). No DMPs were significantly associated with chronic stress in mothers (). All Bonferroni-corrected DMPs and gene annotations are listed in .
Figure 1. Manhattan plots of eight epigenome-wide association tests of maternal stress measures among mothers (a–d) and newborns (e–h). Dashed lines indicate the Bonferroni level of significance (p = 7.07 × 10−8 for mothers and 7.23 × 10−8 for newborns). A total of 706,981 probes for mothers and 691,867 for newborns were included in analyses. N = 145.
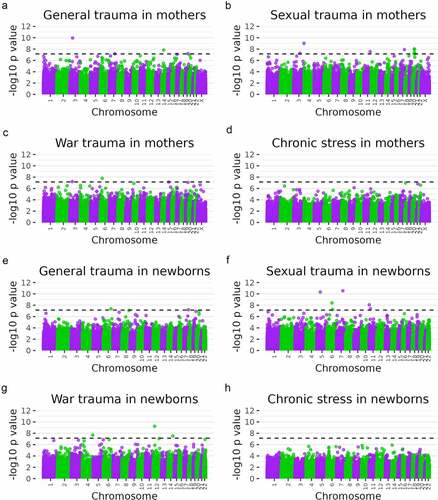
Table 2. Bonferroni-corrected differentially methylated positions in EWAS in mothers and newborns.
Eleven DMPs were identified in newborns that associated with one of the maternal stress measures and met Bonferroni-corrected significance (p < 7.23 × 10−8; ). Two DMPs associated with general trauma (), six DMPs with sexual trauma (), and three DMPs with war trauma (). No DMPs were significantly associated with chronic stress (). All Bonferroni-corrected DMPs and gene annotations are listed in . There was no overlap between significant DMPs in mothers and DMPs in newborns.
Maternal stress and principal components of DNA methylation
No maternal stress measures were significantly associated with the top ten PCs of DNAm in linear multiple regression analyses after Bonferroni correction for multiple testing.
Maternal stress and GMM
No maternal stress measures were significantly associated with GMM in linear multiple regression analyses.
Maternal stress and epigenetic age
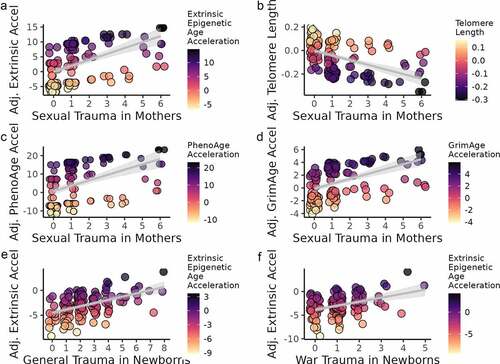
A range of epigenetic clocks were used to test for relationships between biological ageing and maternal stress in mothers and newborns. Among mothers, significant associations were identified between sexual trauma and four different epigenetic clocks (). Sexual trauma was positively associated with epigenetic age acceleration as measured by the extrinsic epigenetic age clock (b = 1.09, p = 0.004), the PhenoAge clock (b = 1.36, p = 0.03), and the GrimAge clock (b = 0.42, p = 0.01) and was negatively associated with the telomere epigenetic clock (b = −0.02, p = 0.02). No other maternal stress measures in mothers were associated with measures of epigenetic age acceleration.
Figure 2. Epigenetic age acceleration and maternal stress measures in mothers and newborns. Colors correspond to the unstandardized beta coefficient of a regression of epigenetic age acceleration measures on maternal stress measures. N = 149 for mothers and N = 145 for newborns.
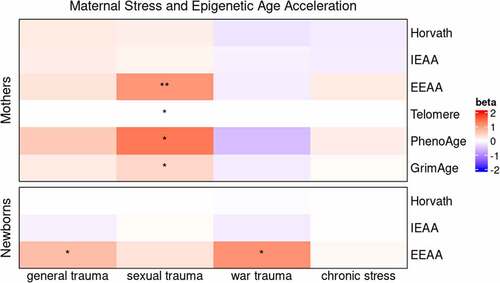
Among newborns, two maternal stress measures were associated with extrinsic epigenetic age acceleration (). General trauma (b = 0.70, p = 0.02) and war trauma (b = 1.12, p = 0.048) were positively associated with extrinsic epigenetic age acceleration. No other maternal stress measures in newborns were associated with measures of epigenetic age acceleration. Plots of the significant associations between maternal stress measures and epigenetic age acceleration in mothers and newborns are shown in .
DNase I enrichment analyses
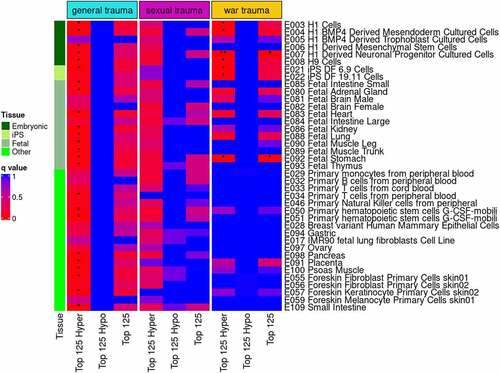
Top DMPs identified in EWAS were tested for enrichment of DNase I hypersensitive sites. In newborns, enrichment of DNase I hypersensitive sites was significant in three analyses (). The top 125 hypermethylated probes from the general trauma EWAS were enriched for DNase I hypersensitive sites across virtually all cell types and tissues tested (q < 0.05). From the war trauma EWAS, the top 125 DMPs were enriched for DNase I hypersensitive sites in derived neuronal progenitor cultured cells and foetal stomach cells (q < 0.05) and the top 125 hypermethylated DMPs were enriched in seven embryonic, foetal, and stem cell types (all q < 0.05). No evidence for enrichment was identified in mothers with any of the top DMPs for general trauma, sexual trauma, and war trauma (data not shown).
Figure 4. eFORGE analysis for enrichment of DNase I hypersensitive sites in newborns. Analyses were performed with the top 125 differentially methylated, hyper-methylated, and hypo-methylated CpG sites for the three maternal stress measures with Bonferroni-corrected significant sites in the EWAS. The top 125 DMPs were compared to the EPIC array distribution of probes to test whether the top 125 DMPs were significantly associated with DNase I hypersensitive locations in genome.
Associations between maternal stress, DNA methylation, and birthweight
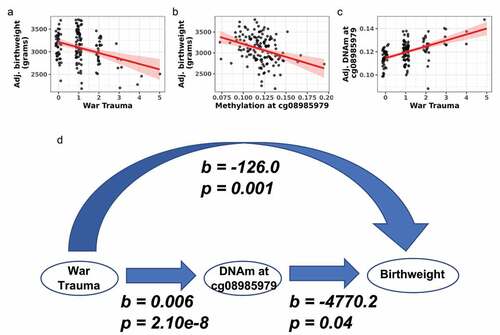
Associations between the four maternal stress measures, DNAm, and birthweight were tested. War trauma was negatively associated with birthweight (b = −126.0, p = 0.001; ). No other maternal stress measure was associated with birthweight. Next, the 26 Bonferroni-significant DMPs from the EWAS analyses were tested for associations with birthweight. Methylation at one DMP (cg08985979) from the newborn war trauma EWAS was negatively associated with birthweight (b = −4770.2, p = 0.04; ). Methylation at cg08985979 was positively associated with war trauma (b = 0.006, p = 2.10e−8; ). Thus, greater war trauma was associated with higher methylation at cg08985979 in newborns and lower birthweight. A model linking war trauma as the exposure, birthweight as the outcome, and DNAm as a possible linking mechanism is depicted in .
Figure 5. War trauma was associated with DNA methylation and birthweight in newborns. (a) War trauma was negatively associated with birthweight. (b) DNA methylation at cg08985979 was negatively associated with birthweight. (c) War trauma was positively associated with DNA methylation at cg08985979 in an EWAS. (d) Model linking war trauma with DNA methylation and birthweight.
Discussion
We report novel associations between multiple measures of maternal stress with maternal and newborn DNAm. It is noteworthy that not only the direct victim, but also the indirect recipient of maternal stress demonstrated epigenetic impacts, consistent with the tenets of DOHaD that predict biological impacts of adverse early life exposures. Furthermore, more than 50% of the DMPs identified in the EWAS were associated with maternal sexual trauma, in both mothers and newborns (), highlighting the devastating and intergenerational impact of rape and related sexual trauma. Interestingly, only mothers showed epigenetic age acceleration in association with sexual trauma and this ageing was manifest as increased ageing of the mother’s immune system (, EEAA), increased mortality risk (PhenoAge and GrimAge), and increased telomere shortening (Telomere), all consistent with accelerated biological ageing in the mother. In contrast, newborns showed increased epigenetic ageing (EEAA) that was associated with general trauma and war trauma, suggesting that trauma-related ageing may start very early in life. Finally, of the 26 stress-associated DMPs we identified, we predicted that just a small number of those would also show phenotypic effects. In fact, one DMP that showed altered newborn DNAm was associated with both increased maternal war trauma and decreased newborn birthweight (), thus providing the foundation for a model where DNAm links maternal trauma with newborn health outcomes. Additional details on our results are presented below.
EWAS results
Associations were detected between general trauma, sexual trauma, and war trauma in separate EWAS in both mothers and newborns. In total, 26 CpG sites met Bonferroni-corrected levels of significance across eight EWAS (four EWAS in mothers and four EWAS in newborns). The top general trauma DMP (cg11408019) is unannotated, but the nearest gene is RAD54L2. This gene interacts with androgen receptors to regulate transcription and is ubiquitously expressed. Our results with RAD54L2 suggest possible effects of maternal stress on the regulation of androgen-driven transcription, which is associated with growth and reproduction in both males and females [Citation56].
In mothers, the top DMP for sexual trauma (cg06308131) is annotated to MUC4. This gene encodes mucin proteins, which protect epithelial surfaces and may be expressed in the endometrium [Citation57,Citation58]. MUC4 polymorphisms have been associated with endometriosis [Citation59] and recurrent pregnancy loss [Citation60]. Furthermore, sexual abuse has been associated with chronic pelvic pain as well as laparoscopically confirmed endometriosis [Citation61], reflecting our association of sexual trauma and DNAm at MUC4.
Finally, the top DMP for war trauma in mothers (cg26486174) is unannotated, but the nearest gene is HMGA1. HMGA1 is reported to bind DNA and regulate transcription through structural modification and transcription factor interaction. HMGA1 has been associated with proliferative embryonic and tumour tissues [Citation62] as well as placental expression [Citation63] and has been implicated in preeclampsia through interference with trophoblast invasion [Citation64]. DNAm at this gene may associate with foetal health outcomes by affecting trophoblast invasion success.
In newborns, the top DMP for general trauma (cg24590750) is annotated to TBPL1. This gene is a transcriptional regulator highly expressed in testis. Early mouse studies found evidence for a role of TBPL1 in spermiogenesis [Citation65]. A candidate gene study discovered nonsynonymous SNPs in infertile men in TBPL1 [Citation66]. Our results suggest that methylation of TBPL1 may represent a possible mechanism for intergenerational effects of prenatal maternal stress on males.
The top DMP for sexual trauma exposure in newborns (cg20807701) is annotated to several genes, the nearest of which is THAP5. THAP5 has been associated with the cell cycle and cell death [Citation67]. Balakrishnan et al. [Citation68] found that THAP5 was primarily expressed in heart tissue, but also in brain and muscle tissue. These authors noted a decrease in THAP5 protein following myocardial infarction, hinting at a role for THAP5 in heart disease. Other studies have found in utero exposures to be associated with adult heart disease [Citation69], and our results hint at DNAm as a possible mechanism for such associations.
Finally, the top DMP for war trauma in newborns (cg21172322) is annotated to BCAT1. In a rat model, BCAT1 expression was upregulated in foetal brain tissue in a maternal immune activation paradigm [Citation70]. Researchers linked BCAT1 expression to changes in foetal brain metabolism, with implications for brain growth and development. Given the similarity in biological pathways for transfer of stress to the foetus between maternal immune activation and psychosocial stress [Citation71], these findings may be relevant to results reported here. Our DNase I hypersensitivity results show that top DMPs in a war trauma EWAS with newborns were enriched for regulatory elements in derived neuronal progenitor cells. The association we report suggests that brain development in newborns is sensitive to prenatal maternal experiences of war trauma.
Epigenetic age acceleration
Accelerated epigenetic ageing, calculated using several different epigenetic clocks, associated with sexual trauma in mothers. These associations controlled for age and represent biological ageing in excess of that expected by chronological age alone. The directionality of effects of maternal stress on biological ageing was consistent across four different epigenetic clocks. The positive association between sexual trauma and extrinsic epigenetic age acceleration suggests that sexual trauma leads to increased ageing of the immune system as measured by the extrinsic epigenetic ageing clock [Citation49]. This finding is consistent with research describing the proinflammatory effects of intimate partner trauma [Citation72]. Furthermore, the positive associations between sexual trauma and the PhenoAge and GrimAge clocks suggest that maternal sexual trauma is associated with increased mortality risk [Citation51,Citation52] due to accelerated biological ageing. Finally, the negative association between sexual trauma and estimated telomere length is consistent with the idea that telomere shortening can be accelerated by stress [Citation73].
Both general trauma and war trauma were associated with accelerated extrinsic epigenetic ageing in newborns. This finding suggests that biological age at birth of newborns with exposure to increased general trauma and war trauma is already increased. These early exposures may shape later life immune responses, possibly associating with greater risk for psychopathology in adulthood [Citation74]. If supported by additional research, trauma-associated DNAm could be used as a prognostic biomarker to ascertain psychopathology risk on an individual basis [Citation75].
DNase I hypersensitivity site results
Enrichment analyses revealed that only newborn DMPs were enriched for DNase I hypersensitive sites, which mark active regulatory regions of the genome. The top 125 hypermethylated DMPs in the newborn EWAS for general trauma were associated with DNase I hypersensitivity sites across a broad range of tissues. Breeze [Citation54] suggested that this type of general enrichment reflects possible enrichment for housekeeping genes, or genes affecting processes fundamental to cell survival in all tissues. These results suggest widespread effects of general trauma in key regulatory regions focused on hypermethylation in newborns. In contrast, enrichment for DNase I hypersensitivity sites from top war trauma DMPs was more focused on embryonic and foetal tissue types, suggesting that war trauma-associated changes in DNAm may be occurring in developmentally critical tissues during gestation.
Association of birthweight with war trauma and cg08985979
Associations were discovered between maternal war trauma, newborn DNAm at cg08985979, and birthweight. From the newborn war trauma EWAS, cg08985979 was found to be negatively associated with war trauma. This site is annotated to a long non-coding RNA (TENM3-AS1) just upstream of TENM3, which is involved in neuronal development [Citation76]. In our study, increased prenatal maternal stress was associated with an increase in newborn cg08985979 methylation and decreased birthweight. These results suggest that DNAm is a possible mechanism by which prenatal maternal stress becomes biologically embedded to affect newborn health outcomes.
Strengths and limitations
Our study had several strengths and limitations. Studies of prenatal maternal stress outside of European and North American contexts are rare, and increasing the diversity of research participants in epigenomic studies fills an important gap in knowledge [Citation77,Citation78]. Relatedly, studies of prenatal maternal stressors in a Western context frequently focus on anxiety or depression. Our study tested a wider range of stressors and identified associations of DNAm with both standardized measures of general trauma and sexual trauma as well as the ethnographically informed measure of war trauma. One limitation of these measures was that they did not allow us to distinguish the effects of educational background and poverty more generally from the effects of psychosocial stress. Our finding of >50% of maternal and newborn DMPs, as well as maternal accelerated epigenetic ageing, that were associated with sexual trauma suggests that efforts to reduce sexual violence, and gender inequity in general, could have outsize effects on improved health for generations to come. Proposed efforts would include improved education for girls and increased support for hospitals like HEAL Africa that have broad agendas that include education and economic opportunities, conflict resolution initiatives, and programmes targeted to rape victims (such as the Sexual Violence Unit in this study) that provide health care as well as psychological and social support in order to improve maternal and infant health [Citation79]. Due to problems associated with working in resource-limited countries, our sample size was small, which may have limited our ability to detect the typically small effect sizes that characterize EWAS. Effect sizes are generally larger in studies of epigenetic age acceleration, so the small sample size may have been less of a limitation in this part of our study. Another important limitation to consider is the use of blood to analyse epigenetic patterns since the relationship between DNAm in a peripheral tissue, such as blood, with DNAm in organs of relevance, such as brain, remains unclear [Citation80].
Conclusion
We provide evidence that changes in DNAm may act as a mechanism to mediate the effects of maternal stress on newborn health outcomes. Specifically, we find evidence that multiple measures of maternal stress are associated with specific changes in DNAm in mothers and newborns, accelerated epigenetic ageing in mothers and newborns, and association with newborn birthweight. Importantly, we find associations between maternal war trauma, newborn DNAm at cg08985979, and birthweight, providing support for the role of DNAm as a mechanism to link maternal stress and offspring health outcomes.
Supplemental Material
Download MS Word (26 KB)Acknowledgments
We thank the mothers and children who participated in this study. The authors also acknowledge the doctors as well as the research team at HEAL Africa Hospital who made this study possible: Bisho Mushagalusa, Georgette Kamate, Chantal Nyaramugisha, Anne Marie Rutega, and Bernard Kitumaini. Sonile Peck processed survey data.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
DNA methylation data are available at gene expression omnibus under record GSE224365. R scripts are available at github.com/edward-quinn/congo_ewas.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15592294.2023.2222473
Additional information
Funding
References
- Coussons-Read ME. Effects of prenatal stress on pregnancy and human development: mechanisms and pathways. Obstet Med. 2013;6(2):52–16. doi:10.1177/1753495x12473751
- Entringer S, Wüst S, Kumsta R, et al. Prenatal psychosocial stress exposure is associated with insulin resistance in young adults. Am J Obstet Gynecol. 2008;199:498.e1–7. doi:10.1016/j.ajog.2008.03.006
- Bianco-Miotto T, Craig JM, Gasser YP, et al. Epigenetics and DOHaD: from basics to birth and beyond. J Dev Orig Health Dis. 2017;8:513–519. doi:10.1017/S2040174417000733
- Bussières E-L, Tarabulsy GM, Pearson J, et al. Maternal prenatal stress and infant birth weight and gestational age: a meta-analysis of prospective studies. Developmental Review. 2015;36:179–199. doi:10.1016/j.dr.2015.04.001
- Belbasis L, Savvidou MD, Kanu C, et al. Birth weight in relation to health and disease in later life: an umbrella review of systematic reviews and meta-analyses. BMC Med. 2016;14(1):147. doi:10.1186/s12916-016-0692-5
- Laplante DP, Brunet A, Schmitz N, et al. Project ice storm: prenatal maternal stress affects cognitive and linguistic functioning in 5 1/2-year-old children. J Am Acad Child Adolesc Psychiatry. 2008;47:1063–1072. doi:10.1097/CHI.0b013e31817eec80
- O’Connor TG, Heron J, Golding J, et al. ALSPAC study team. Maternal antenatal anxiety and behavioural/emotional problems in children: a test of a programming hypothesis. J Child Psychol Psychiatr. 2003;44(7):1025–1036. doi:10.1111/1469-7610.00187
- Cusick SE, Georgieff MK. The role of nutrition in brain development: the golden opportunity of the “first 1000 days”. J Pediatr. 2016;175:16–21. doi:10.1016/j.jpeds.2016.05.013
- Aristizabal MJ, Anreiter I, Halldorsdottir T, et al. Biological embedding of experience: a primer on epigenetics. Proc Natl Acad Sci, USA. 2020;117(38):23261–23269. doi:10.1073/pnas.1820838116
- Greenberg MVC, Bourc’his D. The diverse roles of DNA methylation in mammalian development and disease. Nat Rev Mol Cell Biol. 2019;20(10):590–607. doi:10.1038/s41580-019-0159-6
- Klengel T, Mehta D, Anacker C, et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci. 2013;16:33–41. doi:10.1038/nn.3275
- Mulligan CJ. Early environments, stress, and the epigenetics of human health. Annu Rev Anthropol. 2016;45(1):233–249. doi:10.1146/annurev-anthro-102215-095954
- Mulligan CJ. Insights from epigenetic studies on human health and evolution. Curr Opin Genet Dev. 2018;53:36–42. doi:10.1016/j.gde.2018.06.008
- Sharma R, Frasch MG, Zelgert C, et al. Maternal-fetal stress and DNA methylation signatures in neonatal saliva: an epigenome-wide association study. Clin Epigenetics. 2022;14:87. doi:10.1186/s13148-022-01310-x
- Viuff AC, Sharp GC, Rai D, et al. Maternal depression during pregnancy and cord blood DNA methylation: findings from the avon longitudinal study of parents and children. Transl Psychiatry. 2018;8(1):244. doi:10.1038/s41398-018-0286-4
- Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:R115. doi:10.1186/gb-2013-14-10-r115
- Palma-Gudiel H, Fañanás L, Horvath S, et al. Psychosocial stress and epigenetic aging. Int Rev Neurobiol. 2020;150:107–128 doi:10.1016/bs.irn.2019.10.020.
- Zannas AS, Arloth J, Carrillo-Roa T, et al. Lifetime stress accelerates epigenetic aging in an urban, African American cohort: relevance of glucocorticoid signaling. Genome Biol. 2015;16(1):1–12. doi:10.1186/s13059-015-0828-5
- Jovanovic T, Vance LA, Cross D, et al. Exposure to violence accelerates epigenetic aging in children. Sci Rep. 2017;7(1):8962. doi:10.1038/s41598-017-09235-9
- McGill MG, Pokhvisneva I, Clappison AS, et al. Maternal prenatal anxiety and the fetal origins of epigenetic aging. Biol Psychiatry. 2022;91(3):303–312. doi:10.1016/j.biopsych.2021.07.025
- Oblak L, van der Zaag J, Higgins-Chen AT, et al. A systematic review of biological, social and environmental factors associated with epigenetic clock acceleration. Ageing Res Rev. 2021;69:101348. doi:10.1016/j.arr.2021.101348
- Rodney NC, Mulligan CJ. A biocultural study of the effects of maternal stress on mother and newborn health in the Democratic Republic of Congo. Am J Phys Anthropol. 2014;155:200–209. doi:10.1002/ajpa.22568
- Clukay CJ, Hughes DA, Kertes DA, et al. Associations between maternal psychosocial stress, DNA methylation, and newborn birth weight identified by investigating methylation at individual, regional, and genome levels. Hum Biol. 2019;91:117–131. doi:10.13110/humanbiology.91.2.04
- Mulligan CJ, D’Errico NC, Stees J, et al. Methylation changes at NR3C1 in newborns associate with maternal prenatal stress exposure and newborn birth weight. Epigenetics. 2012;7(8):853–857. doi:10.4161/epi.21180
- Kertes DA, Kamin HS, Hughes DA, et al. Prenatal maternal stress predicts methylation of genes regulating the hypothalamic-pituitary-adrenocortical system in mothers and newborns in the Democratic Republic of Congo. Child Dev. 2016;87:61–72. doi:10.1111/cdev.12487
- Kertes DA, Bhatt SS, Kamin HS, et al. BNDF methylation in mothers and newborns is associated with maternal exposure to war trauma. Clin Epigenetics. 2017;9(1):68. doi:10.1186/s13148-017-0367-x
- Montoya-Williams D, Quinlan J, Clukay C, et al. Associations between maternal prenatal stress, methylation changes in IGF1 and IGF2, and birth weight. J Dev Orig Health Dis. 2018;9(2):215–222. doi:10.1017/S2040174417000800
- Clukay CJ, Hughes DA, Rodney NC, et al. DNA methylation of methylation complex genes in relation to stress and genome-wide methylation in mother-newborn dyads. Am J Phys Anthropol. 2018;165:173–182. doi:10.1002/ajpa.23341
- Bremner JD, Bolus R, Mayer EA. Psychometric properties of the early trauma inventory-self report. J Nerv Ment Dis. 2007;195:211–218. doi:10.1097/01.nmd.0000243824.84651.6c
- Hooper LM, Stockton P, Krupnick JL, et al. Development, use, and psychometric properties of the trauma history questionnaire. J Loss Trauma. 2011;16(3):258–283. doi:10.1080/15325024.2011.572035
- Kanner AD, Coyne JC, Schaefer C, et al. Comparison of two modes of stress measurement: daily hassles and uplifts versus major life events. J Behav Med. 1981;4(1):1–39. doi:10.1007/BF00844845
- Haftorn KL, Lee Y, Denault WRP, et al. An EPIC predictor of gestational age and its application to newborns conceived by assisted reproductive technologies. Clin Epigenetics. 2021;13(1):82. doi:10.1186/s13148-021-01055-z
- Green MR, Sambrook J. Precipitation of DNA with Ethanol. Cold Spring Harb Protoc. 2016;2016:pdb.prot093377. doi:10.1101/pdb.prot093377
- R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2021.
- Min JL, Hemani G, Davey Smith G, et al. Meffil: efficient normalization and analysis of very large DNA methylation datasets. Bioinformatics. 2018;34(23):3983–3989. doi:10.1093/bioinformatics/bty476
- Heiss JA, Just AC. Identifying mislabeled and contaminated DNA methylation microarray data: an extended quality control toolset with examples from GEO. Clin Epigenetics. 2018;10(1):73. doi:10.1186/s13148-018-0504-1
- Salas LA, Koestler DC, Butler RA, et al. An optimized library for reference-based deconvolution of whole-blood biospecimens assayed using the Illumina HumanMethylationEPIC BeadArray. Genome Biol. 2018;19(1):64. doi:10.1186/s13059-018-1448-7
- Gervin K, Salas LA, Bakulski KM, et al. Systematic evaluation and validation of reference and library selection methods for deconvolution of cord blood DNA methylation data. Clin Epigenetics. 2019;11(1):125. doi:10.1186/s13148-019-0717-y
- Zhou W, Triche TJ, Laird PW, et al. SeSAMe: reducing artifactual detection of DNA methylation by Infinium BeadChips in genomic deletions. Nucleic Acids Res. 2018;46:e123. doi:10.1093/nar/gky691
- Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8(1):118–127. doi:10.1093/biostatistics/kxj037
- Leek JT, Johnson WE, Parker HS, et al. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics. 2012;28(6):882–883. doi:10.1093/bioinformatics/bts034
- Zhou W, Laird PW, Shen H. Comprehensive characterization, annotation and innovative use of Infinium DNA methylation BeadChip probes. Nucleic Acids Res. 2017;45:e22. doi:10.1093/nar/gkw967
- Sharp GC, Salas LA, Monnereau C, et al. Maternal BMI at the start of pregnancy and offspring epigenome-wide DNA methylation: findings from the pregnancy and childhood epigenetics (PACE) consortium. Hum Mol Genet. 2017;26(20):4067–4085. doi:10.1093/hmg/ddx290
- Liu C, Marioni RE, Hedman ÅK, et al. A DNA methylation biomarker of alcohol consumption. Mol Psychiatry. 2018;23(2):422–433. doi:10.1038/mp.2016.192
- Jaffe AE, Irizarry RA. Accounting for cellular heterogeneity is critical in epigenome-wide association studies. Genome Biol. 2014;15(2):R31. doi:10.1186/gb-2014-15-2-r31
- Ryan CP, Hayes MG, Lee NR, et al. Reproduction predicts shorter telomeres and epigenetic age acceleration among young adult women. Sci Rep. 2018;8(1):11100. doi:10.1038/s41598-018-29486-4
- Jones MJ, Goodman SJ, Kobor MS. DNA methylation and healthy human aging. Aging Cell. 2015;14(6):924–932. doi:10.1111/acel.12349
- Schlinzig T, Johansson S, Gunnar A, et al. Epigenetic modulation at birth - altered DNA-methylation in white blood cells after Caesarean section. Acta Paediatr. 2009;98:1096–1099. doi:10.1111/j.1651-2227.2009.01371.x
- Horvath S, Gurven M, Levine ME, et al. An epigenetic clock analysis of race/ethnicity, sex, and coronary heart disease. Genome Biol. 2016;17(1):1–22. doi:10.1186/s13059-016-1030-0
- Lu AT, Seeboth A, Tsai P-C, et al. DNA methylation-based estimator of telomere length. Aging. 2019;11(16):5895–5923. doi:10.18632/aging.102173
- Levine ME, Lu AT, Quach A, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging. 2018;10(4):573–591. doi:10.18632/aging.101414
- Lu AT, Quach A, Wilson JG, et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging. 2019;11(2):303–327. doi:10.18632/aging.101684
- Breeze CE, Reynolds AP, van Dongen J, et al. eFORGE v2.0: updated analysis of cell type-specific signal in epigenomic data. Bioinformatics. 2019;35(22):4767–4769. doi:10.1093/bioinformatics/btz456
- Breeze CE. Cell type-specific signal analysis in epigenome-wide association studies. Methods Mol Biol. 2022;2432:57–71.
- Gu Z, Eils R, Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics. 2016;32(18):2847–2849. doi:10.1093/bioinformatics/btw313
- Hammes SR, Levin ER. Impact of estrogens in males and androgens in females. J Clin Invest. 2019;129(5):1818–1826. doi:10.1172/JCI125755
- Alameda F, Mejías-Luque R, Garrido M, et al. Mucin genes (MUC2, MUC4, MUC5AC, and MUC6) detection in normal and pathological endometrial tissues. Int J Gynecol Pathol. 2007;26:61–65. doi:10.1097/01.pgp.0000225837.32719.c1
- Dharmaraj N, Chapela PJ, Morgado M, et al. Expression of the transmembrane mucins, MUC1, MUC4 and MUC16, in normal endometrium and in endometriosis. Hum Reprod. 2014;29(8):1730–1738. doi:10.1093/humrep/deu146
- Chang C-Y, Chang H-W, Chen C-M, et al. MUC4 gene polymorphisms associate with endometriosis development and endometriosis-related infertility. BMC Med. 2011;9:19. doi:10.1186/1741-7015-9-19
- Kim J-H, Park H-S, Lee J-Y, et al. Association study between Mucin 4 (MUC4) polymorphisms and idiopathic recurrent pregnancy loss in a Korean population. Genes (Basel). 2022;13(6):937. doi:10.3390/genes13060937
- Harris HR, Wieser F, Vitonis AF, et al. Early life abuse and risk of endometriosis. Hum Reprod. 2018;33(9):1657–1668. doi:10.1093/humrep/dey248
- Wang L, Zhang J, Xia M, et al. High mobility group A1 (HMGA1): structure, biological function, and therapeutic potential. Int J Biol Sci. 2022;18(11):4414–4431. doi:10.7150/ijbs.72952
- Bamberger A-M, Makrigiannakis A, Röser K, et al. Expression of the high-mobility group protein HMGI(Y) in human trophoblast: potential role in trophoblast invasion of maternal tissue. Virchows Arch. 2003;443:649–654. doi:10.1007/s00428-003-0892-1
- Matsubara K, Matsubara Y, Uchikura Y, et al. HMGA1 is a potential driver of preeclampsia pathogenesis by interference with extravillous trophoblasts invasion. Biomolecules. 2021;11(6):11. doi:10.3390/biom11060822
- Zhang D, Penttila TL, Morris PL, et al. Spermiogenesis deficiency in mice lacking the Trf2 gene. Science. 2001;292(5519):1153–1155. doi:10.1126/science.1059188
- Kuzmin A, Jarvi K, Lo K, et al. Identification of potentially damaging amino acid substitutions leading to human male infertility. Biol Reprod. 2009;81(2):319–326. doi:10.1095/biolreprod.109.076000
- Balakrishnan MP, Cilenti L, Ambivero C, et al. THAP5 is a DNA-binding transcriptional repressor that is regulated in melanoma cells during DNA damage-induced cell death. Biochem Biophys Res Commun. 2011;404(1):195–200. doi:10.1016/j.bbrc.2010.11.092
- Balakrishnan MP, Cilenti L, Mashak Z, et al. THAP5 is a human cardiac-specific inhibitor of cell cycle that is cleaved by the proapoptotic Omi/HtrA2 protease during cell death. Am J Physiol Heart Circ Physiol. 2009;297(2):H643–53. doi:10.1152/ajpheart.00234.2009
- Barker DJ, Gluckman PD, Godfrey KM, et al. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–941. doi:10.1016/0140-6736(93)91224-A
- McColl ER, Piquette-Miller M. Poly(i: c) alters placental and fetal brain amino acid transport in a rat model of maternal immune activation. Am J Reprod Immunol. 2019;81:e13115. doi:10.1111/aji.13115
- Rakers F, Rupprecht S, Dreiling M, et al. Transfer of maternal psychosocial stress to the fetus. Neurosci Biobehav Rev. 2017. doi:10.1016/j.neubiorev.2017.02.019
- Yim IS, Kofman YB. The psychobiology of stress and intimate partner violence. Psychoneuroendocrinology. 2019;105:9–24. doi:10.1016/j.psyneuen.2018.08.017
- Rentscher KE, Carroll JE, Mitchell C. Psychosocial stressors and telomere length: a current review of the science. Annu Rev Public Health. 2020;41(1):223–245. doi:10.1146/annurev-publhealth-040119-094239
- Danese A, Baldwin JR. Hidden wounds? inflammatory links between childhood trauma and psychopathology. Annu Rev Psychol. 2017;68:517–544. doi:10.1146/annurev-psych-010416-044208
- van den Oord CLJD, Copeland WE, Zhao M, et al. DNA methylation signatures of childhood trauma predict psychiatric disorders and other adverse outcomes 17 years after exposure. Mol Psychiatry. 2022;27:3367–3373. doi:10.1038/s41380-022-01597-5
- Zhang X, Lin P-Y, Liakath-Ali K, et al. Teneurins assemble into presynaptic nanoclusters that promote synapse formation via postsynaptic non-teneurin ligands. Nat Commun. 2022;13(1):2297. doi:10.1038/s41467-022-29751-1
- Breeze CE, Wong JYY, Beck S, et al. Diversity in EWAS: current state, challenges, and solutions. Genome Med. 2022;14(1):71. doi:10.1186/s13073-022-01065-3
- Non AL. Social epigenomics: are we at an impasse? Epigenomics. 2021;13(21):1747–1759. doi:10.2217/epi-2020-0136
- D’Errico NC, Wake CM, Wake RM. Healing Africa? Reflections on the peace-building role of a health-based non governmental organization operating in eastern Democratic Republic of Congo. Med Confl Surviv. 2010;26(2):145–159. doi:10.1080/13623699.2010.491390
- Bakulski KM, Halladay A, Hu VW, et al. Epigenetic research in neuropsychiatric disorders: the “tissue issue”. Curr Behav Neurosci Rep. 2016;3(3):264–274. doi:10.1007/s40473-016-0083-4