ABSTRACT
Physical activity is a first-line treatment for polycystic ovary syndrome (PCOS). Resistance or aerobic exercise improves metabolic complications, reproductive outcomes, and quality of life in PCOS. DNA methylation reprogramming during exercise may be the major modifier behind these changes. We sought to evaluate genome-wide DNA methylation changes after supervised resistance and aerobic exercise in women with PCOS. Exercises were performed in 56 women with PCOS (resistance, n = 30; aerobic, n = 26), for 16 weeks (wks), three times per week, in 50-minute to one-hour sessions. Anthropometric indices and hormonal and metabolic parameters were measured before and after training. Genome-wide leukocyte DNA methylation was analysed by Infinium Human MethylationEPIC 850K BeadChip microarrays (Illumina). Both resistance and aerobic exercise improved anthropometric indices, metabolic dysfunction, and hyperandrogenism in PCOS after the training programme, but no differences were observed between the two exercises. Resistance and aerobic exercise increased genome-wide DNA methylation, although resistance changed every category in the CpG island context (islands, shores, shelve, and open sea), whereas aerobic exercise altered CpG shores and the open sea. Using a stringent FDR (>40), 6 significantly differentially methylated regions (DMRs) were observed in the resistance exercise cohort and 14 DRMs in the aerobic cohort, all of which were hypermethylated. The increase in genome-wide DNA methylation may be related to the metabolic and hormonal changes observed in PCOS after resistance and aerobic exercise. Since the mammalian genome is hypermethylated globally to prevent genomic instability and ageing, resistance and aerobic exercise may promote health and longevity through environmentally induced epigenetic changes.
Introduction
PCOS is a heterogenic and complex endocrinopathy with increasing prevalence, affecting 5.5% to 19.9% of women of reproductive age globally [Citation1], depending on the diagnostic criteria [Citation2,Citation3]. The main features underlying PCOS pathophysiology include an androgen excess, oligo-anovulation, and polycystic ovarian morphology (PCOM), being hyperandrogenism the main feature behind ovulatory dysfunction [Citation2,Citation4]. The variability in clinical manifestations includes metabolic dysfunction, mainly insulin resistance (IR), compensatory hyperinsulinemia, dyslipidemia, abnormal glucose metabolism, and chronic inflammation which increase the risk of developing type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD) [Citation3]. Infertility or subfertility is a common condition in PCOS women (~72%) who also present psychological alterations such as depression and anxiety [Citation5]. Women with PCOS are either overweight or obese (38% to 88%) [Citation6], a condition that increases infertility and directly affects reproductive treatment and pregnancy outcomes [Citation7].
Given the variety of clinical manifestations, a recent guideline for the management of PCOS emphasizes healthy lifestyle changes as the primary therapeutic strategy to optimize metabolic and hormonal disturbances, weight management, quality of life, and infertility [Citation8]. Regular practice of physical activity has been beneficial in all aspects of PCOS health [Citation9]. We previously demonstrated that aerobic and resistance exercises improved both hyperandrogenism and body composition [Citation10,Citation11], as well as restore the reproductive function [Citation10] and quality of life [Citation12] of women with PCOS. The induction of physiological changes through physical activity is mainly due to epigenetic reprogramming to promote health and prevent and/or treat diseases [Citation13]. Epigenetic mechanisms are those that alter gene expression by inducing chromatin remodelling through chemical alterations in DNA and histone proteins as well as interactions with non-coding RNAs (ncRNAs) [Citation14]. DNA methylation is a major epigenetic modification through the mammalian genome, and in addition to transcriptional silencing [Citation15], it has been shown to play an important role in genomic stability and integrity, activation of transposable elements, and telomere maintenance [Citation16,Citation17] and participates in the developmental process of genomic imprinting [Citation18] and X-chromosome inactivation [Citation19]. Disturbances in DNA methylation patterns are implicated in the genesis of many human diseases, including developmental disorders [Citation20], neurodegenerative diseases [Citation21], cancer [Citation22], metabolic syndrome, obesity, and associated comorbidities [Citation23].
Although there is a genetic predisposition, the variability of clinical outcomes, suggests a complex network of gene interaction and environmentally induced epigenetic changes involved in PCOS aetiology [Citation24]. The dynamic epigenetic reprogramming, either during early embryonic development, caused by an adverse intrauterine environment, or throughout life due to metabolic, hormonal, and psychological disturbances and lifestyle conditions, may favour the development of PCOS or the worsening of its comorbidities and related infertility [Citation24,Citation25]. It has been shown that physical activity can improve metabolic, cardiovascular, and neurodegenerative diseases through lifetime epigenome reprogramming [Citation13], and the extent of epigenetic modulation is dependent on the type and duration of exercise [Citation13]. However, the DNA methylation changes underlying these beneficial effects of exercise in PCOS were not investigated. As an epigenetic marker for gene expression control and genomic stability maintenance, which is affected by environmental changes, we sought to investigate the effects of supervised aerobic and resistance training protocols on leukocyte DNA methylation patterns in women with PCOS.
Participants, material, and methods
Ethics statement
This is a retrospective study approved by the Institutional Review Board (IRB) of the University Hospital of Ribeirao Preto Medical School – University of São Paulo – USP (Protocol number: 031802/2018). This analysis included the participants of the observational clinical trials designed to evaluate the effects of supervised physical activity in PCOS properly registered as follows: (1) a nonrandomized single-arm, case-control investigation of progressive resistance training approved by our IRB (protocol no. 13475/2009) and registered at the Brazilian Clinical Trials Registry (ReBec, RBR-7p23c3) and (2) a randomized, controlled three-arm investigation of aerobic physical activity approved by our IRB (protocol no. 9640/2014) and registered at the Brazilian Clinical Trials Registry (ReBec, RBR-78qtwy) and the International Standard Randomized Controlled Trial Registry (ISRCTN10416750). Participants and/or their legal guardians provided written informed consent to participate in the Resistance and Aerobic Studies and waived signing a new form.
Participants and study design
This study included 56 women with PCOS from our previously reported clinical trials involving resistance [Citation10,Citation26] and aerobic exercise [Citation11,Citation27]. The clinical and anthropometric characteristics were analysed in this cohort to compare the differences between our previously published data from the resistance and the continuous aerobic training, called aerobic training. DNA methylation, the primary outcome of this investigation, is a retrospective analysis. After checking the quality of the DNA, 30 women with PCOS were included in the resistance training and 26 in the continuous aerobic training (Flow chart, Supplementary Figure S1). For the resistance exercise, all PCOS women performed the exercise, and the results were compared to trained non-PCOS women. For aerobic exercise, only PCOS women were included. The participants were randomly allocated into three groups including the continuous and intermittent aerobic training and non-training control group. The randomization was previously discussed [Citation11], and due to the applicability and the results obtained, the continuous aerobic protocol was chosen as an aerobic activity for this secondary analysis.
The participants were selected from the Gynecological Endocrinology Outpatient Clinic at the University Hospital of Ribeirão Preto Medical School of the USP. Women with PCOS aged between 18 and 39 years, and body mass index (BMI) between 18 and 39.9 kg/m2, with a sedentary lifestyle, regardless of race and social status, were considered eligible for this study. The participants were not using any pharmacological intervention and had no dietary restrictions. PCOS diagnosis was carried out according to the Rotterdam criteria reviewed [Citation28–30] as follows: the presence of irregular menstrual cycles (infrequent cycles of more than 38 days or amenorrhoea), the presence of clinical or laboratory hyperandrogenism and ultrasound findings of polycystic ovaries (≥20 follicles between in diameter or ovarian volume greater than 9 cm3). The ovarian morphology was evaluated using ultrasound examination with the Voluson E8 Expert device (GE Healthcare, Zipf, Austria) equipped with a 5–9 MHz vaginal probe (RIC5-9D). The distribution of PCOS phenotypes was evaluated in each exercise group.
For biochemical measurements and leukocyte DNA analysis, 20 mL of peripheral blood was collected in the morning, after an overnight fast, before and after 16 weeks of training. During the blood collection, all participants were monitored by a medical professional at the University Hospital. In women with PCOS with oligomenorrhea, the blood sample was collected until the eighth day of the menstrual cycle (early follicular phase) for control women and any day when the participant experienced amenorrhoea for more than 90 days (the 95th percentile for cycle length). To confirm PCOS diagnosis the thyroid-stimulating hormone (TSH), 17-hydroxyprogesterone (17-OHP) and prolactin were evaluated to exclude the participants with adrenal hyperplasia, hyperprolactinaemia, and thyroid dysfunction. Participants using drugs that interfere with the hypothalamic-pituitary-ovarian axis, pregnant, smoking, with diabetes or musculoskeletal disorders were excluded, as well as the participation in any regular physical exercise at least three times per week.
Resistance and aerobic training
The resistance and aerobic training followed the American College of Sports Medicine (ACSM) recommendations [Citation31] and all related interventions were previously published [Citation10,Citation11]. Briefly, the resistance and continuous aerobic training were performed three times a week, in 50-minute to one-hour sessions for 16 wks, under supervision by physical education professionals. For the resistance exercise, a linear periodization of training protocol was used, with decreasing volume and increasing intensity according to [Citation32,Citation33] and the resistance exercises included bench press, leg extension, front lat pull-down, leg curl, lateral raise, leg press (45°), triceps pulley, calf leg press, arm curl, and abdominal exercise.
The aerobic exercise was carried out on a treadmill (Embreex 570-L and Embreex 570-Pro, SC, Brazil) with a progressive increase in the duration of the training, from 30 minutes in the first week to 50 minutes in the last [Citation11]. Light (50%-64% HRmax), moderate (64%-77% HRmax), and vigorous (77%-94% HRmax) intensities were considered to calculate the progression of the protocols and the clinical profile of each participant. The HRmax formula (220-age) was used to calculate the intensity of training monitored by a pulse frequency metre (Polar RS800CX, Polar Electro Oy, Kempele, Finland). To obtain the volume of each session, the HRmax (%) was multiplied by the duration of the session in minutes. The adherence was monitored through direct supervision, and data were recorded by physical education professionals. The criterion of non-accession for both resistance and aerobic exercises was the failure of at least 20% of the training sessions. The subjects were instructed not to undertake any regular or supervised exercise during the training periods.
Clinical, anthropometric, and biochemical measurements
Age, diastolic and systolic blood pressure, heart rate, body weight, and height were assessed. Body mass index (BMI) was calculated using the weight in kilograms divided by the square of the height in metres. The waist circumference (WC) was measured at the midpoint between the lateral iliac crest and the lowest rib margin at the end of normal expiration, and hip circumference (HC) was measured in the region where the buttocks are the largest. The waist-to-hip ratio (WHR) was obtained by dividing WC (cm) by HC (cm). The anthropometric index was assessed according to the ‘International Standards for Anthropometric Assessment’ [Citation34]. Body composition was measured using dual-energy X-ray absorptiometry (DXA) using the Hologic Discovery Wi, QDR series (Hologic, Inc., Waltham, MA, USA). The regions of interest (ROIs) for assessment of the total body fat (BF) (fat mass (g) plus lean mass including bone mineral content (g)) and the percentage fat (%) (fat mass/total mass 100) were evaluated. The concentrations of total testosterone, androstenedione, and 17-hydroxyprogesterone (17-OHP) were measured by radioimmunoassay (Perkin Elmer – TriCarb 4910TR) to quantify antigen-antibody reactions. It is based on the competition of two antigens, one labelled with a radioactive isotope and the other unlabelled (patient); thyroid-stimulating hormone (TSH), sex steroid hormone-binding globulin (SHBG), fasting insulin, and, prolactin, oestradiol, homocysteine, follicle stimulating hormone (FSH), luteinizing hormone (LH) and c-reactive protein (CRP) were determined using a solid-phase, two side chemiluminescent immunoassay (Immulite 2000; Immunoassay System; Siemens, Santa Ana, CA, USA) with monoclonal antibody specific for each hormone protein, and the hydrolysis of the chemiluminescent substrate produces emission of light. Fasting blood glucose was determined using the oxidase method (CMD 800X1/CMD 800iX1, Wiener Lab, São Paulo, Brazil). Total cholesterol, high-density lipoprotein (HDL), and triglycerides were evaluated using the enzymatic colorimetric method (CMD 800X1/CMD 800iX1, Wiener Lab, São Paulo, Brazil) using glucose oxidase to catalyse the oxidation of glucose to hydrogen peroxide and peroxidase to generate quinonimine, which is measured at 505 nm. The concentration of quinonimine, and therefore the intensity of the colour, is directly proportional to the concentration of cholesterol in the sample. Low-density lipoprotein (LDL) was measured using the Fried Ewald formula: LDL cholesterol = total cholesterol (HDL cholesterol + triglycerides/5). The Free Androgen Index (FAI) was obtained using the following formula: (total testosterone (nmol/L)/SHBG (nmol/L) 100). The homoeostatic model assessment of insulin resistance (HOMA-IR) was evaluated using the formula: [fasting blood glucose (mg/dL) × 0.05551) × fasting insulin (μUI/mL)/22.5] [Citation35].
Statistical analysis
This study included a convenience sample based on DNA quality control as reported in the flow chart of the study. Data are presented as median, lower, and upper quartiles. Student’s t-test for paired samples was used to test whether there was a statistical difference between age and height at baseline. To assess the effects of time (before and after) on each group and of groups (resistance and aerobic) at each time, a general linear mixed regression model adjusted for BMI. This model takes into account intra- and inter-group variability. Additionally, to evaluate whether the differences observed were carried out by the exercises, the delta was calculated using the difference before and after training (BT – AT) and the median was compared between the RT and ACT using the Mann-Whitney nonparametric test to independently compare the distribution of hormonal and anthropometric variables between the groups as we did not assume normally distributed data. All statistical analyses were performed using SAS® 9.3 software (SAS Institute Inc, University of North Carolina, North Carolina), and p < .05 was considered significant.
Genomic DNA isolation, bisulphite conversion and sequencing
Genomic DNA was isolated from peripheral blood before and after the training protocols. Plasma was removed from whole blood by centrifugation, and leukocytes were separated after lysis of red blood cells (0.32 M sucrose, l%[v/v] Triton X-100, 5 mM MgC12.6H2O, 12 mM Tris-HC1, pH 7.5) and stored until use at −80°C. DNA was extracted using MasterPure Complete DNA and RNA Purification Kit (Epicentre, Illumina Company, San Diego, CA, USA), according to the manufacturer’s instructions. DNA integrity was assessed by agarose gel stained with GelRed (Unisciences, Miami Lakes, FL, USA), and the concentration was determined using the Nanodrop 2000c spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Bisulphite conversion was performed using the Zymo EZ-DNA Methylation kit (Zymo Research) according to the manufacturer’s adjusted protocol specifically for Illumina array platforms. Bisulphite-converted DNA was then processed where it was hybridized to Infinium Human MethylationEPIC 850K BeadChip microarrays.
Data processing
Raw. Idat files produced from array processing were assessed using R. Briefly, raw intensity values for both methylated and unmethylated probes were SWAN normalized utilizing the package minfi [Citation36]. Processing raw data included the generation of beta values which effectively represent fraction methylation. Beta values = methylated intensity/(methylated intensity + unmethylated intensity). Beta values for each probe on the array range from 0 to 1 with 0 being fully unmethylated and 1 being completely methylated. Once produced, the beta values are utilized for all downstream differential methylation analyses.
Differential methylation analysis
Differential methylation analysis was performed in two ways. First, we performed differential methylation analysis across the entire array (by averaging all probes tiled on the array), and in the context of CpG islands (including islands, shores, shelves, and the open sea). We utilized a paired t-test to assess differences between groups, since DNA methylation averages over large regions of the genome are typically normally distributed. The CpG island context designations were made based on annotation provided by Illumina specific for the 850K array which draws from UCSC (HG19 genome build) and many other sources. Statistical analysis of these potential differences was assessed via paired t-tests with typical thresholds for significance between blood collected prior to and following the exercise programme (either resistance or aerobic). In addition to an assessment via t-test, we also plotted averages from each group (pre and post-exercise regimen) on a scatter plot to visualize if any large scale, distinct differences arose as a result of this intervention. Differential DNA methylation was also performed regionally with the use of the MethylationArrayScanner application in the USEQ software package [Citation37]. This application performs a sliding window analysis which identifies the boundaries of differentially methylated regions using a Wilcoxon signed rank analysis and has been used repeatedly to successfully identify regions of significant differential methylation (PMID: 26361204, 28950428, 25010591). Regions were considered to be significantly differentially methylated only if they contained > 3 CpGs, had a log2 ratio greater than 0.2, and had a Phred scaled FDR score of greater than 40. While an FDR of greater than 13 is technically considered a significant finding (it is approximately equivalent to an adjusted p-value of 0.05) we have selected a more rigorous cut-off for significance to ensure that we are not reporting any spurious findings. We have loosened our level of significance once in the study to 13 only for the purpose of attempting to visualize potential enrichment across chromosomes. Enrichment of any pathways or GeneOntology (GO) using significantly differentially methylated regions [Citation38] was performed using the Genomic Regions Enrichment of Annotations Tool (GREAT) and GeneOntology (http://geneontology.org) powered by PANTHER [Citation39]. Exercise-interacting proteins were predicted using the STeu to canRING database (https://string-db.org, version 11.0) [Citation40].
Epigenetic age calculation
We also performed epigenetic age analysis using the Horvath epigenetic age calculator [Citation41]. Using this analysis, we assessed the accuracy of the prediction utilizing a linear regression analysis of predicted and chronological age for both resistance and aerobic exercise. Most importantly, we additionally tested to determine if there was a difference in age acceleration or deceleration as a result of the exercise regimen intervention by subtracting the actual age from the predicted age and averaging these results for both groups to determine if the predicted age increased or decreased. To accommodate the difference in age between the two groups, we ensured that the accurate ages were assessed at both collection time points.
Results
Clinical and anthropometric characteristics
The flow chart of this study is presented in the Supplementary Figure S1. The PCOS phenotypes in the resistance training were 73.08% phenotype A [hyperandrogenism (HA) + ovulatory dysfunction (OD) + polycystic ovaries (PCO)], 23.08% phenotype C (HA+PCO) and 3.85% phenotype D (OD+PCO); in the aerobic training were 43.33% phenotype A, 3.33% phenotype B (HA+OD), 30% phenotype C and 23.33% phenotype D. No differences were observed between the phenotypic distribution in the resistance and aerobic training (p = 0.07). Clinical and anthropometric characteristics of the resistance and aerobic exercise groups are shown in . Mean age and BMI were similar between the exercise groups. Both resistance and aerobic exercise reduced WC and testosterone levels, and resistance exercise reduced HDL. After adjustment for BMI, we did not observe changes in WC in the resistance exercise. Comparing the training protocols, no differences were observed between the aerobic and resistance exercise groups using the median difference (delta) before and after exercise ().
Table 1. Anthropometric and biochemical parameters of women with PCOS before and after the resistance and aerobic training.
Table 2. Estimation of the difference in clinical and anthropometric parameters of women with PCOS before and after resistance and aerobic exercise.
Differential DNA methylation
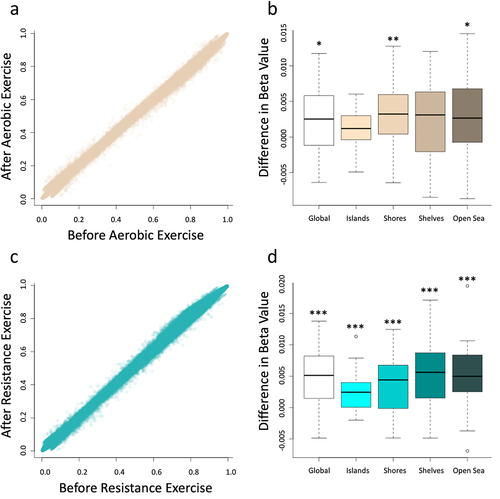
In our assessment of the impact of aerobic exercise, we found that DNA methylation was significantly increased across the genome (, p-value = 0.018) and that this was largely driven by increased methylation at CpG shores (p-value = 0.002) and in the open sea (p-value = 0.035) while neither CpG islands nor shelves were significantly differentially methylated based on paired t-tests (). Similarly, resistance exercise increased genome-wide DNA methylation (, p-value <0.001), and every category in the CpG island context (islands, shores, shelve, and open sea) were found to be significantly differentially methylated (). Importantly, these differences were all extremely subtle in nature with the mean difference of beta values of less than 0.003 across all probes on the array as a result of aerobic exercise and less than 0.005 as a result of resistance exercise. While the genome-wide changes were significant due to the vast number of CpGs assessed by the array, it is important to note that the very small magnitude of change is likely not biologically meaningful. We additionally plotted the average at each CpG on the array prior to, and following, exercise interventions and found that in neither the aerobic nor resistance exercise cohorts did we observe obvious outliers. In fact, the averages were extremely similar which further supports the idea that while there was a subtle change based on paired t-tests, that change was so small that it is almost certainly did not affect cell function.
Figure 1. Average beta values for all probes on the array were compared before and after the implementation of the protocols evidencing an increasing in global DNA before both aerobic exercise (a) and resistance exercise (c). Differential methylation was assessed using paired t-tests for each respective region of the genome. The aerobic exercise (b) increased DNA methylation in global, shores and open sea genomic context, while the resistance exercise (d) increased DNA methylation in all genomic context, including global, islands, shores, shelves and open sea. *designates a p-value of < 0.05, **designates a p-value of < 0.01, and ***designates a p-value of < 0.001. This figure was made with R program version 4.2.2.
To further assess if meaningful alterations could exist, we performed regional differential DNA methylation analysis using a sliding window approach. We identified 14 significantly differentially methylated regions in the aerobic cohort (RPS27, GYPC, GXYLT2, RPL35A, ZNF827, CENPK, RIOK2, SNHG5, C7ORF55, HMBOX1, EMC2, C14ORF119, KIAA0931, WRAP53) and 6 differentially methylated regions in the resistance cohort (RPL14, WRNIP1, NR2C2AP, NANP, DSCR9, RHBDD3) that met our initial criteria of an FDR > 40 (). Interestingly, all of these regions displayed increased methylation (hypermethylation) in both cohorts (). Using the Genomic Regions Enrichment of Annotations Tool (GREAT) we explored the enrichment of any pathways or GO terms in our significantly differentially methylated regions [Citation38]. This tool enables users to simply upload a. Bed file of genomic regions of interest and the output provides enriched pathways. In our aerobic cohort, we were able to identify two GO cellular component terms including intracellular ribonucleoprotein complex and ribonucleoprotein complex which both had a p-value for enrichment of 1.2 × 10−6. Further, we identified one GO biological process term, RNA processing with a p-value of 4.2 × 10−6.
Figure 2. Circos plots display all significantly differentially methylated regions that passed a threshold of FDR > 13 for both aerobic (a) and resistance (c) exercise cohorts. The outer track represents the chromosomes, and the inner track represents the log2 ratio of all differentially methylated regions for both differences that increase following exercise (orange) and decreased following exercise (blue). Additionally, box plots depict the results from the paired sliding window analysis at all sites that were differentially methylated with an FDR > 40 for both the aerobic (b) and the resistance (d) cohorts. The specific location of these regions can be found in . Circular plots were made with RCircos (a package in R version 4.2.2).
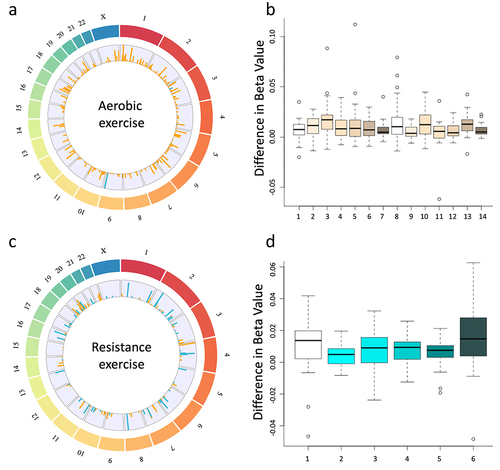
Table 3. Differentially methylated regions as a result of exercise: all regions determined to be altered based on the sliding window analysis are listed. Each site met the basic criteria of FDR of > 40 and a log2 ratio of > 0.2.
Using the GeneOntology (http://geneontology.org) powered by PANTHER [Citation39] we found that the genes identified in aerobic training participate in biological regulation, cellular and metabolic processes, and molecular functions were binding, catalytic, and structural molecule activity. The resistance training genes participate in biological regulation, cellular and metabolic processes, and response to stimulus, and the molecular functions were ATP-dependent activity, binding, catalytic activity, molecular function regulator, and structural molecule activity (Supplementary Figure S2). Exercise-interacting proteins were predicted using the STRING database (https://string-db.org, version 11.0, Supplementary Figure S2) [105]. The network interactions were built with a score of 0.4 or greater, using multiple proteins, selecting Homo sapiens as an organism, and an initial input of 13 protein-coding genes for the aerobic and six for the resistance cohort. The enrichment for aerobic exercise indicates that the proteins RPS27, RPL35A, and RIOK2 are biologically connected (PPI enrichment p-value: 0.194). For resistance exercise, no interaction was observed between the proteins analysed (PPI enrichment p-value: 0.1).
We additionally softened our thresholds for significance to an FDR of 13 and evaluated the results to determine if there are any chromosomal regions of enriched DNA methylation alterations (). In the aerobic exercise cohort, we identified 4 hypomethylated regions and 356 hypermethylated regions at this threshold, while in the resistance exercise cohort, we identified 70 hypomethylated regions and 100 hypermethylated regions at this threshold. These data suggest that the patterns of differential methylation are quite widespread throughout the genome in both the resistance exercise and aerobic exercise cohorts.
Epigenetic age assessment
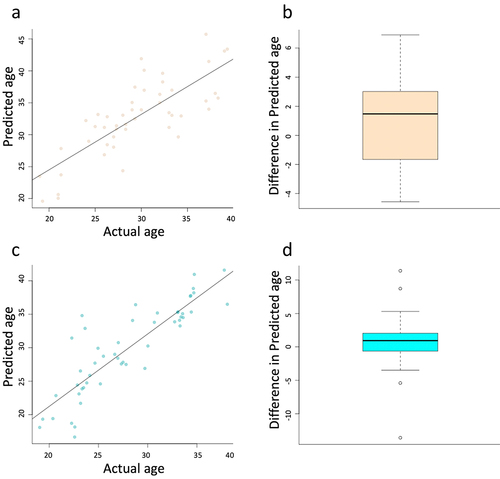
To predict age acceleration and how genetic and environmental factors regulate the PCOS ageing process, we used Horvath’s DNA methylation age, one of the most promising epigenetic clocks [Citation42]. We found that the age calculation was quite effective in age prediction with an r2 of 0.61 and p-value of < 0.001 in the aerobic cohort and an r2 of 0.74 and p-value of < 0.001 in the resistance cohort (). In addition to ensuring the relative accuracy of prediction, we assessed the predicted values for differences in age acceleration and deceleration. We found no significant difference in epigenetic age as a result of exercise intervention, in either the aerobic or resistance cohorts ().
Figure 3. Scatter plots depict the accuracy of the Horvath ageing calculator in our cohort of samples for both aerobic (a) and resistance (c) exercise. Boxplots depict the change in predicted age following the exercise regimen for both aerobic (b) and resistance (d). No significant difference was identified for either aerobic (p-value = 0.14) or resistance (p-value = 0.34). This figure was made with R program version 4.2.2.
Discussion
Physical activity is a non-pharmacological therapy for women with PCOS that improves metabolic disorders, hormonal alterations, infertility, and quality of life. Previously we reported that both aerobic and resistance exercises improved body composition and hyperandrogenism in women with PCOS [Citation10,Citation11,Citation43]. In this reduced cohort, resistance and aerobic training reduced WC and testosterone levels, while HDL was reduced only in the resistance training. In the model adjusted for BMI, all differences remained except for WC in resistance exercise. No differences were observed between the two classes of exercise in the clinical and anthropometric variables, as well as the distribution of PCOS phenotypes. As changes in the epigenetic landscape are one of the main molecular modifications that contribute to the beneficial effects of physical activity on health, we observed that both aerobic and resistance exercises increased average genome-wide DNA methylation in women with PCOS, although the resistance cohort seemed to have a slightly higher magnitude of changes. To our knowledge, this was the first study to evaluate epigenetic modifications in women with PCOS after supervised resistance and aerobic exercises, with the absence of structured dietary energy restrictions, that could impact the effects of physical activity.
Despite the heterogeneity of clinical outcomes, physical activity improved PCOS-related symptoms, reducing hyperandrogenism and metabolic disturbance [Citation44,Citation45]. We did not observe differences in the distribution of PCOS phenotypes between the exercises group, and both exercises reduced testosterone levels and WC after training, and the resistance training specifically reduced HDL cholesterol. However, after adjustment for BMI, we did not observe differences in WC in the resistance exercise. Reduction of androgens, such as testosterone, is important for reducing IR and obesity index and improving reproductive function in PCOS women [Citation46]. Booth exercises reduced WC, irrespective of weight loss, however, BMI in the resistance exercise may play a role in the magnitude of abdominal obesity even if it is related to a modest change in these variables [Citation47]. Besides the type of exercise and training protocol, HDL levels depend on several factors, including age, race, BMI, diet, and baseline levels [Citation48]. Not only the quantity but also the quality of HDL particles may be important for their action [Citation49]. In addition, low levels or inefficient action of HDL are usually accompanied by high triglyceride levels, and the risk of developing disease should be adjusted by the ratio between these two biomarkers [Citation50]. As we did not observe an increase in triglycerides after the resistance exercise, and a reduction in central obesity was observed (WC), resistance exercise is still effective in treating some characteristics of PCOS.
Despite the reduction in HDL levels after training in this cohort, we previously observed that the resistance exercise reduced testosterone and glucose levels in both PCOS and non-PCOS women [Citation10]. Additionally, resistance exercise decreased WC, SHBG levels, and FAI, and increased muscle mass index, lean mass (LM/height [Citation2], and androstenedione levels in PCOS when compared to non-PCOS women. Nevertheless, resistance exercise improved reproductive function since one woman became pregnant and 17 reported changes in menstrual cyclicity [Citation10], a result that was not observed for the continuous or intermittent aerobic training protocol [Citation11]. However, aerobic exercise was also an important lifestyle change for women with PCOS. Comparing training and non-training PCOS women, the randomized controlled study showed that continuous aerobic exercise reduced WC, HC, total cholesterol, LDL, and testosterone, whereas intermittent aerobic exercise reduced WC, WHC, testosterone, and FAI. On the other hand, the non-training PCOS control group increased WC and almost all regions of interest (ROIs) for assessment of the total body mass [Citation11]. Also, resistance and aerobic exercise improved various aspects of well-being in women with PCOS, with intermittent aerobic exercise being the most effective for the quality of life (QoL) and female sexual function index (FSFI) domains and the hospital anxiety and depression scale (HADS) score [Citation12].
Lifetime changes promoted by physical exercise to improve the overall health of PCOS, including associated infertility, are complex and multifactorial, with both genetic and environmentally induced epigenetic contributions to a given phenotype [Citation10,Citation45]. However, each type of physical activity can have a different effect on PCOS-related comorbidities that may be mostly modulated by lifestyle-induced changes in the epigenome [Citation51]. DNA methylation is a repressive epigenetic marker essential for the maintenance of genomic stability and the control of gene expression in the mammalian genome [Citation52]. Jacques and collaborators (2019) showed epigenetic changes in DNA methylation in response to exercise with potential effects on skeletal muscle metabolism in healthy participants. However, the specific genes that were altered, and their associated pathways depended upon the type and duration of the intervention, and the strategy for DNA methylation analysis [Citation53].
We observed that 16 wks of resistance and aerobic physical activity induced epigenetic alterations by changing average genome-wide DNA methylation as well as regional DNA methylation. The aerobic exercise increased average DNA methylation specifically at CpG shores and in the open sea, while resistance exercise induced alterations in every category in the CpG island context, such as CpG islands, shores, shelves, and open sea, in addition to the average methylation across all probes and regional methylation differences. While CpG island methylation changes are known to be capable of directly affecting gene expression, the methylation status of surrounding regions (shores, shelves, and open sea) impacts genomic stability and chromatin remodelling [Citation54]. The mammalian genome is usually highly methylated [Citation55] and this modification contributes to the establishment of condensed heterochromatin domains, preventing rearrangements and chromosomal translocations, inactivating repetitive DNA elements, and regulating DNA repair mechanisms [Citation56]. Moran et al. (2008) reported increased lymphocyte genomic instability with more micronuclei and chromosome mal segregation in women with PCOS, which may be related to IR [Citation57]. Obesity is linked to DNA damage and genomic instability due to increased inflammation and induced IR [Citation58]. By increasing global DNA methylation, physical activity may prevent genomic instability due to metabolic and hormonal disturbance in PCOS women.
Despite the differences observed, the changes in average DNA methylation across all probes were quite small and likely not biologically significant. Previous work has demonstrated that both types of physical activity can contribute to an important lifestyle change that attenuates ageing effects on the genome such as DNA damage, telomere shortening, and global DNA hypomethylation [Citation59]. We also evaluated the epigenetic effects of ageing, and no differences were observed before and after the resistance and aerobic training. Similarly, we did not observe changes in telomere length, a molecular marker of ageing and genomic stability [Citation26,Citation27]. Since epigenetic ageing is a progressive and time-dependent process, 16 wks of intervention may not be sufficient to observe age-related modifications. The epigenetic clock based on DNA methylation to predict healthy ageing is an accurate method to predict biological age and lifestyle changes and acts as an epigenetic modifier to reversibly slow down epigenetic ageing [Citation60].
Using an FDR of 13, we identified 4 hypomethylated regions and 356 hypermethylated regions in the aerobic cohort and 70 hypomethylated regions and 100 hypermethylated regions in the resistance cohort. By increasing the confidence of our test (FDR >40) and incurring a relatively low proportion of false positives, the number of DMRs, which were 14 for the aerobic and six for the resistance exercise, were all hypermethylated. These DMRs are related to ribosomal structure, transcriptional regulation, binding proteins, centromere function, mitochondrial protein, kinase activity, and telomeric DNA binding activity, among others, that have not been previously associated with PCOS genetic and/or epigenetic markers [Citation61,Citation62], but are involved in important biological processes such as DNA replication, genomic stability, and cell division. We observed changes in two long non-coding RNAs (lncRNAs), the SNHG5 in the aerobic cohort and the DSCR9 in the resistance cohort. Overexpression of the lncRNA SNHG5 is related to the carcinogenesis process and metastasis [Citation63], and downregulation of the SNHG5 May be associated with triglycerides and T2DM susceptibility [Citation64,Citation65]. The lncRNA DSCR9, Down syndrome (DS) critical regions (DSCRs) are responsible for most features of DS, including metabolic disease and insulin regulation [Citation66], and are associated with cancer progression [Citation67]. These changes in DNA methylation levels in specific CpG contexts and chromosomal regions in combination contribute to the hormonal, metabolic, and anthropometric changes observed after aerobic and resistance exercise [Citation61,Citation62].
Aerobic exercises change DNA methylation profiles, however, these changes seem to be intensity-dependent, as the effects of epigenetic alterations need to be maintained throughout the lifetime [Citation59]. In our specific aerobic exercise protocol, participants used progressive moderate-intensity training that impacts the hormonal and metabolic parameters of PCOS [Citation11]. The GO analysis showed that the DMRs were found to participate in the biological process of RNA processing and as cellular components including the intracellular ribonucleoprotein complex and ribonucleoprotein complex. The protein interaction network revealed an interaction between the ribosomal proteins RPS27 and RPL35A and the kinase RIOK2 in aerobic exercise, without interaction between the other protein-coding genes (PPI enrichment p-value 0.194). Barres and collaborators (2012) showed that acute aerobic exercise reduced global methylation, with hypomethylation in promoter regions (PGC-1α, PDK4, and PPAR-δ) and reduced gene expression in skeletal muscle, and it seems to be an early event in contraction-induced gene activation [Citation68]. Another study showed that vigorous interval aerobic activity (26–30 min/day, increasing the physical effort per day) increased global DNA methylation in skeletal muscle irrespective of gender differences [Citation69]. Six months of aerobic activity showed beneficial effects on health and induced DNA methylation changes in the skeletal muscle of mild cognitively impaired elderly in a more recent study, especially around genes related to amyloid biology, intracellular protein trafficking, and lipoprotein regulations [Citation70]. Although aerobic exercise is beneficial to health, high-intensity activity may lead to DNA damage in specific tissues, especially in skeletal muscle, but this adverse effect is reversible and may persist for less than three days [Citation71,Citation72].
Resistance training is an effective strategy to improve body composition. We previously demonstrated a reduction in SHBG levels, visceral fat, and WC and an increase in lean muscle mass and androstenedione levels after progressive resistance protocol in PCOS [Citation10]. Irrespective of PCOS diagnosis, resistance exercise increased inflammatory biomarkers and decreased telomere length in our previous study [Citation26]. We observed an increased DNA methylation in all CpG contexts, including the islands, shores, shelves, and the open sea after resistance exercise in PCOS women. However, no significant GO terms were observed for the hypermethylated regions in resistance training and the network does not have significant interactions. Lindholm and collaborators (2014) evaluated the effects of three months of endurance exercise (45 min, 4 sessions per week) and reported global changes in DNA methylation on skeletal muscle in healthy males and females and these changes were mostly in enhancers, gene bodies and intergenic regions, then in CpG islands. These regions included muscle-related genes, such as the MRF, MEF2, and EST [Citation73].
Bagley et al. (2020) showed hypomethylation of the retrotransposon LINE-1 and hypermethylation of metabolic genes (GPAM and SREBF2) after acute resistance exercise [Citation74]. Hypertrophy training (load), followed by the return of muscle mass to baseline (unloading) and later hypertrophy (reloading), was associated with decreased methylation at 18,816 CpG sites after reloading and 9,153 CpG sites after loading, including the genes AXIN1, GRIK2, CAMK4, TRAF1 in the male population [Citation75]. Of those studies evaluating DNA methylation and resistance exercise, none were performed specifically in the female population, and the epigenetic impacts of resistance versus endurance exercises were not compared.
Epigenetic reprogramming is a dynamic and reversible process that controls gene expression in a time and cell-specific manner [Citation76]. Changes in DNA methylation induced by physical activity are observed in many tissues throughout the body, thereby impacting muscular activity, adiposity index, and cardiovascular and immune physiology [Citation59]. Increased global lymphocyte DNA methylation and target IL-17A and IFN-γ promoter methylation were observed in aged women who performed unsupervised resistance, aerobic, or the combination of both exercises at least for three months, 2–3 times per week [Citation77]. Six months of aerobic exercises on a treadmill induced global changes in DNA methylation in blood samples from mild cognitively impaired elderly, as 214 CpG sites displayed reduced DNA methylation levels and 34 CpG sites displayed increased DNA methylation after exercise. The majority of those modifications were noted to be near genes responsible for amyloid biology, intracellular traffic, and lipoprotein regulation [Citation70]. Ronn et al. (2013) also reported altered DNA methylation in adipose tissue after six months and the greatest changes (difference in DNA methylation 0.8%) included ITPR2 and TSTD1 for increased and LTBP4 for decreased DNA methylation [Citation78]. Adipose changes in DNA methylation were observed after 6 weeks of 45-minute cycling sessions, 5 days per week [Citation79].
Only one supervised study evaluated DNA methylation after 12 weeks of high-intensity aerobic interval (HIIT), resistance, and combined exercise in young and old males and females. The authors reported changes in body composition and insulin sensitivity, as well as an increased expression of transcripts and proteins involved in translation machinery, especially in HIIT exercise, with small changes in DNA methylation in the promoter region [Citation80]. We showed significant differences in DNA methylation after 16 wks of intervention in both exercises, with an increased sample size compared to other studies [Citation73,Citation75,Citation80] without a specific diet, as participants were instructed to maintain their daily diet during the intervention. The strengths of this study include the supervised exercise in the absence of structured dietary energy restrictions. We have previously observed that hyperandrogenism improves muscle strength in women with PCOS [Citation43]. Because the hormonal and metabolic changes of PCOS affect the response to physical activity and the pattern of DNA methylation, only women with PCOS were used to avoid additional bias in our study. Also, the genome-wide methylation array is a high-throughput platform that covers over 850,000 CpG sites in the human methylome, without the bias of PCR amplification using qPCR analysis or targeted sequencing. Other platforms as Whole-genome bisulphite sequencing (WGBS) and Methylation Capture Sequencing (MC-seq) increase the coverage of the methylome but still have some limitations as the high cost and large DNA input (WGBS) and the bias due to the presence of PCR duplicates (MC-seq) [Citation81].
Despite the strengths of our study, one limitation is that we did not evaluate the VO2max or the metabolic equivalent (METS) of participants prior to and following the intervention, although it is important to note that there is a linear relationship between VO2max and HRmax at submaximal exercise intensities [Citation82]. In addition, the use of chemiluminescence for the measurement of steroid sex hormones may not be as sensitive as mass spectrometry for detecting androgen levels in women, on the other hand, all women were evaluated using the same equipment, assays and methodology. Also, we did not evaluate the DNA methylation contribution to transcriptome changes, especially for those chromosomal differentially methylated regions evaluated. Even though we could not control other environmental changes on DNA methylation, we used Horvath’s DNA methylation clocks to predict age acceleration and how genetic and environmental factors regulate an individual’s ageing process [Citation41], even in the white blood cells with their constant which are constantly renewing themselves [Citation83]. Furthermore, by using the same participant as a control for themselves (paired samples), the difference observed between pre- and post-training is more likely due to the exercise rather than an individual variation.
The underlying biological mechanisms whereby physical activity influences epigenome remodelling and prevents diseases are complex and multifactorial. Hormonal and metabolic disturbances, either during embryonic/foetal life or adulthood, are combined factors that contribute to genetic and epigenetic susceptibility to PCOS or the worsening of its related comorbidities, including impaired fertilization [Citation61]. Although other tissues will have their epigenetic signature after the intervention, changes in body fluids, such as in blood cells and circulating molecules, have been widely used as minimally invasive biomarkers for the diagnosis and prognosis of many diseases [Citation84]. Further analysis using adipose tissue, granulosa cells, ovaries, oocytes, and other tissues should provide a better understanding of the specific phenotypes associated with PCOS, such as obesity and chronic anovulation.
DNA methylation is a repressive epigenetic mark that alters chromatin structure and DNA accessibility, thereby establishing a differential gene expression programme and maintaining genomic stability [Citation52]. Since global hypomethylation is associated with human disease and the ageing process, the increase in DNA methylation in the genomic contexts throughout the aerobic and resistance exercises reinforces the idea that physical activity promotes health and longevity. These benefits have been observed in the improvement of metabolic, hormonal, and body composition in women with PCOS (), as well as reproductive outcomes and quality of life, even over a short period of time; both exercises can modulate the epigenome of leukocyte cells. Both aerobic and resistance exercises should be considered effective non-pharmacological treatments in the management of PCOS with implications for epigenomic reprogramming. Given that the epigenome can be reprogrammed throughout life, the reversible characteristic of epigenetic changes suggests that physical activity is a life-long change.
Figure 4. Resistance and aerobic training have different effects on the metabolic, hormonal and body composition of women with PCOS after 16 weeks of intervention [Citation10,Citation11]. The resistance exercise reduces waist circumference, testosterone levels and HDL, while the aerobic exercises reduced waist circumference and testosterone levels. These changes are reflected in epigenetic reprogramming through different exercises, in which the resistance exercises increased average DNA methylation in all CpG island contexts, including global, islands, shores, shelves and open sea and the aerobic exercise increased average DNA methylation across all probes (global), as well as at shores and open sea. This figure was made in part using BioRender (https://biorender.com).
![Figure 4. Resistance and aerobic training have different effects on the metabolic, hormonal and body composition of women with PCOS after 16 weeks of intervention [Citation10,Citation11]. The resistance exercise reduces waist circumference, testosterone levels and HDL, while the aerobic exercises reduced waist circumference and testosterone levels. These changes are reflected in epigenetic reprogramming through different exercises, in which the resistance exercises increased average DNA methylation in all CpG island contexts, including global, islands, shores, shelves and open sea and the aerobic exercise increased average DNA methylation across all probes (global), as well as at shores and open sea. This figure was made in part using BioRender (https://biorender.com).](/cms/asset/65b0552b-631d-4212-bd23-2a4539eb7ee7/kepi_a_2305082_f0004_oc.jpg)
Author contributions
C.L.M.F., G.S.K. and R.M.R. conceived and designed the study. C.L.M.F, analysed and interpreted the data and wrote the manuscript; M.H., N.T. and T.J, performed bisulphite sequencing analysis; G.S.K. and V.B.R, supervised the physical activity in the participants; M.R.S, performed sample collection; R.A.F. and K.I.A., contribute to participant’s measurements and data analysis; RMR, provided project oversight. All authors critically revised the manuscript and approved the final version and submission of this manuscript.
Ethics statement
All the protocols were in accordance with relevant guidelines and regulations for human studies and all experimental protocols were approved by the Institutional Review Board of the University Hospital (UH) of Ribeirao Preto Medical School – University of São Paulo (FMRP-USP) (Protocol number: 031802/2018). In addition, all interventions were registered as follows: (1) Non-randomized Resistance Clinical Trial: Brazilian Clinical Trials Registry (ReBec) RBR-7p23c3; (2) Randomized Controlled Aerobic Clinical Trial: ReBec: RBR-78qtwy and International Standard Randomized Controlled Trial Registry (ISRCTN10416750). All participants provided written informed consent.
-(Supplemental Material.docx
Download MS Word (1.9 MB)Acknowledgments
We would like to thank the participants who volunteered for this study and their willingness to exercise with unwavering intensity and to sacrifice many of their normal activities to adhere to the described protocol. We also thank the members of the Human Reproduction. Division at the Department of Gynecology and Obstetrics of the Ribeirao Preto Medical School, University of Sao Paulo, especially Cristiana Carolina Padovan and Océlia de Vasconcelos for the technical support. We would like to thank Sofia Reis Moura for the English review.
Disclosure statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data availability statement
Data is publicly available at the gene expression omnibus (GEO) under accession number #GSE213366.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15592294.2024.2305082
Additional information
Funding
References
- Belenkaia LV, Lazareva LM, Walker W, et al. Criteria, phenotypes and prevalence of polycystic ovary syndrome. Minerva Ginecol. 2019;71(3):211–19. doi: 10.23736/S0026-4784.19.04404-6
- Azziz R, Carmina E, Chen Z, et al. Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2(1):16057. doi: 10.1038/nrdp.2016.57
- Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8(1):41. doi: 10.1186/1741-7015-8-41
- Azziz R, Carmina E, Dewailly D, et al. The androgen excess and PCOS society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91(2):456–488. doi: 10.1016/j.fertnstert.2008.06.035
- Moran LJ, Deeks AA, Gibson-Helm ME, et al. Psychological parameters in the reproductive phenotypes of polycystic ovary syndrome. Hum Reprod. 2012;27(7):2082–8. doi: 10.1093/humrep/des114
- Barber TM, McCarthy MI, Wass JA, et al. Obesity and polycystic ovary syndrome. Clin Endocrinol (Oxf). 2006;65(2):137–45. doi: 10.1111/j.1365-2265.2006.02587.x
- Fichman V, Costa R, Miglioli TC, et al. Association of obesity and anovulatory infertility. Einstein (Sao Paulo). 2020;18:eAO5150. doi: 10.31744/einstein_journal/2020AO5150
- Teede HJ, Tay CT, Laven J, et al. Recommendations from the 2023 International Evidence-based Guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2023;120(4):767–93. doi: 10.1016/j.fertnstert.2023.07.025
- Butt MS, Saleem J, Zakar R, et al. Benefits of physical activity on reproductive health functions among polycystic ovarian syndrome women: a systematic review. BMC Public Health. 2023;23(1):882. doi: 10.1186/s12889-023-15730-8
- Kogure GS, Miranda-Furtado CL, Silva RC, et al. Resistance exercise impacts lean muscle mass in women with polycystic ovary syndrome. Med Sci Sports Exerc. 2016;48(4):589–598. doi: 10.1249/MSS.0000000000000822
- Ribeiro VB, Kogure GS, Lopes IP, et al. Effects of continuous and intermittent aerobic physical training on hormonal and metabolic profile, and body composition in women with polycystic ovary syndrome: a randomized controlled trial. Clin Endocrinol (Oxf). 2020;93(2):173–186. doi: 10.1111/cen.14194
- Kogure GS, Lara L, Ribeiro VB, et al. Distinct protocols of physical exercise may improve different aspects of well-being in women with polycystic ovary syndrome. Am J Lifestyle Med. 2023;17(1):140–151. doi: 10.1177/15598276211001330
- Grazioli E, Dimauro I, Mercatelli N, et al. Physical activity in the prevention of human diseases: role of epigenetic modifications. BMC Genomics. 2017;18(S8):802. doi: 10.1186/s12864-017-4193-5
- Kim JK, Samaranayake M, Pradhan S. Epigenetic mechanisms in mammals. Cell Mol Life Sci. 2009;66(4):596–612. doi: 10.1007/s00018-008-8432-4
- Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293(5532):1089–93. doi: 10.1126/science.1063443
- Benayoun BA, Pollina EA, Brunet A. Epigenetic regulation of ageing: linking environmental inputs to genomic stability. Nat Rev Mol Cell Biol. 2015;16(10):593–610. doi: 10.1038/nrm4048
- Hu H, Li B, Duan S. The alteration of subtelomeric DNA methylation in aging-related diseases. Front Genet. 2018;9:697. doi: 10.3389/fgene.2018.00697
- Reik W, Walter J. Genomic imprinting: parental influence on the genome. Nat Rev Genet. 2001;2(1):21–32. doi: 10.1038/35047554
- Cotton AM, Price EM, Jones MJ, et al. Landscape of DNA methylation on the X chromosome reflects CpG density, functional chromatin state and X-chromosome inactivation. Hum Mol Genet. 2015;24(6):1528–39. doi: 10.1093/hmg/ddu564
- Buiting K. Prader–Willi syndrome and Angelman syndrome. Am J Med Genet C Semin Med Genet. 2010;154C(3):365–376. doi: 10.1002/ajmg.c.30273
- Martinez-Iglesias O, Carrera I, Carril JC, et al. DNA methylation in neurodegenerative and cerebrovascular disorders. Int J Mol Sci. 2020;21(6):21. doi: 10.3390/ijms21062220
- Locke WJ, Guanzon D, Ma C, et al. DNA methylation cancer biomarkers: translation to the clinic. Front Genet. 2019;10:1150. doi: 10.3389/fgene.2019.01150
- Samblas M, Milagro FI, Martinez A. DNA methylation markers in obesity, metabolic syndrome, and weight loss. Epigenetics. 2019;14(5):421–44. doi: 10.1080/15592294.2019.1595297
- Eiras MC, Pinheiro DP, Romcy KAM, et al. Polycystic ovary syndrome: the epigenetics behind the disease. Reprod Sci. 2022;29(3):680–694. doi: 10.1007/s43032-021-00516-3
- Sagvekar P, Kumar P, Mangoli V, et al. DNA methylome profiling of granulosa cells reveals altered methylation in genes regulating vital ovarian functions in polycystic ovary syndrome. Clin Epigenetics. 2019;11(1):61. doi: 10.1186/s13148-019-0657-6
- Miranda-Furtado CL, Ramos FK, Kogure GS, et al. A nonrandomized trial of progressive resistance training intervention in women with polycystic ovary syndrome and its implications in telomere content. Reprod Sci. 2016;23(5):644–654. doi: 10.1177/1933719115611753
- Ribeiro VB, Pedroso DCC, Kogure GS, et al. Short-term aerobic exercise did not change telomere length while it reduced testosterone levels and obesity indexes in PCOS: a randomized controlled clinical trial study. Int J Environ Res Public Health. 2021 18(21):11274. doi: 10.3390/ijerph182111274
- Rotterdam EA-SPCWG. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004; 81:19–25. doi: 10.1016/j.fertnstert.2003.10.004
- Teede HJ, Legro RS, Norman RJ. A vision for improving the assessment and management of PCOS through international collaboration. Semin Reprod Med. 2018;36:3–4. doi: 10.1055/s-0038-1667158
- Teede HJ, Misso ML, Costello MF, et al. International PN. Erratum. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2019;34:388. doi: 10.1093/humrep/dey363
- American College of Sports M. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41(3):687–708. doi: 10.1249/MSS.0b013e3181915670
- Fleck SJ, Kraemer WJ. Fundamentos do Treinamento de Forca Muscular. Porto Alegre: Artmed; 2006.
- Rhea MR, Ball SD, Phillips WT, et al. A comparison of linear and daily undulating periodized programs with equated volume and intensity for strength. J Strength Cond Res. 2002;16(2):250–5. doi: 10.1519/00124278-200205000-00013
- Kinanthropometry ISftAo. Adelaide, Australia: University of South Australia. 2001.
- Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883
- Fortin JP, Triche TJ Jr., Hansen KD, et al. Preprocessing, normalization and integration of the Illumina HumanMethylationEPIC array with minfi. Bioinformatics. 2017;33(4):558–60. doi: 10.1093/bioinformatics/btw691
- Nix DA, Courdy SJ, Boucher KM. Empirical methods for controlling false positives and estimating confidence in ChIP-seq peaks. BMC Bioinf. 2008;9(1):523. doi: 10.1186/1471-2105-9-523
- McLean CY, Bristor D, Hiller M, et al. GREAT improves functional interpretation of cis-regulatory regions. Nat Biotechnol. 2010;28(5):495–501. doi: 10.1038/nbt.1630
- Mi H, Muruganujan A, Casagrande JT, et al. Large-scale gene function analysis with the PANTHER classification system. Nat Protoc. 2013;8(8):1551–66. doi: 10.1038/nprot.2013.092
- Szklarczyk D, Morris JH, Cook H, et al. The STRING database in 2017: quality-controlled protein–protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45(D1):D362–D368. doi: 10.1093/nar/gkw937
- Horvath S. Erratum to: DNA methylation age of human tissues and cell types. Genome Biol. 2015;16:96. doi: 10.1186/s13059-015-0649-6
- Sillanpaa E, Ollikainen M, Kaprio J, et al. Leisure-time physical activity and DNA methylation age—a twin study. Clin Epigenetics. 2019;11(1):12. doi: 10.1186/s13148-019-0613-5
- Kogure GS, Silva RC, Miranda-Furtado CL, et al. Hyperandrogenism enhances muscle strength after progressive resistance training, independent of body composition, in women with polycystic ovary syndrome. J Strength Cond Res. 2018;32(9):2642–51. doi: 10.1519/JSC.0000000000002714
- Lua ACY, How CH, King TFJ. Managing polycystic ovary syndrome in primary care. Singapore Med J. 2018;59:567–71. doi: 10.11622/smedj.2018135
- Moran LJ, Hutchison SK, Norman RJ, et al. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2011:CD007506. doi: 10.1002/14651858.CD007506.pub4
- Pasquali R, Oriolo C. Obesity and Androgens in Women. Front Horm Res. 2019;53:120–134. doi: 10.1159/000494908
- Palomba S, Giallauria F, Falbo A, et al. Structured exercise training programme versus hypocaloric hyperproteic diet in obese polycystic ovary syndrome patients with anovulatory infertility: a 24-week pilot study. Hum Reprod. 2008;23(3):642–50. doi: 10.1093/humrep/dem391
- Franczyk B, Gluba-Brzozka A, Cialkowska-Rysz A, et al. The impact of aerobic exercise on HDL quantity and quality: a narrative review. Int J Mol Sci. 2023;24(5):24. doi: 10.3390/ijms24054653
- Cho KH. The Current status of research on high-density lipoproteins (HDL): a paradigm shift from HDL quantity to HDL quality and HDL functionality. Int J Mol Sci. 2022;23(7):23. doi: 10.3390/ijms23073967
- Varbo A, Nordestgaard BG. Commentary: triglycerides or HDL cholesterol in cardiovascular disease—which is the true culprit? Int J Epidemiol. 2019;48(5):1407–1408. doi: 10.1093/ije/dyy292
- Barron-Cabrera E, Ramos-Lopez O, Gonzalez-Becerra K, et al. Epigenetic modifications as outcomes of exercise interventions related to specific metabolic alterations: a systematic review. Lifestyle Genom. 2019;12(1–6):25–44. doi: 10.1159/000503289
- Nishiyama A, Nakanishi M. Navigating the DNA methylation landscape of cancer. Trends Genet. 2021;37(11):1012–27. doi: 10.1016/j.tig.2021.05.002
- Jacques M, Hiam D, Craig J, et al. Epigenetic changes in healthy human skeletal muscle following exercise– a systematic review. Epigenetics. 2019;14(7):633–648. doi: 10.1080/15592294.2019.1614416
- Visone R, Bacalini MG, Di Franco S, et al. DNA methylation of shelf, shore and open sea CpG positions distinguish high microsatellite instability from low or stable microsatellite status colon cancer stem cells. Epigenomics. 2019;11(6):587–604. doi: 10.2217/epi-2018-0153
- Yong WS, Hsu FM, Chen PY. Profiling genome-wide DNA methylation. Epigenet Chromatin. 2016;9(1):26. doi: 10.1186/s13072-016-0075-3
- Zhou D, Robertson KD. Role of DNA Methylation in Genome Stability. In: Kovalchuk I, Kovalchuk O, editors. Genome Stability From Virus to Human Application. 1st ed. Academic Press; 2016. p. 409–424. doi: 10.1016/B978-0-12-803309-8.00024-0
- Moran LJ, Noakes M, Clifton PM, et al. Genome instability is increased in lymphocytes of women with polycystic ovary syndrome and is correlated with insulin resistance. Mutat Res. 2008;639(1–2):55–63. doi: 10.1016/j.mrfmmm.2007.11.007
- An C, Pipia I, Ruiz AS, et al. The molecular link between obesity and genomic instability in cancer development. Cancer Lett. 2023;555:216035. doi: 10.1016/j.canlet.2022.216035
- Sellami M, Bragazzi N, Prince MS, et al. Regular, intense exercise training as a healthy aging lifestyle strategy: preventing DNA damage, telomere shortening and adverse DNA methylation changes over a lifetime. Front Genet. 2021;12:652497. doi: 10.3389/fgene.2021.652497
- Fiorito G, Caini S, Palli D, et al. DNA methylation-based biomarkers of aging were slowed down in a two-year diet and physical activity intervention trial: the DAMA study. Aging Cell. 2021;20(10):e13439. doi: 10.1111/acel.13439
- Eiras MC, Pinheiro DP, Romcy KAM, et al. Polycystic ovary syndrome: the epigenetics behind the disease. Reprod Sci. 2022;29(3):680–94. doi: 10.1007/s43032-021-00516-3
- Khan MJ, Ullah A, Basit S. Genetic basis of polycystic ovary syndrome (PCOS): Current perspectives. Appl Clin Genet. 2019;12:249–60. doi: 10.2147/TACG.S200341
- Zhang M, Li Y, Wang H, et al. LncRNA SNHG5 affects cell proliferation, metastasis and migration of colorectal cancer through regulating miR-132-3p/CREB5. Cancer Biol Ther. 2019;20(4):524–36. doi: 10.1080/15384047.2018.1537579
- He X, Ou C, Xiao Y, et al. LncRNAs: key players and novel insights into diabetes mellitus. Oncotarget. 2017;8(41):71325–41. doi: 10.18632/oncotarget.19921
- Mohamadi M, Ghaedi H, Kazerouni F, et al. Deregulation of long noncoding RNA SNHG17 and TTC28-AS1 is associated with type 2 diabetes mellitus. Scand J Clin Lab Invest. 2019;79(7):519–23. doi: 10.1080/00365513.2019.1664760
- Dierssen M, Fructuoso M, Martinez de Lagran M, et al. Down syndrome is a metabolic disease: altered insulin signaling mediates peripheral and brain dysfunctions. Front Neurosci. 2020;14:670. doi: 10.3389/fnins.2020.00670
- Li M, Lin C, Cai Z. Downregulation of the long noncoding RNA DSCR9 (down syndrome critical region 9) delays breast cancer progression by modulating microRNA-504-5p-dependent G protein-coupled receptor 65. Hum Cell. 2023;36(4):1516–1534. doi: 10.1007/s13577-023-00916-4
- Barres R, Yan J, Egan B, et al. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab. 2012;15(3):405–11. doi: 10.1016/j.cmet.2012.01.001
- Zhang FF, Cardarelli R, Carroll J, et al. Physical activity and global genomic DNA methylation in a cancer-free population. Epigenetics. 2011;6(3):293–9. doi: 10.4161/epi.6.3.14378
- Ngwa JS, Nwulia E, Ntekim O, et al. Aerobic exercise training-induced changes on DNA methylation in mild cognitively impaired elderly African Americans: gene, exercise, and memory study - GEMS-I. Front Mol Neurosci. 2021;14:752403. doi: 10.3389/fnmol.2021.752403
- Tryfidou DV, McClean C, Nikolaidis MG, et al. Correction to: DNA damage following acute aerobic exercise: a systematic review and meta-analysis. Sports Med. 2020;50(1):129–32. doi: 10.1007/s40279-019-01197-4
- Tryfidou DV, McClean C, Nikolaidis MG, et al. DNA damage following acute aerobic exercise: a systematic review and meta-analysis. Sports Med. 2020;50(1):103–27. doi: 10.1007/s40279-019-01181-y
- Lindholm ME, Marabita F, Gomez-Cabrero D, et al. An integrative analysis reveals coordinated reprogramming of the epigenome and the transcriptome in human skeletal muscle after training. Epigenetics. 2014;9(12):1557–69. doi: 10.4161/15592294.2014.982445
- Bagley JR, Burghardt KJ, McManus R, et al. Epigenetic Responses to Acute Resistance Exercise in Trained vs Sedentary Men. J Strength Cond Res. 2020;34(6):1574–1580. doi: 10.1519/JSC.0000000000003185
- Seaborne RA, Strauss J, Cocks M, et al. Human skeletal muscle possesses an Epigenetic memory of hypertrophy. Sci Rep. 2018;8(1):1898. doi: 10.1038/s41598-018-20287-3
- Waddington CH. Canalization of development and genetic assimilation of acquired characters. Nature. 1959;183(4676):1654–5. doi: 10.1038/1831654a0
- Machado OAS, Diniz VLS, Passos MEP, et al. Physical exercise increases global and gene-specific (interleukin-17 and interferon-γ) DNA methylation in lymphocytes from aged women. Exp Physiol. 2021;106(9):1878–1885. doi: 10.1113/EP089673
- Ronn T, Volkov P, Davegardh C, et al. A six months exercise intervention influences the genome-wide DNA methylation pattern in human adipose tissue. PLoS Genet. 2013;9(6):e1003572. doi: 10.1371/journal.pgen.1003572
- Fabre O, Ingerslev LR, Garde C, et al. Exercise training alters the genomic response to acute exercise in human adipose tissue. Epigenomics. 2018;10(8):1033–50. doi: 10.2217/epi-2018-0039
- Robinson MM, Dasari S, Konopka AR, et al. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and Old Humans. Cell Metab. 2017;25(3):581–92. doi: 10.1016/j.cmet.2017.02.009
- Shu C, Zhang X, Aouizerat BE, et al. Comparison of methylation capture sequencing and infinium MethylationEPIC array in peripheral blood mononuclear cells. Epigenet Chromatin. 2020;13(1):51. doi: 10.1186/s13072-020-00372-6
- Greenwood EA, Noel MW, Kao CN, et al. Vigorous exercise is associated with superior metabolic profiles in polycystic ovary syndrome independent of total exercise expenditure. Fertil Sterility. 2016;105(2):486–93. doi: 10.1016/j.fertnstert.2015.10.020
- Gibbs WW. Biomarkers and ageing: the clock-watcher. Nature. 2014;508(7495):168–70. doi: 10.1038/508168a
- Martins I, Ribeiro IP, Jorge J, et al. Liquid biopsies: applications for cancer diagnosis and monitoring. Genes (Basel). 2021;12(3):12. doi: 10.3390/genes12030349
