ABSTRACT
Cattle skeletal muscle development is a complex and highly coordinated biological process mediated by a series of myogenic regulators, which plays a critical role in beef yield and quality. Long non-coding RNAs (lncRNAs) have been shown to regulate skeletal muscle development. However, the molecular mechanism by which lncRNAs regulate skeletal muscle development is largely unknown. We performed transcriptome analysis of muscle tissues of adult and embryo Angus cattle to investigate the mechanism by which lncRNA regulates skeletal muscle development between adult and embryo cattle. A total of 37,115 candidate lncRNAs were detected, and a total of 1,998 lncRNAs were differentially expressed between the muscle tissue libraries of adult and embryo cattle, including 1,229 up-regulated lncRNAs and 769 down-regulated lncRNAs (adult cattle were the control group). We verified the expression of 7 differentially expressed lncRNAs by quantitative real-time PCR (RT-qPCR), and analysed the tissue expression profile of lnc000100, which is down-regulated in the longest dorsal muscle during foetal life and which is highly specifically expressed in muscle tissue. We found that the interference of lnc000100 significantly inhibited cell proliferation and promoted cell differentiation. Lnc000100 was located in the nucleus by RNA-FISH. Our research provides certain resources for the analysis of lncRNA regulating cattle skeletal muscle development, and may also provide new insights for improving beef production and breed selection.
Highlight
Identification of lncRNAs associated with muscle development and skeletal muscle disease that are differentially expressed between embryo and adult cattle. We identified 1,998 differentially expressed lncRNAs between the muscle tissue libraries of adult and embryo. GO analysis showed that these lncRNAs were involved in muscle development.
Construction of co-expression networks and competitive endogenous networks related to muscle development. We constructed the co-expression networks and lncRNA-miRNA-mRNA interaction networks of four differentially expressed lncRNAs.
A newly identified lncRNA lnc000100 promoted myoblast proliferation and inhibited myoblast differentiation during muscle development. GO analysis showed that lnc000100 was associated with muscle development (such as muscle structure development, etc.) and skeletal muscle diseases (such as muscle hypertrophy, etc.). FISH analysis suggests that lnc000100 is localized in the nucleus and may regulate muscle development at the transcriptional/post-transcriptional level.
KEYWORDS:
Introduction
In animal husbandry, the development of skeletal muscle determines the quality and quantity of meat [Citation1,Citation2]. Skeletal muscle development is a highly complex and coordinated biological process, involving the proliferation and differentiation of myoblasts at various developmental stages. The growth and development of skeletal muscle begin in embryonic mesoderm. After the mesenchymal stem cells derived from embryonic mesoderm differentiate into myoblasts, myoblasts multiply and differentiate to form muscle tubes [Citation3], which further mature to form muscle fibres. Eventually, muscle fibres grow and mature into skeletal muscle [Citation4,Citation5], this process depends on the close coordination of coding gene and non-coding RNAs, such as myogenic regulatory factor (MRF) [Citation3,Citation6], the paired box protein 3/7 (Pax3/7) and myocyte enhancer factor 2 (MEF2) family [Citation7,Citation8]. However, there are still a lot of unknown areas in the research of muscle development in livestock, and some new regulatory factors and their mechanisms of regulating meat quality need to be further explored. This, in turn, will improve the meat quality and yield of farm animals, promote genetic improvement of livestock and poultry meat, and contribute to the food value of livestock products.
A large number of non-coding RNAs have been reported to have important roles in skeletal muscle development, including microRNA (miRNA) [Citation9], long non-coding RNA (lncRNA) [Citation10] and circular RNAs (circRNA) [Citation11]. LncRNA plays a variety of roles in multilevel regulation of skeletal muscle generation and regeneration [Citation12–15]. In 2002, Okazaki described lncRNAs for the first time in large-scale sequencing of mouse full-length cDNA libraries [Citation16]. LncRNAs localized to different subcellular components regulate gene expression at different levels through epigenetic, transcriptional and post-transcriptional levels. For instance, lncRNAs act as ‘molecular scaffolds,’ decoys, guide molecules and signalling molecules to activate or inhibit transcription [Citation17]. In addition, lncRNA can act as a competitive endogenous RNAs (ceRNA) to isolate miRNA from its target messenger RNA (mRNA) and participate in the regulation of muscle development [Citation15,Citation18–21]. IGF2-AS [Citation22] and lncMGPF [Citation13] are well-known ceRNAs, which bind to miR-503 and miR-135a-5p respectively, indicating that they play an important role in post transcriptional regulation. However, there are still a large number of unknown lncRNAs in studies on muscle development in livestock.
Angus cattle are known for their superior meat quality and are one of the best sources of beef cattle. Angus cattle are high yielding, with excellent carcass quality and marbled muscle. It also has the characteristics of fast growth, early maturity, easy fattening and easy mating. The objective of this study was to identify key lncRNA candidates during bovine muscle development and to explore the role of lncRNA in the proliferation and differentiation of bovine myoblasts. These results may provide valuable transcriptional regulatory resources for understanding the mechanisms of bovine muscle development and may provide new insights for improving beef production.
Materials and methods
Sample preparation
Six longissimus dorsi muscles of Angus cattle were selected from adult (24 months of age, n = 3) and foetal (3 months of age, n = 3) for transcriptomic sequencing and RT-qPCR analysis. The cattle used in this experiment were obtained from a local slaughterhouse in Xi’an, China. Longissimus dorsi tissue is collected when cattle are unintentionally slaughtered. Foetal longissimus dorsi, liver, lung, kidney, and leg muscles were also collected during the slaughter of adult cattle. The samples collected were washed in saline, rinsed in 75% alcohol and washed in saline again. Subsequently, the samples were packed into labelled lyophilization tubes and stored in liquid nitrogen. Total RNA was extracted from liver, lung, kidney, longissimus dorsi muscle and leg muscle. This experimental animal care research programme has been approved by the Animal Care Professional Committee of the College of Animal Science and Technology of Guangxi University.
Library preparation and Illumina sequencing
Total RNA was extracted from longissimus dorsi muscle samples of 3 foetal and 3 adult Angus cattle using Takara RNAiso Plus and determined by gel electrophoresis. Those with a RIN of 7 or more were accepted for library preparation. The specific RIN values are shown in Table S1. Quantification was performed by NanoDrop spectrophotometer (NanoDrop, Wilmington, USA) and Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). Ribosomal RNA was removed by a probe prior to the construction of RNA-seq libraries, cDNA library preparation and Illumina sequencing were performed as in previous researches [Citation23].
Identification of lncRNAs
Potential lncRNAs were filtered using the following highly rigorous criteria: (1) Transcript length not less than 200 bp. (2) Transcripts with more than two exons. (3) Cuffcompare software was used to screen out transcripts that overlap with the exon area of database annotations. (4) Cuffquant calculated the expression level of each transcript, and selected transcripts with FPKM ≥ 0.5. (5) Coding potential was predicted by CNCI, CPC, PFAM and phyloCSF software, and the software parameters are defaulted.
GO and KEGG pathway analysis
Gene ontology (GO) analysis (http://www.geneontology.org) was performed to explore potential functions of lncRNA-hosting genes. Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis (http://www.kegg.jp) was performed to analyse the biological pathways involved in host genes. The GO and KEGG pathways with q-value <0.05 were considered significant.
Co-expression and CeRNA network analysis
LncRNA can cis-regulate the expression of adjacent genes. The co-expression networks of candidate lncRNAs and their upstream or downstream 100 kb mRNA were constructed. According to the miRNA binding sites detected in mRNA and lncRNA sequences, three lncRNA-miRNA-mRNA interaction networks were constructed. The TargetScan (http://www.targetscan.org/vert_71/) and miRbase (https://www.mirbase.org/) was used to predict the miRNA – mRNA and miRNA – lncRNA interaction.
Quantitative real-time PCR (RT-qPCR)
Total RNA was extracted using Trizol reagent (TaKaRa, Dalian, China) from longissimus dorsi muscle, liver, lung, kidney, leg and muscle cells, and cDNA was obtained by reverse transcription using HiScript R II One Step kit (Vazyme, Nanjing, China). RT-QPCR was performed on three biological replicates using ChamQ SYBR qPCR Master Mix (Vazyme, Nanjing, China). The internal control was β-actin, and the relative expression of RT-qPCR data was analysed by 2−ΔΔCt [Citation24]. All primer sequences are shown in Table S2.
Cell culture, treatments and transfection
Primary muscle cells were isolated from longissimus dorsi muscle (about 15 cm long) of embryo Angus cattle (3 months old). Bovine muscle primary cells were isolated and cultured by the digestion method of collagenase (TyPeI). The skin of longissimus dorsi muscle of foetal bovine was cut open, the fascia was removed, and the muscle was cut and washed with PBS containing 2% penicycin, 75% alcohol and 1% penicycin. The tissue was cut and added 0.2% Roche type I collagenase, twice the volume, digested at 37°C for 1 h. After centrifugation for 5 min, the supernatant was discarded and appropriate amount of 0.25% pancreatin was added for digestion at 37°C for 25 min. After centrifugation for 6 min, the supernatant was discarded, the primary medium was added, and the cells were re-suspended and cultured in an incubator at 37°C and 5% CO2. After 2 hours of culture in the cell incubator, the cell fluid was transferred to a new culture dish for further culture. According to the requirements of CCK-8, EdU, WB, qPCR, immunofluorescence and other different tests, the cells were inoculated into different well plates, and cultured in medium containing 10% foetal bovine serum and 1% double antibody. The lnc_000100 was interfered and si-lnc_000100 was sent to Guangzhou Ruibo Biological Technology Co., Ltd. for synthesis. Si-lnc_000100 was transfected with liP2000 (11668–019, Invitrogen, USA) when the muscle cell fusion reached 30–50%. When the convergence of muscle cells in growth medium (GM) reached 80%, DMEM containing 2% horse serum (Gibco, MA, USA) was used instead of growth medium to induce cell differentiation.
CCK-8 and EdU assays
Bovine skeletal muscle cells were inoculated in 96-well plates and transfected with si- lnc000100 when the cell density reached 30%-50%. The medium was changed to complete medium after 4 hours and the cells were incubated in the incubator until the cell density was approximately 80% and the cells were observed to increase in value (approximately 24-36 h). The proliferation status of bovine skeletal muscle cells was studied using the Cell Counting Kit-8 (CCK-8) (Tiande, Beijing, China) and the Cell-Light EdU Apollo 567 in vitro imaging kit (RiboBio, Guangzhou, China). Detailed procedures are described in the manufacturer’s instructions. The results were observed and photographed under fluorescence microscope.
Fluorescence in situ hybridization
Fluorescence in situ hybridization (FISH) was performed using an in situ hybridization reagent (Servicebio, Wuhan, China). Cattle myoblasts were cultured on slides and incubated at room temperature with FISH fixation solution for 30 min. The cells were incubated with Triton X-100 to penetrate the cell membrane and then incubate with pre-hybrid solution at 37°C for 1 h. The fixed cell hybrid solution and fluorescently labelled lnc000100 probe were then placed in a black wet box and incubated overnight at 4°C. On the second day, the cells were washed with PBS solution for 5 times, and then 4 ,’ 6-diamino-2-phenyllindo (DAPI, 1:1000) solution was added and incubated in the dark at room temperature for 5 min. All of the above processes were performed in order to keep the cells moist. Finally, the cells were observed using a fluorescence microscope (Nikon, Tokyo, Japan) and fluorescent photographs were taken.
Western blot analysis
Protein was extracted from cell samples using RIPA (Radio Immuno Precipitation Assay) lysate buffer containing 1% PMSF (Solarbio, Beijing, China). Protein concentrations were determined with a BCA kit (Beyotime, Shanghai, China). The proteins were isolated by electrophoresis with 10% SDS-polyacrylamide gel (Bio-Rad, Hercules, CA, USA) and transferred to polyvinylidene fluoride membranes. The membrane was incubated with CDK2, PCNA, MyoD, MyHC, MyoG and β-actin (Abcam, Cambridge, MA, USA) specific primary antibodies, and then with the corresponding secondary antibodies. The membranes were exposed to ECL Plus (Solarbio, Beijing, China) and the images generated were captured with the ChemiDoc XRS+ system (Bio-Rad, California, USA). We used Image J software for WB grey-scale value analysis without normalization.
Immunofluorescence staining
Primary cattle myoblasts differentiated for four days were washed with PBS (pH 7.4) for three times, and then fixed with 4% paraformaldehyde for 30 min. The cells were then infused with 0.5% Triton X-100 for 10 min, incubated overnight with MyoD antibody (Wanlei, Shen yang, China) at 4°C, and then dilute goat anti-mouse IgG (H&L)-Alexa Fluor 594 (1:500; RS3608, Immunoway, USA) with 1% cattle serum albumin. The nuclei were stained with DAPI. Finally, the cells were rinsed three times with PBS and observed under a fluorescence microscope (DM5000B, Leica, Germany).
Statistical analysis
All data were expressed as mean ± SEM. The difference between the groups was determined by the multiple t test of GraphPad prism v6.01 software. The corrected p value < 0.05 was a significant difference, with statistical significance.
Data availability
The data that supports the findings of this study are available in the supplementary material of this article.
Result
Expression profile of lncRNA in skeletal muscle of Angus cattle
By ribosomal RNA-seq analysis of embryonic and adult bovine longissimus dorsi muscle samples (Figure S1a), (), 476 known lncRNAs, as well as 25,537 new lncRNAs with no coding potential and 11,102 new lncRNAs with coding potential (TUCP) transcripts were identified (Figure S1b, Table S3). In order to identify lncRNAs with potential functions in cattle muscle development, we conducted statistics on the lncRNAs found. We found that 19 lncRNAs were specifically expressed in the embryo library and 317 in the adult library (Figure S1c). Statistical results showed that 44.9% of lncRNAs were derived from intergenic regions and 48.7% from introns, but only a few lncRNAs (6.4%) were antisense lncRNAs (Figure S1d).
Table 1. Summary of reads mapping to the Bos taurus reference genome.
Genomic characterization of lncRNA
Previous studies have shown that lncRNAs are shorter in length than protein-encoded transcripts. As shown in the Figure S2, the mean length of lncRNA transcripts (1,608 nucleotides) was smaller than the mean length of mRNA (2,470 nucleotides) (Figure S2a). In addition, lncRNA had fewer exons (about 4.17) than protein-coding genes (about 11.25) (Figure S2b). LncRNA had an average ORF length of about 210 nt and an mRNA of 605nt (Figure S2C). The average expression level of lncRNA without coding potential was 3.94 (FPKM), and that of lncRNA with coding potential was 2.06 (FPKM) (Figure S2d). Compared with the mRNA expression, lncRNA expression was lower, which was consistent with the general characteristics of lncRNA (Figure S2d). As shown, the date of lncRNA showed a good correlation between embryo and adult muscle samples in cattle (Figure S2e).
Differentially expressed lncRNAs
We identified 1,963 differentially expressed mRNA (p < 0.05) () and 1,998 differentially expressed lncRNA (p < 0.05) () in Angus cattle embryo and adult libraries, of which 496 have coding potential and 1,502 have no coding potential. All differentially expressed lncRNAs were listed in Table S4. Compared with the muscle tissue of adult cattle, 1,229 lncRNAs were up-regulated and 769 lncRNAs were down-regulated (adult cattle were the control group) ( Tables S4). Genes tend to be distributed regularly on chromosomes, and some genes that are closer to each other on chromosomes may perform similar biological functions. shows the chromosomal distribution of the differentially expressed transcripts. In order to further clarify the potential function of lncRNAs, we conducted cluster analysis of differentially expressed lncRNAs. showed the abundance of maternal gene transcripts with no coding potential and lncRNAs with coding potential (TUCP) respectively. The GO analysis results of lncRNAs with () and without () coding potential are shown in the figure. In addition to involving with general metabolic processes, differentially expressed lncRNAs and their target genes are also enriched in skeletal muscle contraction, muscle cell development, striated muscle contraction and other pathways related to muscle generation, as well as pathways related to skeletal muscle diseases (dilated cardiomyopathy, hypertrophic cardiomyopathy). LncRNAs may regulate the transcription of distant genes by cis regulation of adjacent genes or trans regulation. The top 20 KEGG pathways in which lncRNA is enriched through cis and trans regulation are shown in the figure (.
Figure 1. Differentially expressed lncRNAs.
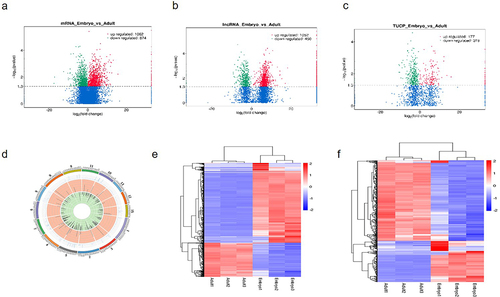
Figure 2. GO and KEGG analysis of differentially expressed lncRNAs.
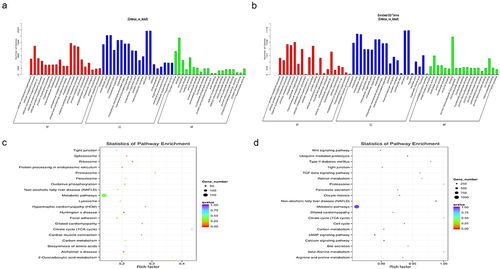
Table 2. Differentially expressed lncRNAs in cattle muscle tissues.
Co-expression and ceRNAs network
Researches have shown that lncRNAs are involved in epigenetic or transcriptional regulation of adjacent genes in a cis or trans manner. In order to further explore the cis regulatory relationship of lncRNAs, co-expression networks were constructed for adjacent coding genes of 100 kb upstream and downstream of candidate lncRNAs. The four differentially expressed lncRNAs were selected to search for their adjacent coding genes ( and ). Each lncRNA has different number of adjacent coding genes. For example, lnc007411 has 14 adjacent coding genes, while lnc015714 has only 8 neighbouring coding genes. Interestingly, lnc0074111 is down-regulated in foetus longissimus dorsi compared with adult muscle tissues. Its four adjacent (co-located) coding genes (ENO3, SLC25A11, KIF1C, SPAG7) are at a high level in the longissimus dorsi of adult cattle. Most importantly, we found a differentially expressed lncRNA: lnc000100, whose co-expression gene has been shown to be involved in muscle development, such as Pax7, Myf5, and IGF2 (). Researches have shown that lncRNA can competitively bind miRNA, thereby eliminating the negative effects of miRNA on target gene expression. We performed bioinformatics analysis of all differentially expressed lncRNAs and predicted their binding miRNAs, which appear to be associated with muscle development. We selected 3 differentially expressed lncRNAs with high expression levels and may be related to muscle development, 31 differentially expressed mRNAs and 9 miRNAs to construct interaction network: lncRNA-miRNA-mRNA interaction network (). For example, lnc007411, one of the most down-regulated lncRNAs in foetal life, has multiple miRNA binding sites (bta-miR-27, bta-miR-29, bta-miR-125, bta-miR-195, bta-miR-214, bta-miR-224). These miRNAs have been shown to regulate muscle development.
Figure 3. Co-expression and competitive endogenous RNA networks in cattle muscle tissue.
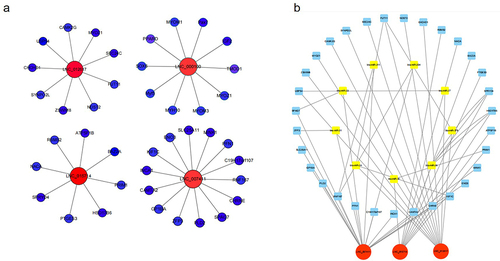
Table 3. Co-expression mRNA of lncRNA.
Lnc000100 was identified as a candidate lncRNA
In order to confirm the expression profile of lncRNAs obtained from the results of RNA-seq, 7 lncRNAs with differential expression that may be involved in the regulation of muscle development. In the RNA-seq data, the expression levels of these seven lncRNAs in adult longissimus dorsi muscle were significantly higher than those in embryo (). RT-qPCR results showed that the expression trend of these lncRNAs was similar to that of RNA-Seq sequencing results (. LncRNA expression in different tissues of foetal cattle () showed that lnc000100 were highly expressed in the muscle tissues (longissimus dorsi muscle and leg muscle) and low expressed in other tissues. Previous analysis results showed that lnc000100 was co-expressed in genes related to muscle development, such as Pax7, Myf5 and IGF2 (). Therefore, lnc000100 was selected as the candidate lncRNA, whose full length was 1368 nt (Text S1). Fluorescence in situ hybridization (FISH) indicated that lnc000100 was distributed in the nucleus (). In order to analyse the effect of lnc000100 on myoblast development, we interfered with lnc000100, and the results showed that lnc000100 expression level was significantly decreased (p < 0.05; ).
Figure 4. Expression of lnc000100 in cattle.
Effects of lnc000100 on cell proliferation
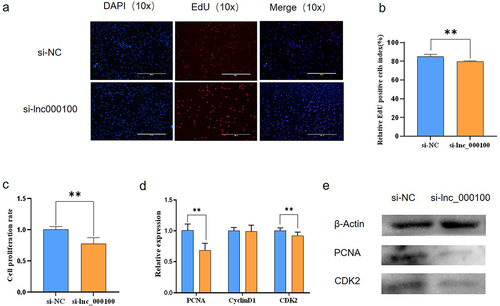
In order to explore the function of lnc00100 in the process of cattle muscle cell proliferation, we interfered with lnc000100. EdU staining analysis showed that si-lnc000100 significantly reduced the proportion of EdU positive cells, indicating that si-lnc000100 had an inhibitory effect on cell proliferation (. CCK-8 assay was used to detect the effect of lnc000100 on cell proliferation, and si-lnc000100 significantly inhibited cell viability (p < 0.05; ). Real-time quantitative PCR and Western blot showed that the expression of proliferation-related genes such as proliferating cell nuclear antigen (PCNA) and CDK2 decreased significantly after interference with lnc000100 (p < 0.05; . These results suggest that lnc000100 promotes cattle myoblast proliferation.
Figure 5. Effects of lnc000100 on cell proliferation.
Effects of lnc000100 on cell differentiation
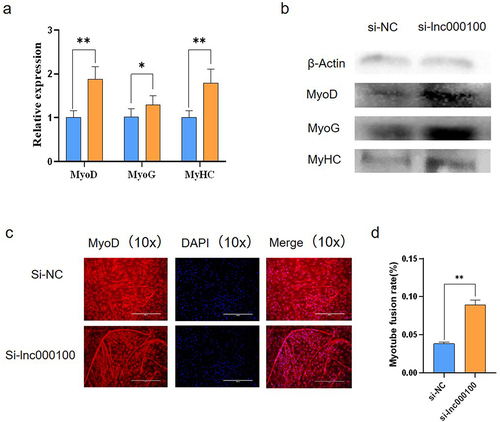
In order to explore the potential regulatory effect of lnc000100 on bovine muscle cell differentiation, we interfered with lnc000100 to reduce lnc000100 expression level. RT-qPCR analysis showed that si-lnc000100 significantly increased the expression of MyoD, MyoG and MyHC at mRNA levels (p < 0.05; ). Western blot analysis showed that si-lnc000100 significantly increased the expression of MyoD, MyoG and MyHC at protein levels (p < 0.05; ). Immunofluorescence assay results showed that si-lnc000100 significantly induced myotubule formation (. These results suggest that lnc000100 inhibits cattle myoblast differentiation.
Figure 6. Effect of lnc000100 on cell differentiation.
Discussion
At present, there are few researches on the muscle development of livestock. Therefore, this study used RNA-Seq sequencing of the longest dorsal muscles of Angus cattle at foetal and adult ages, three cattle each at foetal and adult ages, to ensure a high level of confidence [Citation25]. Compared to mRNAs, lncRNA transcripts in Angus cattle skeletal muscle tissue were shorter in length, expressed at lower levels, had fewer exons and shorter ORFs, similar to studies in other domestic animals [Citation26,Citation27].
Most researches exploring cattle muscle development and growth focused on protein-coding genes until a large number of non-coding RNAs were found, including circRNA [Citation28] and lncRNA [Citation29]. However, the role of lncRNAs in cattle muscle remains largely unknown, and there are relatively few studies on their molecular regulatory mechanisms and functions in cattle muscle development. Therefore, this research aims to explore the function of lncRNA in cattle muscle development, and provide a certain theoretical basis for improving beef cattle meat yield, breed selection. In this research, lncRNA associated with cattle muscle development were analysed and annotated by RNA-seq analysis. We identified some lncRNAs with coding potential through bioinformatics analysis, and we named this part TUCP for analysis. These lncRNAs may provide new insights into the regulatory mechanisms of skeletal muscle development in cattle. In order to further explore the function of lncRNAs, we performed GO analysis on differentially expressed lncRNAs and found that differentially expressed lncRNAs and their target genes were enriched in GO pathways related to muscle generation, such as skeletal muscle contraction, muscle cell development and striated muscle contraction. The GO analysis results suggest that lncRNA may play an important role in the process of muscle development. In addition, we mapped the co-expression networks and competitive endogenous networks of four significantly differentially expressed lncRNAs. This co-expression network and ceRNA network may provide new insights into the regulatory mechanisms of cattle skeletal muscle generation. Most researches exploring cattle muscle development and growth focused on protein-coding genes until a large number of non-coding RNAs were found, including circRNA [Citation28] and lncRNA [Citation29]. However, the role of lncRNAs in cattle muscle remains largely unknown, and there are relatively few studies on their molecular regulatory mechanisms and functions in cattle muscle development. Therefore, this research aims to explore the function of lncRNA in cattle muscle development, and provide a certain theoretical basis for improving beef cattle meat yield, breed selection. In this research, lncRNA associated with cattle muscle development were analysed and annotated by RNA-seq analysis. We identified some lncRNAs with coding potential through bioinformatics analysis, and we named this part TUCP for analysis. These lncRNAs may provide new insights into the regulatory mechanisms of skeletal muscle development in cattle. In order to further explore the function of lncRNAs, we performed GO analysis on differentially expressed lncRNAs and found that differentially expressed lncRNAs and their target genes were mostly enriched in GO pathways related to muscle generation, such as skeletal muscle contraction, muscle cell development and striated muscle contraction. The GO analysis results suggest that lncRNA may play an important role in the process of muscle development. In addition, we mapped the co-expression networks and competitive endogenous networks of four significantly differentially expressed lncRNAs. This co-expression network and ceRNA network may provide new insights into the regulatory mechanisms of cattle skeletal muscle generation.
LncRNA may interact with multiple target genes. In this study, we found that lnc000100 simultaneously targeted MYOF, MYOZ1, MYH10, Pax7, MYOM3, MYOM1, SOX6, Myf5, PPARD, IGF2, TMOD1 and Myf6. LncRNA can competitively bind miRNA and release the inhibition effect of miRNA on target genes. According to the ceRNA theory, three lncRNA-miRNA-mRNA ceRNA networks were constructed. Lnc007411 contains multiple miRNA binding sites related to skeletal muscle development, contestable combination bta-miR-27a-3p, bta-miR-29d-3p, bta-miR-29b, bta-miR-29c, bta-miR-125a, bta-miR-125b, bta-miR-195, bta-miR-214 and bta-miR-224. These miRNAs are known to be associated with muscle development, suggesting that lnc007411 May be involved in the growth and development of bovine skeletal muscle by targeting miRNAs. This ceRNA network may provide a new perspective for understanding the growth and development of bovine skeletal muscle [Citation30].
It has previously been reported that long non-coding RNAs are involved in the regulation of gene expression during muscle development, which is related to their location in cells. In the nucleus, lncRNAs regulate transcription through chromatin interaction [Citation31–33], and established the spatial organization of nuclear compartments through scaffolds [Citation34]. LncRNA Gtl2 can be used as a co-factor of PRC2 to promote the binding of PRC2 and Dlk1 gene, inhibit its expression, and regulate skeletal muscle development [Citation35]. In cytoplasm, lncRNAs mediate post-transcriptional regulation, isolate miRNAs [Citation36] and proteins [Citation37] regulate their activity and levels, and mediate mRNA translation and stability [Citation38,Citation39]. For example, lncMGPF increases the expression of myocyte enhancement factor 2C (MEF2C) by acting as a molecular sponge for miR-135a-5p. It can also enhance the stability of mRNA of myogenic regulatory gene MyoD and myogenic protein (MyoG) mediated by human antigen R, thus promoting muscle growth and regeneration [Citation13]. However, current studies on the molecular mechanism of lncRNA on cattle skeletal muscle development are mostly focused on the post-transcriptional level, and there are few studies on epigenetic and transcriptional regulation. In this study, we found that lncRNA was widely expressed in various tissues (longissimus dorsi muscle, leg muscle, lung, liver and kidney), which was consistent with previous results [Citation40]. Lnc000100 has high expression levels in the muscle tissues (longissimus dorsi muscle and leg muscle) of foetal cattle. We identified lnc000100, which is co-expressed with genes related to muscle development and highly expressed in muscle tissue, as a candidate molecule and interfered with it. The results showed that the expressions of PCNA and CDK2 were significantly down-regulated at mRNA and protein levels. Proliferative nuclear antigen (PCNA), as a protein in proliferative cells, is closely related to cellular DNA replication [Citation41] and other important cellular processes, such as chromatin remodelling, DNA repair and cell cycle control [Citation42], and is a good indicator for detecting cell proliferation. CDK2 protein plays a key role in regulating the cell cycle and is involved in many biological processes [Citation43]. Therefore, the downregulation of PCNA and CDK2 detected in this study suggests that cell proliferation is inhibited. In addition, the expression of genes related to muscle cell differentiation (such as MyHC, MyoD and MyoG) increased significantly after interference with lnc000100. Myogenin (MyoG) plays a key regulatory role in cell fusion and mature muscle cell formation [Citation44]. MyoG plays a unique role in foetal myogenesis and plays a key role in terminal differentiation [Citation45]. MyoG is essential for myogenic differentiation [Citation46]. Homozygous mice with targeted mutations in the myoietin gene died immediately after birth and showed severe loss of all skeletal muscles [Citation47]. Myf5 and MyoD play overlapping roles in myogenesis. In the absence of Myf5 and MyoD, progeners no longer form myoblasts, but divide into mesenchymal cells. Gene knockout experiments showed that neonatal mice lacking MyoD and Myf5 genes were completely deficient in myoblasts and skeletal muscle fibres, and died shortly after birth [Citation48]. These results suggest that the interference of lnc000100 can significantly inhibit the proliferation of myoblasts and promote the differentiation of myoblasts. Previous studies have shown that meat production can be directly increased by promoting muscle cell cell proliferation and differentiation [Citation49] as well as muscle cell hypertrophy [Citation50,Citation51]. This suggests that lnc000100 is important for muscle production.
In this research, we found that the interference of lnc000100 in foetal bovine myoblasts significantly inhibited the proliferation of muscle cells and significantly promoted the differentiation of muscle cells, suggesting that lnc000100 plays an important role in the formation of bovine skeletal muscle. We found that lnc000100 was mostly located in the nucleus by RNA fluorescence in situ hybridization (RNA-FISH), suggesting that lnc000100 may regulate the expression of genes related to the proliferation and differentiation of bovine myoblasts at the epigenetic or transcriptional level and regulate the growth and development of bovine skeletal muscle. Studies have shown that lncRNAs can regulate cis-or trans-gene expression. For example, lnc403 [Citation52] regulates the growth and development of bovine skeletal muscle by cis-regulating the expression of Myf6. We found that lnc000100 was co-expressed with Myf5, Myf6, MEF2B, Pax7 and IGF2 genes, which are related to muscle development. This suggests that lnc000100 may be involved in muscle development by trans-regulating the expression of distant genes.
Skeletal muscle dysplasia is the cause of a large number of muscle diseases (muscle hypertrophy, atrophy). Previous studies have shown that non-coding RNAs are critical for muscle development and disease [Citation53], and lncRNAs may serve as biomarkers and therapeutic targets for skeletal system diseases including osteoarthritis, osteoporosis, and cancer of the skeletal system [Citation54]. In this report, we identified differentially expressed lncRNAs that are enriched in pathways associated with skeletal muscle diseases, such as hypertrophic cardiomyopathy and dilated cardiomyopathy. This suggests that these differentially expressed lncRNAs may play an important role in the regulation of skeletal muscle diseases.
Conclusion
Our study provides a catalogue of lncRNAs expression in bovine muscle tissue. We annotated thousands of lncRNAs, many of which showed highly different abundance in foetal and adult muscle samples. We then focused on one of the lnRNA, lnc000100, which is down-regulated in the longest dorsal muscle during foetal life and which is highly specifically expressed in muscle tissue. Our results suggest that lnc000100, which is highly expressed in muscle tissue and localized in the nucleus, can significantly promote the proliferation and differentiation of myoblasts. We anticipate that these results will be a stepping stone to identifying the genetic mechanisms that control muscle formation.
Author contributions
QL, DS and HL designed the study. MM and MC performed the experiments. RS helped to draft the manuscript. XW and JW helped to collect tissue samples. All the authors read and approved the final manuscript. All authors contributed to the article and approved the submitted version.
Supplemental Material
Download Zip (3.6 MB)Acknowledgments
The authors thank the State Key Laboratory for Conservation and Utilization of Subtropical Agro-Bioresources of Guangxi University for providing the experimental platform and conditions.
Disclosure statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15592294.2023.2270864.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Buckingham M. Myogenic progenitor cells and skeletal myogenesis in vertebrates. Curr Opin Genet Dev. 2006;16(5):525–15. doi: 10.1016/j.gde.2006.08.008
- Cagnazzo M, Te Pas MF, Priem J, et al. Comparison of prenatal muscle tissue expression profiles of two pig breeds differing in muscle characteristics. J Anim Sci. 2006;84(1):1–10. doi: 10.2527/2006.8411
- Hernández-Hernández JM, García-González EG, Brun CE, et al. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin Cell Dev Biol. 2017;72:10–18. doi: 10.1016/j.semcdb.2017.11.010
- Bailey P, Holowacz T, Lassar AB. The origin of skeletal muscle stem cells in the embryo and the adult. Curr Opin Cell Biol. 2001;13(6):679–689. doi: 10.1016/S0955-0674(00)00271-4
- Dirks A, Leeuwenburgh C. Apoptosis in skeletal muscle with aging. Am J Physiol Regul Integr Comp Physiol. 2002;282(2):R519–27. doi: 10.1152/ajpregu.00458.2001
- Braun T, Gautel M. Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nat Rev Mol Cell Biol. 2011;12(6):349–361. doi: 10.1038/nrm3118
- Naya FJ, Olson E. MEF2: a transcriptional target for signaling pathways controlling skeletal muscle growth and differentiation. Curr Opin Cell Biol. 1999;11(6):683–688. doi: 10.1016/S0955-0674(99)00036-8
- Taylor MV, Hughes SM. Mef2 and the skeletal muscle differentiation program. Semin Cell Dev Biol. 2017;72:33–44. doi: 10.1016/j.semcdb.2017.11.020
- Mok GF, Lozano-Velasco E, Münsterberg A. A münsterberg, microRnas in skeletal muscle development. Semin Cell Dev Biol. 2017;72:67–76. doi: 10.1016/j.semcdb.2017.10.032
- Zhang ZK, Li J, Guan D, et al. A newly identified lncRNA MAR1 acts as a miR-487b sponge to promote skeletal muscle differentiation and regeneration. J Cachexia Sarcopenia Muscle. 2018;9(3):613–626. doi: 10.1002/jcsm.12281
- Legnini I, Di Timoteo G, Rossi F, et al. Circ-ZNF609 is a Circular RNA that can be Translated and functions in myogenesis. Mol Cell. 2017;66(1):22–37 e9. doi: 10.1016/j.molcel.2017.02.017
- Matsumoto A, Pasut A, Matsumoto M, et al. mTORC1 and muscle regeneration are regulated by the LINC00961-encoded SPAR polypeptide. Nature. 2017;541(7636):228–232. doi: 10.1038/nature21034
- Lv W, Jin J, Xu Z, et al. lncMGPF is a novel positive regulator of muscle growth and regeneration. J Cachexia Sarcopenia Muscle. 2020;11(6):1723–1746. doi: 10.1002/jcsm.12623
- Wang Y, Zhao ZJ, Kang XR, et al. LncRNA DLEU2 acts as a miR-181a sponge to regulate SEPP1 and inhibit skeletal muscle differentiation and regeneration. Aging (Albany NY). 2020;12(23):24033–24056. doi: 10.18632/aging.104095
- Song C, Yang Z, Jiang R, et al. lncRNA IGF2 as regulates Bovine Myogenesis through different pathways. Mol Ther Nucleic Acids. 2020;21:874–884. doi: 10.1016/j.omtn.2020.07.002
- Okazaki Y, Furuno M, Kasukawa T, et al. Analysis of the mouse transcriptome based on functional annotation of 60,770 full-length cDnas. Nature. 2002;420(6915):563–573.
- Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43(6):904–914. doi: 10.1016/j.molcel.2011.08.018
- Cheng X, Li L, Shi G, et al. MEG3 promotes differentiation of porcine satellite cells by sponging miR-423-5p to relieve inhibiting effect on SRF. Cells. 2020;9(2):449. doi: 10.3390/cells9020449
- Zou C, Li L, Cheng X, et al. Identification and functional analysis of long intergenic non-coding RNAs underlying intramuscular fat content in pigs. Front Genet. 2018;9:102. doi: 10.3389/fgene.2018.00102
- Wu T, Wang S, Wang L, et al. Long Noncoding RNA (lncRNA) CTTN-IT1 Elevates Skeletal Muscle Satellite Cell Proliferation and Differentiation by Acting as ceRNA for YAP1 Through Absorbing miR-29a in Hu Sheep. Front Genet. 2020;11: 843. doi: 10.3389/fgene.2020.00843
- Li ZH, Cai BL, Abdalla BA, et al. LncIRS1 controls muscle atrophy via sponging miR-15 family to activate IGF1-PI3K/AKT pathway. J Cachexia Sarcopenia Muscle. 2019;10(2):391–410. doi: 10.1002/jcsm.12374
- Huang J, Chen YX, Zhang B. IGF2-AS affects the prognosis and metastasis of gastric adenocarcinoma via acting as a ceRNA of miR-503 to regulate SHOX2. Gastric Cancer. 2020;23(1):23–38. doi: 10.1007/s10120-019-00976-2
- Li H, Wei X, Yang J, et al. Developmental transcriptome profiling of bovine muscle tissue reveals an abundant GosB that regulates myoblast proliferation and apoptosis. Oncotarget. 2017;8(19):32083–32100. doi: 10.18632/oncotarget.16644
- Zhang L, Dong L, Lu C, et al. Methylation of SDC2/TFPI2 and its diagnostic value in colorectal tumorous lesions. Front Mol Biosci. 2021;8:706754. doi: 10.3389/fmolb.2021.706754
- Liu R, Han M, Liu X, et al. Genome-wide Identification and characterization of long non-coding RNAs in longissimus dorsi skeletal muscle of Shandong black cattle and Luxi cattle. Front Genet. 2022;13:849399. doi: 10.3389/fgene.2022.849399
- Sun J, Xie M, Huang Z, et al. Integrated analysis of non-coding RNA and mRNA expression profiles of 2 pig breeds differing in muscle traits. J Anim Sci. 2017;95(3):1092–1103. doi: 10.2527/jas2016.0867
- Zhan S, Dong Y, Zhao W, et al. Genome-wide identification and characterization of long non-coding RNAs in developmental skeletal muscle of fetal goat. BMC Genomics. 2016;17(1):666. doi: 10.1186/s12864-016-3009-3
- Chen M, Wei X, Song M, et al. Circular RNA circMYBPC1 promotes skeletal muscle differentiation by targeting MyHC. Mol Ther Nucleic Acids. 2021;24:352–368. doi: 10.1016/j.omtn.2021.03.004
- Li H, Yang J, Jiang R, et al. Long non-coding RNA profiling reveals an abundant MDNCR that promotes differentiation of myoblasts by sponging miR-133a. Mol Ther Nucleic Acids. 2018;12:610–625. doi: 10.1016/j.omtn.2018.07.003
- Huang C, Ge F, Ma X, et al. Comprehensive analysis of mRNA, lncRNA, circRNA, and miRNA expression profiles and their ceRNA networks in the longissimus dorsi muscle of cattle-yak and yak. Front Genet. 2021;12:772557. doi: 10.3389/fgene.2021.772557
- Kugel JF, Goodrich JA. Non-coding RNAs: key regulators of mammalian transcription. Trends Biochem Sci. 2012;37(4):144–151. doi: 10.1016/j.tibs.2011.12.003
- Melé M, Rinn JL. “Cat’s cradling” the 3D Genome by the act of LncRNA transcription. Mol Cell. 2016;62(5):657–664. doi: 10.1016/j.molcel.2016.05.011
- Saxena A, Carninci P. Long non-coding RNA modifies chromatin: epigenetic silencing by long non-coding RNAs. BioEssays. 2011;33(11):830–839. doi: 10.1002/bies.201100084
- Clemson CM, Hutchinson JN, Sara SA, et al. An architectural role for a nuclear noncoding RNA: NEAT1 RNA is essential for the structure of paraspeckles. Mol Cell. 2009;33(6):717–726. doi: 10.1016/j.molcel.2009.01.026
- Zhao J, Ohsumi TK, Kung JT, et al. Genome-wide identification of polycomb-associated RNAs by RIP-seq. Mol Cell. 2010;40(6):939–953. doi: 10.1016/j.molcel.2010.12.011
- Cesana M, Cacchiarelli D, Legnini I, et al. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147(2):358–369. doi: 10.1016/j.cell.2011.09.028
- Lee S, Kopp F, Chang TC, et al. Noncoding RNA NORAD regulates genomic stability by sequestering PUMILIO proteins. Cell. 2016;164(1–2):69–80. doi: 10.1016/j.cell.2015.12.017
- Carrieri C, Cimatti L, Biagioli M, et al. Long non-coding antisense RNA controls Uchl1 translation through an embedded SINEB2 repeat. Nature. 2012;491(7424):454–457. doi: 10.1038/nature11508
- Gong C, Maquat LE. lncRnas transactivate STAU1-mediated mRNA decay by duplexing with 3’ UTRs via Alu elements. Nature. 2011;470(7333):284–288. doi: 10.1038/nature09701
- Li H, Huang K, Wang P, et al. Comparison of long non-coding RNA expression profiles of cattle and buffalo differing in muscle characteristics. Front Genet. 2020;11:98. doi: 10.3389/fgene.2020.00098
- Bravo R, Frank R, Blundell PA, et al. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987;326(6112):515–517. doi: 10.1038/326515a0
- Maga G, Hubscher U. Proliferating cell nuclear antigen (PCNA): a dancer with many partners. J Cell Sci. 2003;116(Pt 15):3051–3060. doi: 10.1242/jcs.00653
- Tadesse S, Anshabo AT, Portman N, et al. Targeting CDK2 in cancer: challenges and opportunities for therapy. Drug Discov Today. 2020;25(2):406–413. doi: 10.1016/j.drudis.2019.12.001
- Asfour HA, Allouh MZ, Said RS. Myogenic regulatory factors: the orchestrators of myogenesis after 30 years of discovery. Exp Biol Med (Maywood). 2018;243(2):118–128. doi: 10.1177/1535370217749494
- Venuti JM, Morris JH, Vivian JL, et al. Myogenin is required for late but not early aspects of myogenesis during mouse development. J Cell Bio. 1995;128(4):563–576. doi: 10.1083/jcb.128.4.563
- Zammit PS. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin Cell Dev Biol. 2017;72:19–32. doi: 10.1016/j.semcdb.2017.11.011
- Hasty P, Bradley A, Morris JH, et al. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature. 1993;364(6437):501–506. doi: 10.1038/364501a0
- Rudnicki MA, Schnegelsberg PNJ, Stead RH, et al. MyoD or myf-5 is required for the formation of skeletal muscle. Cell. 1993;75(7):1351–1359. doi: 10.1016/0092-8674(93)90621-V
- Xu X, Ji S, Li W, et al. LncRNA H19 promotes the differentiation of bovine skeletal muscle satellite cells by suppressing Sirt1/FoxO1. Cell Mol Biol Lett. 2017;22(1):10. doi: 10.1186/s11658-017-0040-6
- Bernat JL, Ochoa JL. Muscle hypertrophy after partial denervation: a human case. J Neurol Neurosurg Psychiatry. 1978;41(8):719–725. doi: 10.1136/jnnp.41.8.719
- Walters J. Muscle hypertrophy and pseudohypertrophy. Pract Neurol. 2017;17(5):369–379. doi: 10.1136/practneurol-2017-001695
- Zhang X, Chen M, Liu X, et al. A novel lncRNA, lnc403, involved in bovine skeletal muscle myogenesis by mediating KRAS/Myf6. Gene. 2020;751:144706. doi: 10.1016/j.gene.2020.144706
- Li D, Yang C, Yin C, et al. LncRNA, important player in bone development and disease. Endocr Metab Immune Disord Drug Targets. 2020;20(1):50–66. doi: 10.2174/1871530319666190904161707
- Huynh NPT, Anderson BA, Guilak F, et al. Emerging roles for long noncoding RNAs in skeletal biology and disease. Connect Tissue Res. 2017;58(1):116–141. doi: 10.1080/03008207.2016.1194406
