ABSTRACT
Long noncoding RNAs have been identified as important regulators of gene expression and animal development. The expression of natural antisense transcripts (NATs) transcribed in the opposite direction to protein-coding genes is usually positively correlated with the expression of homologous sense genes and is the key factor for expression. Here, we identified a conserved noncoding antisense transcript, CFL1-AS1, that plays an important role in muscle growth and development. CFL1-AS1 overexpression and knockout vectors were constructed and transfected into 293T and C2C12 cells. CFL1-AS1 positively regulated CFL1 gene expression, and the expression of CFL2 was also downregulated when CFL1-AS1 was knocked down. CFL1-AS1 promoted cell proliferation, inhibited apoptosis and participated in autophagy. This study expands the research on NATs in cattle and lays a foundation for the study of the biological function of bovine CFL1 and its natural antisense chain transcript CFL1-AS1 in bovine skeletal muscle development. The discovery of this NAT can provide a reference for subsequent genetic breeding and data on the characteristics and functional mechanisms of NATs.
KEYWORDS:
Introduction
Long noncoding RNAs (lncRNAs), as key epigenetic regulators, play an important role in cell growth and differentiation [Citation1]. LncRNAs can be divided into different categories according to their genomic location compared with protein-coding genes. RNAs that overlap other genes, but are transcribed from the opposite strand are classified as antisense noncoding RNAs or natural antisense transcripts (NATs) [Citation2]. NATs are endogenous RNAs produced in organisms under natural conditions and are an important class of lncRNA molecules [Citation3] that can be paired with their corresponding complementary RNAs through base pairing to form double-stranded RNA, enhancing or inhibiting transcription at the local level or regulating the stability of mRNA [Citation4]. NATs have been proven to be capable of cis-regulation of the expression level of the sense strand gene [Citation5] and also of trans-regulating the expression of other genes at a long distance from the sense strand gene [Citation6]. They are also important regulators of gene expression and play important roles in various physiological and pathological processes, such as organ formation, cell differentiation and disease occurrence [Citation7–9].
NATs have been gradually confirmed to be widely present in the transcriptomes of many common species, such as humans [Citation10,Citation11], mice [Citation12,Citation13], pigs [Citation14], and chickens [Citation15,Citation16], suggesting that they may play an important role in gene expression and regulatory functions in these species. However, compared with human and mouse NATs, relevant studies in livestock are lacking. We identified a new lncRNA with low specific expression in adult bovine muscle tissue, and the newly identified lncRNA overlapped with the antisense chain composed of 4 exons of actin binding protein gene 1 (cofilin-1, CFL1) on chromosome 29 of cattle. Therefore, this newly identified NAT was named CFL1-AS1 (antisense CFL1) according to the long noncoding RNA naming principle. Trans prediction showed that CFL1-AS1 could regulate the expression of the CFL2 gene. Both the CFL2 gene and CFL1-AS1 parent gene CFL1 are closely related to muscle growth and development and play an important role in normal muscle function and regeneration. Previous studies have shown that the CFL1 and CFL2 genes are specifically expressed in different growth and development stages of bovine muscle and show opposite expression patterns during muscle cell differentiation [Citation17,Citation18]. RNAhybrid analysis identified a miR-125b binding site on CFL1-AS1, and studies have shown that miR-125b can regulate the proliferation and differentiation of myoblasts [Citation19]. Therefore, it is speculated that CFL1-AS1 may also play an important role in the regulation of muscle growth and development, but the mechanism by which CFL1-AS1 regulates the proliferation and differentiation of bovine myoblasts and which signalling molecules regulate CFL1-AS1 remain to be further studied.
To further study CFL1-AS1, the full-length sequence of CFL1-AS1 was obtained by rapid amplification of cDNA ends (RACE) and analysed by bioinformatics. The tissue expression profile was analysed by real-time PCR, and three sgRNAs targeting CFL1-AS1 were designed by the CRISPR/CAS9 system. pX458-sgRNA-CFL1-AS1 knockout vectors were constructed, and a pcDNA3.1-CFL1-AS1 overexpression vector was also constructed. The full-length sequence of CFL1-AS1 was 616 bp, and it was mainly localized to the cytoplasm. CFL1-AS1 is an RNA with no coding capacity. After knocking down CFL1-AS1, the mRNA expression levels of CFL1 and CFL2 decreased. After overexpression of CFL1-AS1, the expression of CFL1 increased, and the expression of CFL2 did not change significantly. CFL1-AS1 promotes cell proliferation, inhibits apoptosis and participates in autophagy. These results indicate that CFL1-AS1 can positively regulate CFL1 gene expression and participate in cell growth and development. This revealed the expression pattern between CFL1-AS1 and CFL1. This study provided basic data for further study of the functional mechanism of natural antisense lncRNA CFL1-AS1 molecules.
Materials and methods
Nuclear and cytoplasmic RNA fractionation
Nuclear and cytoplasmic RNA fractionation was performed in muscle cells using the Invitrogen™ PARIS™ Kit (AM1921, Waltham, MA, USA). The detailed separation method is described in the manufacturer’s instructions. Nuclear and cytoplasmic RNA was extracted and transcribed into cDNA for further analysis. The cellular localization of CFL1-AS1 was analysed by qRT‒PCR. The primer sequences used for qRT‒PCR are listed in Table S2.
Amplification of full-length CFL1-AS1 by rapid amplification of cDNA ends
According to the predicted CFL1-AS1 gene sequence (attachment-sequence) by high-throughput sequencing, 5’ and 3’ gene-specific primers (GSPs) were designed. The 5’ end of CFL1-AS1 was amplified using CFL1-AS1-5’GSP downstream primers, and the 3’ end of CFL1-AS1 was amplified using CFL1-AS1-3’GSP upstream primers. The 5’ end of CFL1-AS1 was amplified using the traditional 5’ rapid amplification of cDNA ends (5‘RACE) method [Citation20]. Using 5“RACE Ready cDNA as a template, QT universal primers as upstream primers, and CFL1-AS1-5’GSP1 as downstream primers, a round of amplification was performed using a touchdown PCR program (95°C for 10 min; 5 PCR cycles of 94°C for 30 s, 72°C for 3 min; 5 PCR cycles of 94°C for 30 s, 70°C for 30 s,72°C for 3 min; 25 PCR cycles of 94°C for 30 s, 68°C for 30 s,72°C for 3 min;72°C for 10 min). Then, a round of PCR product was used as the template, QI universal primer was used as the upstream primer, CFL1-AS1-5’GSP2 was used as the downstream primer, and the 5” end of CFL1-AS1 was amplified by an ordinary PCR procedure. The 3’ end of CFL1-AS1 was amplified using the kit’s 3‘RACE method (Takara Bio USA, Inc. SMARTer ® RACE 5’/3’ Kit User Manual). 3‘RACE was performed using 3‘RACE Ready cDNA as the template, CFL1-AS1-3’GSP as the upstream primer and universal primer (UPM) as the downstream primer. The primer sequences used for PCR are listed in Table S3. The PCR products were detected by 2% agarose gel electrophoresis and sequenced.
Bioinformatics analysis of the CFL1-AS1 sequence
The full-length sequence of CFL1-AS1 was obtained by splicing the sequence obtained by the RACE experiment (attachment-sequence). DNAMAN software was used to analyse the base content of the sequence. The location of CFL1-AS1 in the bovine genome was queried in UCSC Genome Explorer (http://genome-asia.edu/in-dex.html). Sequence similarity comparison was carried out by the BLAST program in NCBI. The RNAfold network server was used to calculate the minimum free energy (MFE) (http://rna.tbi.univie.ac.at/cgi-bin/RNAWebSuite/RNAfold.cgi) to predict the secondary structure of CFL1-AS1. The LncTar tool was used to predict RNA targets of lncRNAs (LncRNA-RNA interaction (cuilab.cn)). The coding ability of the CFL1-AS1 gene was predicted by CPC software (CPC2 @ CBI, PKU (gao-lab.org)).
Construction of the CFL1-AS1 overexpression vector
According to the restriction site map of the pcDNA3.1-GFP vector, the CFL1-AS1 target fragment was inserted between the KpnI and HindIII restriction sites, and CFL1-AS1-HindIII-F and CFL1-AS1-KpnI-R primers (Table S3) were designed. CFL1-AS1 was amplified from bovine muscle cDNA. The purified PCR product and pcDNA3.1-GFP plasmid were digested with KpnI and HindIII for 30 min at 37°C, respectively. The digested pcDNA3.1-GFP plasmid was mixed with CFL1-AS1 at a ratio of 1:3, and they were ligated with T4 ligase at 16°C for 1 h and then incubated at 4°C overnight. The ligation product was transferred into DH5α competent cells by the heat shock method. After coating the plate, the cells were cultured in an incubator at 37°C for 12 h. Monoclonal colonies were selected and identified by PCR amplification using CFL1-AS1 full-length primers.
Construction of the CFL1-AS1 knockout vector
Three sgRNAs (Table S2) were designed for the CFL1-AS1 sequence, cloned into the pSpCas9 (BB)-2A-GFP (pX458) expression vector, and then transfected into 293T cells to detect the activity of sgRNA. After 36 hours of continuous culture, the cells were placed under a fluorescence microscope to observe the expression of green fluorescence to determine the transfection efficiency. Genomic DNA was extracted from each group of cells for PCR amplification of mutant regions, and mutations were determined at the DNA level by sequencing. The expression of CFL1-AS1 at the mRNA level was determined by qRT‒PCR.
Cell treatment
HEK293T and C2C12 cells were cultured in growth medium consisting of Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% foetal bovine serum and 1% penicillin/streptomycin (Gibco, Carlsbad, CA, USA) at 37°C in a 5% CO2 humidified incubator. HEK293T and C2C12 cells under good growth conditions were uniformly plated in 6-well plates to reach a cell density of 70%. To detect the transfection efficiency of the recombinant vector, pcDNA3.1-CFL1-AS1, pcDNA3.1 (control group), sgRNA1-CFL1-AS1, sgRNA2-CFL1-AS1, sgRNA3-CFL1-AS1 and sgRNA negative control pX458 expression vector (negative control) was transfected into HEK293T cells using Lipo293TM (Beyotime, China) and C2C12 cells using ExFect Transfection Reagent (Vazyme, China). RNA was collected after culturing cells for 36 h.
Isolation of total RNA and real-time PCR analysis
Total RNA was extracted from tissues and cells using TRIzol reagent (TaKaRa, Dalian, China), and then 500 ng RNA was transcribed into cDNA using the PrimeScript RT reagent kit (TaKaRa, Dalian, China) for use in qRT‒PCR with the SYBR Green Master Mix reagent kit (Vazyme, Nanjing, China). Gene-specific primers for the CFL1-AS1 gene (CFL1-AS1-F/R) were designed based on published mRNA sequences using Primer Premier 5.0 software (PREMIER Biosoft International, USA). Transcription levels were normalized to the levels of the housekeeping gene β-actin (β-actin-F/R). The primer sequences used for qRT‒PCR are listed in Table S2.
Statistical analysis
All data are presented as the mean ± SE of 3 independent experiments performed in triplicate. The relative gene expression levels were determined using the 2−∆∆Ct method and analysed by SPSS software (version 18.0). GraphPad Prism 7.0 software was used to generate figures. A difference with p < 0.05 was regarded as statistically significant.
Results
Identification of CFL1-AS1
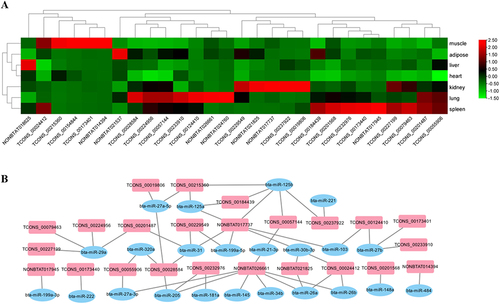
Long noncoding RNAs are abundantly expressed in bovine tissue. We initially screened 28 lncRNAs that were specifically expressed in bovine muscle tissue by sequencing, and they all exhibited differential expression in various bovine tissues (muscle, adipose, liver, heart, kidney, lung and spleen). We found that CFL1-AS1 (TCONS_00184439) is specifically expressed at low levels in muscle tissue (), and the CFL1-AS1 sequence contains the binding site of miR-125a/b (). Both miR-125a and miR-125b can exert biological functions by regulating the expression of the downstream target gene IGF2, and miR-125b can inhibit differentiation. Therefore, we speculated that the identified lncRNA CFL1-AS1 might regulate the proliferation and differentiation of myoblasts through competitive adsorption of miR-125a/b.
Figure 1. Identification of long non-coding RNA CFL1-AS1. (a) Heatmap analysis of differentially expressed lncRnas between adult cattle and embryo cattle. RNA was extracted from the muscle, adipose, liver, heart, kidney, lung, and spleen tissues of three embryo and adults for reverse transcription. qPCR was performed with lncRnas quantitative primer (Table S1). (b) the lncRNA-miRNA network interaction map.
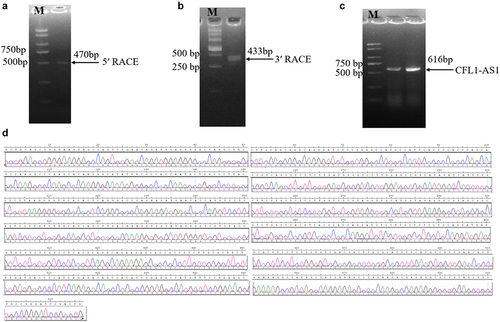
Cloning the long noncoding RNA CFL1-AS1
Using cDNA obtained by reverse transcription of RNA from muscle tissue of cattle as a template, the 470 bp 5’ end of CFL1-AS1 was obtained by the 5‘RACE amplification method (), and the 433 bp 3’ end of CFL1-AS1 was obtained by the 3‘RACE amplification method (). The full-length sequence of bovine lncRNA CFL1-AS1 was obtained by splicing the sequences obtained by 5‘RACE and 3‘RACE, and the total length of the sequence was 616 bp ().
Molecular characterization of CFL1-AS1
The results showed that the CFL1-AS1 gene is located in the interval of cattle chr29: 43973775 ~ 43976501, which overlaps with the position of the antisense strand of the actin binding protein gene 1 (cofilin-1, CFL1) composed of four exons on chromosome 29 (). The results of DNAMAN software analysis showed that the contents of A, C, G and T in CFL1-AS1 were 20.13% (124), 30.36% (187), 27.60% (170) and 21.92% (135), respectively. The GC content (57.96%) was significantly higher than the AT content (42.05%), indicating that the DNA double strands in the coding region were relatively stable. The sequence alignment analysis using the BLAST program in NCBI showed that the CFL1-AS1 gene sequence of cattle had a sequence similarity of more than 90% with Ovis aries, Phacochoerus africanus, Equus quagga, Homo sapiens, musculus and other species, indicating that CFL1-AS1 was highly conserved.
Figure 3. Molecular characterization of CFL1-AS1. (a) UCSC Genome Browser localizing CFL1-AS1. (b) Coding ability of CFL1-AS1 prediction. (c) Secondary structure of CFL1-AS1. (d) Bioinformatics prediction of binding sites in CFL1-AS1 to bta-miR-125a. (e) KEGG enrichment analysis of CFL1 and CFL2.
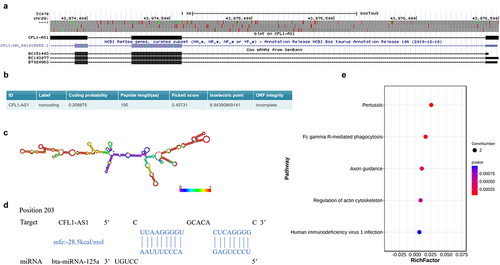
The lncRNA CFL1-AS1 sequence obtained a Fickett score of 0.45731 with an incomplete putative ORF of 195 AA and a pI of 6.94390869141, and these findings classify it as a noncoding sequence with a coding probability of 0.208875 (). Through secondary structure prediction analysis by the RNAfold network server, CFL1-AS1 was found to have a folded secondary structure with multiple hairpin rings (), and the structure is relatively stable. RNAhybrid analysis showed that CFL1-AS1 has a miR-125a binding site (), and miR-125b can regulate the proliferation and differentiation of myoblasts. Complementary nucleotide sequences between CFL1-AS1 and CFL1 and CFL2 were predicted by the LncTar tool. KEGG pathway enrichment analysis showed that the predicted target genes, CFL1 and CFL2, were significantly enriched in the signalling pathways related to muscle development in the regulation of the actin cytoskeleton and the Fc gamma R-mediated phagocytosis signalling pathway ().
Spatial expression characteristics of CFL1-AS1
To further explore the mechanism of CFL1-AS1, we performed subcellular localization analysis. The isolated RNA products were detected by RT‒qPCR; GAPDH was used as the cytoplasmic reference gene, and NEAT1 was used as the nuclear reference gene to determine whether nuclear separation was successful. The results showed that the content of CFL1-AS1 in the cytoplasm was 0.915, and the relative content in the nucleus was 0.085 (), indicating that CFL1-AS1 was mainly localized in the cytoplasm.
Figure 4. Spatial expression patterns of CFL1-AS1. (a) Subcellular localization of CFL1-AS1. (b) Analysis of tissue expression profile of CFL1-AS1 in Qinchuan cattle. CFL1-AS1 was normalized to β-actin. The spatial expression levels of liver tissues were considered as 1. Data are means±SE of n = 3 independent experiments, each performed in triplicate. ** p < 0.01, ***, p < 0.001, two-tailed t test.
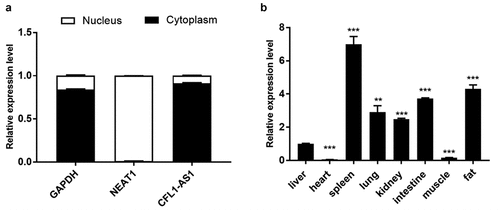
CFL1-AS1 was expressed in the heart, liver, spleen, lung, kidney, small intestine, muscle and fat of adult cattle to varying degrees, with the highest expression level in the spleen (p < 0.001) and the second highest expression level in fat (p < 0.001). Significantly low expression was observed in heart and muscle tissues (p < 0.001) ().
Construction of CFL1-AS1 knockout expression vectors
Three pairs of sgRNA oligos designed for CFL1-AS1 were annealed to form double strands, ligated to the pX458 plasmid after it was digested by BbsI (), and then transformed into stbl3 competent cells to construct the pX458-sgRNA-CFL1-AS1 vector. Ten monoclonal bacteria were selected from each group, and four bacterial solutions were selected for sequencing. The bacterial solution was sequenced using the U6 universal primers contained in the vector. One, three and four positive bacteria were detected in the G1, G2 and G3 groups, respectively. The sequencing results showed that three pairs of sgRNAs were correctly inserted into the pX458 plasmid digestion site (), and the pX458-sgRNA-CFL1-AS1 vector was successfully constructed.
Figure 5. Construction knockout expression vector of CFL1-AS1. (a) Map of gene knockout vector pSpcas9(bb)-2A-GFP (pX458). (b) Sequencing peak of pX458-sgRNA-CFL1-AS1 vector. (c) Expression efficiency of pX458-sgRNA-CFL1-AS1 vector in 293T cells. (d) the PCR product of pX458-sgRNA-CFL1-AS1 DNA in 293T cell. (e) Peak plots of pX458-sgRNA-CFL1-AS1 PCR products. (f) Relative expression levels of CFL1-AS1 mRNA in 293T cell. Three CFL1-AS1 knockdown vectors were constructed using three sgRNA pairs of CFL1-AS1 primers (sgRNA1-CFL1-AS1, sgRNA2-CFL1-AS1, sgRNA3-CFL1-AS1). They were pX458-sgRNA1-CFL1-AS1(G1), pX458-sgRNA2-CFL1-AS1(G2) and pX458-sgRNA3-CFL1-AS1(G3) expression vectors. The mRNA expression level was normalized to β-actin. Data are means±SE of n = 3 independent experiments, each performed in triplicate. ***, p < 0.001, two-tailed t test.
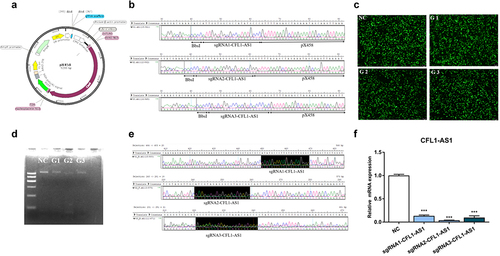
The successfully constructed CFL1-AS1 knockout vectors pX458-sgRNA1-CFL1-AS1(G1), pX458-sgRNA2-CFL1-AS1(G2) and pX458-sgRNA3-CFL1-AS(G3) and the control vector pX458(NC) were transfected into 293T cells. After transfection for 36 h, 293T cells were placed under a fluorescence microscope to observe the expression of green fluorescence. Both the control group (NC) and the experimental groups (G1, G2, G3) had high expression levels of green fluorescent protein (GFP) (), suggesting that the transfection efficiency of the pX458-sgRNA-CFL1-AS1 expression vector was relatively high. The genomic DNA of each transfected group was extracted and identified by PCR, and the PCR products were detected by 2% agarose gel electrophoresis as a band indicating a sequence length of 616 bp. The bands of G1, G2 and G3 in the experimental group were weaker than that of NC in the control group (). The target fragments were amplified by PCR and purified for sequencing. The results showed that the pX458-sgRNA1-CFL1-AS1(G1) and pX458-sgRNA3-CFL1-AS1(G3) groups had a large nested peak, that is, an insertion/deletion mutation, and pX458-sgRNA2-CFL1-AS1(G2) had a single peak without mutation (). The results of RT‒qPCR showed that the expression of CFL1-AS1 in 293T cells was significantly decreased (p < 0.001) (). The constructed pX458-sgRNA-CFL1-AS1 expression vector can be used for subsequent cell experiments to explore the regulatory mechanism after knockdown of CFL1-AS1.
Construction of the CFL1-AS1 overexpression vector
The target fragment was amplified using the designed pcDNA3.1-CFL1-AS1 primers (). The bacterial solutions No. 2 and No. 4 contained massive amounts of the recombinant vector pcDNA3.1-CFL1-AS1, and the extracted plasmids were sent for sequencing (). The sequence obtained by sequencing with the universal primer T7 was compared with the sequence of CFL1-AS1 in NCBI Blast. The sequencing result was completely matched with the target sequence of CFL1-AS1, and the matching result was 100% (). The pcDNA3.1-CFL1-AS1 overexpression vector was successfully constructed.
Figure 6. Construction overexpression vector of CFL1-AS1. (a) the PCR product of CFL1-AS1. (b) the PCR product of pcDNA3.1-CFL1-AS1 DH5α bacterial liquid. (c) Sequence alignment of pcDNA3.1-CFL1-AS1. Query represents the sequence of CFL1-AS1, another is the sequence of pcDNA3.1-CFL1-AS1 sequencing. (d) Expression efficiency of pcDNA3.1-CFL1-AS1 vector in 293T cells. (e) Relative expression levels of CFL1-AS1 mRNA in 293T cell. The mRNA expression level was normalized to β-actin. Data are means±SE of n = 3 independent experiments, each performed in triplicate. ***, p < 0.001, two-tailed t test.
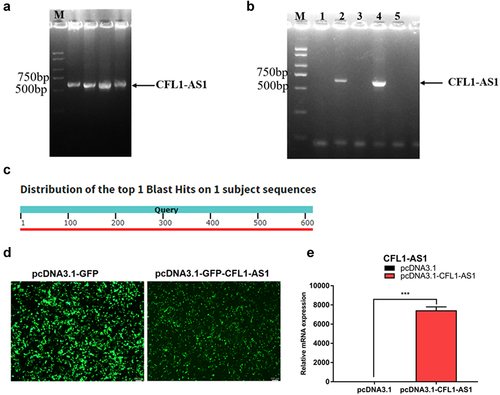
The successfully constructed CFL1-AS1 overexpression vector pcDNA3.1-CFL1-AS1 and the control vector pcDNA3.1 were transfected into 293T cells. After transfection for 36 h, 293T cells were placed under a fluorescence microscope to observe the expression of green fluorescence. The expression of GFP in the control group (pcDNA3.1) and the experimental group (pcDNA3.1-CFL1-AS1) were high (), indicating that the transfection efficiency is higher. RT‒qPCR results showed that the expression of CFL1-AS1 at the mRNA level in the pcDNA3.1-CFL1-AS1 group was significantly increased compared to the control group (p < 0.001) (), and the expression level of CFL1-AS1 was successfully upregulated. The constructed pcDNA3.1-CFL1-AS1 overexpression vector can be used for subsequent cell experiments to explore the regulatory mechanism of CFL1-AS1.
Functional analysis of CFL1-AS1 in 293T cells
NAT-lncRNAs regulate the expression of cognate sense genes. To investigate whether the expression of CFL1-AS1 affects the expression of its cognate sense genes, we studied the expression of CFL1 and CFL2 in 293T cells when CFL1 was upregulated or downregulated. The expression level of CFL1 in 293T cells transfected with pcDNA3.1-CFL1-AS1 was significantly higher (p < 0.001) than that in the pcDNA3.1 group. The expression levels of CFL1 and CFL2 in 293T cells transfected with pX458-sgRNA-CFL1-AS1 (sgRNA-CFL1) were significantly lower (p < 0.001 and p < 0.05, respectively) than that in cells transfected with pX458(NC) (). These results indicate that CFL1-AS1 positively regulates CFL1. The upregulation of CFL1-AS1 promotes the expression of CFL1, and the downregulation of CFL1-AS1 inhibits the expression of CFL1. The CFL2 gene and CFL1-AS1 parent gene CFL1 are closely related to muscle growth and development and play an important role in normal muscle function and regeneration. The expression levels of the myogenic regulatory factors (MRFs) MYOG, MYOD and myosin heavy chain (MYHC) in 293T cells transfected with sgRNA-CFL1-AS1 were significantly lower (p < 0.01) () than those in 293T cells transfected with NC, which indicated that CFL1-AS1 might interact with CFL1 and CFL2 to regulate muscle growth and development.
Figure 7. Functional analysis of CFL1-AS1 in 293T cells. The 293T cells were being transfected with overexpression (pcDNA3.1-CFL1-AS1) or knockout (sgRNA2-CFL1-AS1 and sgRNA3-CFL1-AS1) vectors for 36 h. (a) Real-time PCR analysis of expression of target genes CFL1 and CFL2. (b) Real-time PCR analysis of expression of myogenic markers MYOD, MYOG, and MYHC. (c) Real-time PCR analysis of expression of proliferation marker gene CCND1 and PCNA, apoptosis marker genes Bcl-2 and Caspase9 (d). (e-f) the mRNA levels of Belin1, p53, LC3 and Sod2 were analysed by qRT-PCR. The mRNA expression level was normalized to β-actin. The relative mRNA expression levels of control cell were considered as 1. The control group was transfected with pcDNA3.1 or pX458(NC) vector. Data are means±SE of n = 3 independent experiments, each performed in triplicate. *, p < 0.05, **, p < 0.01, ***, p < 0.001, two-tailed t test.
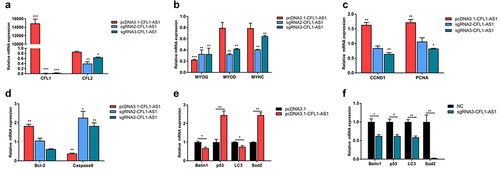
RT‒qPCR showed that PCNA and CCND1 were significantly upregulated (p < 0.01) when CFL1-AS1 was overexpressed, and PCNA and CCND1 were significantly downregulated (p < 0.05) when CFL1-AS1 was knocked down in the sgRNA3-CFL1-AS1 group (). Bcl-2 was significantly upregulated (p < 0.01) and caspase9 was significantly downregulated (p < 0.01) when CFL1-AS1 was overexpressed, and caspase9 was significantly upregulated (p < 0.05) when CFL1-AS1 was knocked down (). When CFL1-AS1 was overexpressed, p53 and Sod2 mRNA levels were increased (p < 0.01) (), and when CFL1-AS1 was knocked down, p53 and Sod2 mRNA levels were decreased (p < 0.05) (). These results indicated that CFL1-AS1 promoted cell proliferation, inhibited cell apoptosis and participated in autophagy, suggesting that CFL1-AS1 May be involved in the processes of cell growth and development.
Functional analysis of CFL1-AS1 in C2C12 cells
Myoblast C2C12 is a widely used to study the muscle cell proliferation and differentiation of cell lines. The successfully constructed CFL1-AS1 overexpression vector and knockout expression vectors were transfected into C2C12 cells. After transfection for 36 h, C2C12 cells were placed under a fluorescence microscope to observe the expression of green fluorescence. The normal expression of GFP in the control group (pcDNA3.1/NC) and the experimental group (PCDNA3.1-CFL1-AS1/sgRNA-CFL1-AS1) () indicated successful transfection. RT‒qPCR showed that the expression of CFL1-AS1 at the mRNA level in the pcDNA3.1-CFL1-AS1 group was significantly increased compared to the control group (p < 0.001), and the expression of CFL1-AS1 at the mRNA level in the sgRNA-CFL1-AS1 group was significantly decreased (p < 0.01) ().
Figure 8. Functional analysis of CFL1-AS1 in C2C12 cells. The C2C12 cells were being transfected with overexpression (pcDNA3.1-CFL1-AS1) or knockout (sgRNA2-CFL1-AS1 and sgRNA3-CFL1-AS1) vectors for 36 h. (a) Expression efficiency of CFL1-AS1 vector in C2C12 cells. (b) Relative expression levels of CFL1-AS1 in C2C12 cell. (c) Real-time PCR analysis of expression of target genes CFL1 and CFL2. (d) Real-time PCR analysis of expression of myogenic markers MYOG, MYOD, and MYHC. (e) Relative mRNA expression levels of CCND1 and Caspase9 in C2C12 cell. The mRNA expression level was normalized to β-actin. The relative mRNA expression levels of control cell were considered as 1. The control group was transfected with pcDNA3.1 or pX458(NC) vector. Data are means±SE of n = 3 independent experiments, each performed in triplicate. *, p < 0.05, **, p < 0.01, ***, p < 0.001, two-tailed t test.
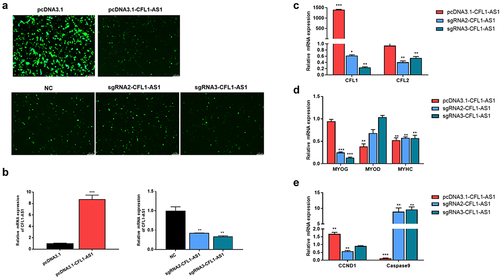
RT‒qPCR showed that when CFL1-AS1 was upregulated, the mRNA level of parent gene CFL1 was significantly increased (p < 0.001), while CFL2 had no significant change. When CFL1-AS1 was downregulated, the mRNA expression of parent gene CFL1 was significantly decreased (p < 0.05), CFL2 was significantly decreased (p < 0.05) (), and myogenic regulatory factors MYOG and MYHC were also significantly decreased (p < 0.01) (). CCND1 was significantly upregulated (p < 0.01) and caspase9 was significantly downregulated (p < 0.001) when CFL1-AS1 was overexpressed. CCND1 was significantly downregulated (p < 0.01) and caspase9 was significantly upregulated (p < 0.01) when CFL1-AS1 was knocked down (). These results further demonstrated that CFL1-AS1 positively regulates CFL1, promotes cell proliferation, and inhibits cell apoptosis, suggesting that CFL1-AS1 is involved in the process of cell growth and development.
Discussion
At present, most NATs are lncRNAs, which can directly interact with sense strand transcripts and regulate the expression of sense strand genes at the transcriptional and epigenetic levels, thus participating in various biological processes, which is of great significance to the growth and development of organisms [Citation21,Citation22]. Studies have shown that approximately 20% of the genes in the human cell transcriptome can produce NATs [Citation23], and more than 70% of the transcription units in the mouse genome can produce antisense transcripts [Citation3,Citation24]. At present, there are many studies on the relationship between NATs and their sense strand transcripts in mammals, such as p15AS [Citation21] and EVA1A-AS [Citation25], while there are few related studies in livestock, such as studies of FUT3-AS1 [Citation26], IGF2 AS [Citation27] and LncNONO-AS [Citation28]. The wide expression of NATs in species and their characteristics in the antisense strand of coding genes suggest that they play an important role in the regulation of gene expression. The study of gene NATs has become a research hotspot to explain the molecular mechanism of the formation of important economic traits in livestock and poultry. Therefore, the analysis of the regulatory role of NATs in the process of livestock muscle development can not only supplement the theory of the regulatory role of lncRNAs in animal muscle but also provide a theoretical basis for bovine muscle development and the development of molecular markers for livestock.
Many long noncoding RNAs have conserved secondary structures [Citation29], splicing forms and subcellular localization [Citation30]. This conservation and specificity indicate that they are functional. However, the function of lncRNAs is more difficult to determine than that of microRNAs and proteins because their functions cannot be speculated only based on sequence or structure. In many ways, lncRNAs are similar to mRNAs that encode proteins; for example, they both contain sites for alternative splicing. However, lncRNAs have low transcriptional levels and are less conserved in the middle of their sequences [Citation31]. Nevertheless, a series of functional elements in the sequence of lncRNA molecules and secondary structures have been confirmed. LncRNAs regulate gene function in various ways [Citation32], with one of the most important ways being binding to the target gene by base complementary pairing to directly regulate the transcription and translation of the target gene or indirectly regulate the transcription and translation of the upstream or downstream target gene [Citation33]. The basis of base pairing is the primary structure of lncRNAs. The secondary structure of lncRNAs, such as stem loops and hairpins formed by posttranscriptional modification, allows for interaction with proteins and plays a very important role in the broad function of lncRNAs [Citation34]. The function and scope of lncRNA-mediated regulation in animals are receiving increasing attention [Citation35]. LncRNAs at different positions in the genome play different roles. The expression of noncoding RNAs that are transcribed in the opposite direction of protein-coding genes is often positively or negatively correlated with their cognate sense gene.
NATs regulate the coding potential of sense RNAs by direct antisense-sense RNA interaction or by encoding proteins coexpressed with sense strand RNA to regulate sense transcripts. The effect of translated transcripts on sense transcripts is manifested in the following two aspects: one is negative feedback regulation or inconsistent regulation, in which antisense transcripts silence or inhibit homologous sense RNAs or proteins through negative regulation [Citation21]; the other is positive feedback regulation or concerted regulation, in which sense and reverse transcript coexpression and antisense transcripts increase sense RNA levels or corresponding protein levels [Citation36,Citation37]. In our study, CFL1-AS1 localization mainly existed in the cytoplasm. Overexpression of CFL1-AS1 promoted the upregulation of CFL1 mRNA, while knockdown of CFL1-AS1 inhibited the expression of CFL1 mRNA, suggesting that CFL1-AS1 May positively regulate CFL1. In addition to serving as cis-antisense transcripts, lncRNAs also function through trans-acting mechanisms in animals and can regulate multiple targets [Citation38]. DsrA, previously known as an 87 nt noncoding RNA containing sequences with complementary regions to at least five different genes in E. coli, has been shown to regulate two of those five genes, hns and rpoS, through RNA‒RNA interactions [Citation39]. Our results show that CFL1-AS1 can regulate the expression of target genes, such as CFL1 and CFL2. Therefore, the trans-acting mechanism of noncoding RNA seems to be able to regulate multiple targets.
In this study, we first found a new natural antisense transcript of the CFL1 gene in cattle named CFL1-AS1. Furthermore, we found that bovine CFL1-AS1 encodes a polypeptide of 195 AA residues. It is transcribed at a different position from the CFL1 gene (an intermediate position), and the sequence is conserved, suggesting that its function may be similar to those of NATs of the CFL1 gene in other species. NAT transcript sequences partially or completely overlap and complement mRNA, promoter or regulatory regions of coding genes and participate in gene expression regulation at the transcriptional and posttranscriptional levels [Citation40]. CFL1 and CFL2 play an important role in the growth and differentiation of skeletal muscle [Citation18]. We predicted that the sequence of CFL1-AS1 can complement the partial sequences of CFL1 and CFL2, suggesting that CFL1-AS1 May regulate the development and differentiation of skeletal muscle by affecting the expression of CFL1 and CFL2. Secondary structure prediction analysis by the RNAfold network server showed that CFL1-AS1 has many stem‒loop motifs, indicating that CFL1-AS1 has the ability to bind to proteins. However, further research and investigation are needed to determine the specific regulatory mechanism.
Regulation of the actin cytoskeleton seems to be related to regulation of cell proliferation [Citation41,Citation42]. To study whether CFL1-AS1 plays a role in cell proliferation, apoptosis and other developmental growth processes, we detected the mRNA levels of proliferating cell antigen PCNA, cyclin CCND1, anti-apoptotic protein Bcl-2 and caspase9 when CFL1-AS1 was overexpressed or knocked down. PCNA is indispensable for DNA replication, and it induces cell cycle progression [Citation43,Citation44]. Bcl-2 can inhibit apoptosis by a mitochondria-dependent caspase9 pathway [Citation45]. When CFL1-AS1 was overexpressed, PCNA, CCND1 and Bcl-2 were significantly upregulated, and caspase9 was significantly downregulated (p < 0.05); when CFL1-AS1 was downregulated, PCNA, CCND1 and Bcl-2 were significantly downregulated, and caspase9 was significantly upregulated (p < 0.05). These results indicate that CFL1-AS1 is involved in cell growth, development and proliferation. However, the mechanism by which CFL1-AS1 regulates cell growth and development needs further analysis and exploration.
Conclusion
The present study demonstrated that CFL1-AS1, an antisense transcript located within the CFL1 gene body, can positively regulate the expression of CFL1 and participate in cell proliferation, apoptosis and autophagy. These findings lay a foundation for further exploration of the functional mechanism of the CFL1-AS1 molecule to enrich our understanding of how antisense transcripts regulate their sense genes.
Supplemental Material
Download Zip (51 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15592294.2023.2231707.
Additional information
Funding
References
- Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet. 2014;15(1):7–14. doi: 10.1038/nrg3606
- Khorkova O, Myers AJ, Hsiao J, et al. Natural antisense transcripts. Hum Mol Genet. 2014;23(R1):R54–63. doi: 10.1093/hmg/ddu207
- Katayama S, Tomaru Y, Kasukawa T, et al. Antisense transcription in the mammalian transcriptome. Science. 2005;309(5740):1564–1566. doi: 10.1126/science.1112009
- Tufarelli C, Stanley JA, Garrick D, et al. Transcription of antisense RNA leading to gene silencing and methylation as a novel cause of human genetic disease. Nat Genet. 2003;34:157–165. doi: 10.1038/ng1157
- Cheng H, Zhang ES, Shi X, et al. A novel ATM antisense transcript ATM-AS positively regulates ATM expression in normal and breast cancer cells. Curr Med Sci. 2022;42:681–691. doi: 10.1007/s11596-022-2585-5
- Ren L, Fang X, Shrestha SM, et al. LncRNA SNHG16 promotes development of oesophageal squamous cell carcinoma by interacting with EIF4A3 and modulating RhoU mRNA stability. Cell Mol Biol Lett. 2022;27(1):89. doi: 10.1186/s11658-022-00386-w
- Faghihi MA, Modarresi F, Khalil AM, et al. Expression of a noncoding RNA is elevated in Alzheimer’s disease and drives rapid feed-forward regulation of beta-secretase. Nat Med. 2008;14:723–730. doi: 10.1038/nm1784
- Wang C, Yu H, Lu S, et al. LncRNA Hnf4αos exacerbates liver ischemia/reperfusion injury in mice via Hnf4αos/Hnf4α duplex-mediated PGC1α suppression. Redox Biol. 2022;57:102498. doi: 10.1016/j.redox.2022.102498
- Vujovic F, Rezaei-Lotfi S, Hunter N, et al. The fate of notch-1 transcript is linked to cell cycle dynamics by activity of a natural antisense transcript. Nucleic Acids Res. 2021;49(18):10419–10430. doi: 10.1093/nar/gkab800
- Yuan XH, Li J, Cao Y, et al. Long non-coding RNA AFAP1-AS1 promotes proliferation and migration of gastric cancer by downregulating KLF2. Eur Rev Med Pharmacol Sci. 2020;24(2):673–680. doi: 10.26355/eurrev_202001_20044
- Zhang CL, Zhu KP, Ma XL. Antisense lncRNA FOXC2-AS1 promotes doxorubicin resistance in osteosarcoma by increasing the expression of FOXC2. Cancer Lett. 2017;396:66–75. doi: 10.1016/j.canlet.2017.03.018
- Alfano G, Vitiello C, Caccioppoli C, et al. Natural antisense transcripts associated with genes involved in eye development. Hum Mol Genet. 2005;14(7):913–923. doi: 10.1093/hmg/ddi084
- Watanabe T, Totoki Y, Toyoda A, et al. Endogenous siRnas from naturally formed dsRnas regulate transcripts in mouse oocytes. Nature. 2008;453(7194):539–543. doi: 10.1038/nature06908
- Wang Y, Xue S, Liu X, et al. Analyses of long non-coding RNA and mRNA profiling using RNA sequencing during the pre-implantation phases in pig endometrium. Sci Rep. 2016;6(1):20238. doi: 10.1038/srep20238
- Wang Z, Xu H, Li T, et al. Chicken GHR antisense transcript regulates its sense transcript in hepatocytes. Gene. 2019;682:101–110. doi: 10.1016/j.gene.2018.10.001
- Lin S, Zhang Z, Xie T, et al. Identi?cation of a novel antisense RNA that regulates growth hormone receptor expression in chickens. RNA Biol. 2019;16(5):626–638. doi: 10.1080/15476286.2019.1572440
- Sun Y, Ma Y, Zhao T, et al. Epigenetic regulation mechanisms of the cofilin-1 gene in the development and differentiation of bovine primary myoblasts. Genes (Basel). 2022;13(5). doi: 10.3390/genes13050723
- Sun Y, Ma Y, Wu X, et al. Functional and comparative analysis of two subtypes of cofilin family on cattle myoblasts differentiation. Agriculture. 2022;12(9):1420. doi: 10.3390/agriculture12091420
- Song C, Wang J, Ma Y, et al. Linc-smad7 promotes myoblast differentiation and muscle regeneration via sponging miR-125b. Epigenetics. 2018;13(6):591–604. doi: 10.1080/15592294.2018.1481705
- Scotto-Lavino E, Du G, Frohman MA. 5’ end cDNA amplification using classic RACE. Nat Protoc. 2006;1:2555–2562. doi: 10.1038/nprot.2006.480
- Yu W, Gius D, Onyango P, et al. Epigenetic silencing of tumour suppressor gene p15 by its antisense RNA. Nature. 2008;451(7175):202–206. doi: 10.1038/nature06468
- Parnigoni A, Caon I, Teo WX, et al. The natural antisense transcript HAS2-AS1 regulates breast cancer cells aggressiveness independently from hyaluronan metabolism. Matrix Biol. 2022;109:140–161. doi: 10.1016/j.matbio.2022.03.009
- Chen J, Sun M, Kent WJ, et al. Over 20% of human transcripts might form sense-antisense pairs. Nucleic Acids Res. 2004;32:4812–4820. doi: 10.1093/nar/gkh818
- Carninci P, Kasukawa T, Katayama S, et al. The transcriptional landscape of the mammalian genome. Science. 2005;309(5740):1559–1563. doi: 10.1126/science.1112014
- Niehus SE, Allister AB, Hoffmann A, et al. Myc/Max dependent intronic long antisense noncoding RNA, EVA1A-AS, suppresses the expression of Myc/Max dependent anti-proliferating gene EVA1A in a U2 dependent manner. Sci Rep. 2019;9(1):17319. doi: 10.1038/s41598-019-53944-2
- Wu Z, Fan H, Jin J, et al. Insight into mechanisms of pig lncRNA FUT3-AS1 regulating E. coli F18-bacterial diarrhea. PLOS Pathog. 2022;18(6):e1010584. doi: 10.1371/journal.ppat.1010584
- Song C, Yang Z, Jiang R, et al. lncRNA IGF2 as regulates bovine myogenesis through different pathways. Mol Ther Nucleic Acids. 2020;21:874–884. doi: 10.1016/j.omtn.2020.07.002
- Zhao Z, Qiao L, Dai Z, et al. LncNONO-AS regulates AR expression by mediating NONO. Theriogenology. 2020;145:198–206. doi: 10.1016/j.theriogenology.2019.10.025
- Johnsson P, Lipovich L, Grandér D, et al. Evolutionary conservation of long non-coding RNAs; sequence, structure, function. Biochim Biophys Acta. 2014;1840(3):1063–1071. doi: 10.1016/j.bbagen.2013.10.035
- Chen LL. Linking long noncoding RNA localization and function. Trends Biochem Sci. 2016;41(9):761–772. doi: 10.1016/j.tibs.2016.07.003
- Kour S, Rath PC. Long noncoding RNAs in aging and age-related diseases. Ageing Res Rev. 2016;26:1–21. doi: 10.1016/j.arr.2015.12.001
- Statello L, Guo CJ, Chen LL, et al. Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol. 2021;22(2):96–118. doi: 10.1038/s41580-020-00315-9
- Yoon JH, Abdelmohsen K, Gorospe M. Posttranscriptional gene regulation by long noncoding RNA. J Mol Biol. 2013;425(19):3723–3730. doi: 10.1016/j.jmb.2012.11.024
- Novikova IV, Hennelly SP, Sanbonmatsu KY. Sizing up long non-coding RNAs: do lncRnas have secondary and tertiary structure? Bioarchitecture. 2012;2:189–199. doi: 10.4161/bioa.22592
- Ma Y, Zhao T, Wu X, et al. Expression profile and functional prediction of novel LncRNA 5.8S rRNA-OT1 in cattle. Anim Biotechnol. 2022;1–11. doi: 10.1080/10495398.2022.2066540
- Zhao F, Li S, Liu J, et al. Long non-coding RNA TRIM52-AS1 sponges microRNA-577 to facilitate diffuse large B cell lymphoma progression via increasing TRIM52 expression. Hum Cell. 2022;35(4):1234–1247. doi: 10.1007/s13577-022-00725-1
- Zhu L, Wei Q, Qi Y, et al. PTB-AS, a novel natural antisense transcript, promotes glioma progression by improving PTBP1 mRNA stability with SND1. Mol Ther. 2019;27(9):1621–1637. doi: 10.1016/j.ymthe.2019.05.023
- Li JT, Zhang Y, Kong L, et al. Trans-natural antisense transcripts including noncoding RNAs in 10 species: implications for expression regulation. Nucleic Acids Res. 2008;36(15):4833–4844. doi: 10.1093/nar/gkn470
- Lease RA, Belfort M. A trans-acting RNA as a control switch in Escherichia coli: DsrA modulates function by forming alternative structures. Proc Natl Acad Sci U S A. 2000;97(18):9919–9924. doi: 10.1073/pnas.170281497
- Cabili MN, Trapnell C, Goff L, et al. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 2011;25(18):1915–1927. doi: 10.1101/gad.17446611
- Bozal-Basterra L, Gonzalez-Santamarta M, Muratore V, et al. LUZP1 controls cell division, migration and invasion through regulation of the actin cytoskeleton. Front Cell Dev Biol. 2021;9:624089. doi: 10.3389/fcell.2021.624089
- Asfour HA, Allouh MZ, Said RS. Myogenic regulatory factors: the orchestrators of myogenesis after 30 years of discovery. Exp Biol Med (Maywood). 2018;243(2):118–128. doi: 10.1177/1535370217749494
- Juríková M, Danihel Ľ, Polák Š, et al.: Ki67, PCNA, and MCM proteins: markers of proliferation in the diagnosis of breast cancer. Acta Histochem 2016, 118:544–552.
- Belső N, Gubán B, Manczinger M, et al. Differential role of D cyclins in the regulation of cell cycle by influencing Ki67 expression in HaCaT cells. Exp Cell Res. 2019;374(2):290–303. doi: 10.1016/j.yexcr.2018.11.030
- McNutt MC, Lagace TA, Horton JD. Catalytic activity is not required for secreted PCSK9 to reduce low density lipoprotein receptors in HepG2 cells. J Biol Chem. 2007;282(29):20799–20803. doi: 10.1074/jbc.C700095200