ABSTRACT
While transgenerational epigenetic inheritance has been extensively documented in plants, nematodes, and fruit flies, its existence in mammals remains controversial. Several factors have contributed to this debate, including the lack of a clear distinction between intergenerational and transgenerational epigenetic inheritance (TEI), the inconsistency of some studies, the potential confounding effects of in-utero vs. epigenetic factors, and, most importantly, the biological challenge of epigenetic reprogramming. Two waves of epigenetic reprogramming occur: in the primordial germ cells and the developing embryo after fertilization, characterized by global erasure of DNA methylation and remodelling of histone modifications. Consequently, TEI can only occur if specific genetic regions evade this reprogramming and persist through embryonic development. These challenges have revived the long-standing debate about the possibility of inheriting acquired traits, which has been strongly contested since the Lamarckian and Darwinian eras. As a result, coupled with the absence of universally accepted criteria for transgenerational epigenetic studies, a vast body of literature has emerged claiming evidence of TEI. Therefore, the goal of this study is to advocate for establishing fundamental criteria that must be met for a study to qualify as evidence of TEI. We identified five criteria based on the consensus of studies that critically evaluated TEI. To assess whether published original research papers adhere to these criteria, we examined 80 studies that either claimed or were cited as supporting TEI. The findings of this analysis underscore the widespread confusion in this field and highlight the urgent need for a unified scientific consensus on TEI requirements.
Introduction
An animal’s genome undergoes two distinct waves of epigenetic reprogramming. The first occurs in developing primordial germ cells (PGCs), and the second occurs in the developing embryo after fertilization [Citation1]. These two waves are characterized by the erasure of DNA methylation and the remodelling of histone modifications [Citation2,Citation3]. DNA methylation is a key epigenetic mechanism that adds methyl groups to DNA, primarily at cytosine bases followed by guanine (CpG sites). It plays important roles in regulating gene expression, genomic imprinting, X-chromosome inactivation, and maintaining genomic stability [Citation4]. Histone proteins package DNA into chromatin. Chemical changes, like adding or removing acetyl, methyl, or phosphate groups, modify these proteins and affect chromatin structure and function, influencing gene expression, DNA repair, and replication [Citation4]. Epigenetic reprogramming is necessary to establish totipotency from the two differentiated germ cells [Citation1]. However, despite these two waves of epigenetic reprogramming, some genomic regions, such as imprinted control regions and transposable elements, resist this reprogramming. Additionally, although many germ cell epigenetic modifications are erased after fertilization, some epigenetic modifications persist through embryonic development, supporting the hypothesis that parental epigenetic marks outside of imprinted regions and transposable elements can be heritable and transmitted to subsequent generations [Citation5]. In Caenorhabditis elegans, the ingestion of environmental double-stranded RNA (dsRNA) triggers the RNA interference (RNAi) pathway, which processes the dsRNA into small interfering RNAs (siRNAs) that silence complementary genes [Citation1]. These secondary siRNAs propagate the effects of the RNAi pathway to subsequent generations, mediated by germline epigenetic modifications such as PIWI-interacting RNAs (piRNAs) and the histone modifications H3K9me3 and H3K36 [Citation1].
Evidence for inherited epigenetic alterations due to environmental factors in mammals
The last two decades of epigenetic research have revealed that both intrinsic and extrinsic environmental factors, including diet, toxicant exposure, inflammation, social stress, physical activity, ageing, medication, and metabolic and hormonal disorders, can influence epigenetic modifications in germ cells, which may subsequently be inherited by multiple generations [Citation6–8]. This phenomenon has been called intergenerational or transgenerational epigenetic inheritance (TEI) interchangeably. Major groundbreaking studies demonstrating epigenetic inheritance in mammals include the Dutch famine in humans [Citation9], the agouti locus in mice [Citation10–12], and the endocrine disruptors in rats [Citation13] studies. These studies provided robust evidence that environmental factors, such as maternal diet or endocrine disruptor exposure, during pregnancy can induce physiological and epigenetic alterations in the offspring, influencing postnatal phenotypes.
The Dutch famine of 1944–1945 had a profound and lasting impact on the health of both the women who experienced it in the womb and their offspring [Citation14]. Prenatal exposure to famine was linked to a range of adverse health outcomes, including reduced birth weight, elevated risks of cardiovascular and metabolic diseases in adulthood, increased susceptibility to schizophrenia, and transgenerational effects associated with elevated neonatal adiposity [Citation14]. Furthermore, Heijmans et al. [Citation9] demonstrated that periconceptional exposure to famine was associated with hypomethylation of the insulin-like growth factor 2 (IGF2) gene six decades later in the offspring. These studies provided the first evidence that transient environmental exposures early in gestation can be recorded as persistent epigenetic alterations.
Another seminal study is on the epigenetic inheritance in mice of the agouti viable yellow (Avy) epiallele, where maternal methyl donor supplementation during pregnancy influenced coat colour and obesity in the F1 and F2 generations [Citation11]. These studies revealed that the offspring of supplemented mothers displayed increased DNA methylation at six CpG sites in a retrotransposon upstream of the Agouti gene [Citation11,Citation12]. One of the earliest examples of epigenetic inheritance induced by endocrine disruptors is the study by Anway et al. [Citation13], in which prenatal exposure of female rats to the fungicide vinclozolin resulted in infertility and altered DNA methylation patterns in subsequent generations.
These groundbreaking studies sparked a surge of research exploring the effects of environmental exposures on the transgenerational inheritance of epigenetic marks and phenotypes. These studies spanned a wide range of species and environmental factors, including diet, stress, toxicants, trauma, pollution, radiation, and countless others. For example, paternal diet has been shown to perturb the methylation and acetylation patterns in the offspring leading to altered cholesterol and lipid metabolism [Citation15] and gene expression in the offspring [Citation16]. Ryan et al. [Citation17] demonstrated that paternal consumption of methyl donor supplements for six weeks prior to mating impaired cognitive and neural function in the offspring. Specifically, mice born to supplemented fathers displayed deficits in hippocampus-dependent spatial learning, decreased hippocampal synaptic plasticity, and impaired contextual fear conditioning. Interestingly, these mice exhibited increased DNA methylation patterns and decreased expression of the Kcnmb2 gene, which is involved in the regulation of neural learning and memory. This study highlighted the increased susceptibility to epigenetic modifications during critical windows of germ cell development, such as the pre-mating period. In a separate study, Wen et al. [Citation18] employed a rat model to assess the impact of maternal vitamin D deficiency on phenotypic performance and DNA methylation in the offspring. They observed that maternal vitamin D deficiency during pregnancy impaired body weight regulation, body fat composition, glucose and lipid metabolism in adulthood, and pre-adipocyte proliferation and differentiation in the F1 generation. DNA methylation and RNA-sequencing analyses revealed differential methylation of over 600 promoters and 204 CpG islands between the vitamin D deficient and control groups. Ponomarev et al. [Citation19] evaluated post-mortem human brains to investigate the effects of chronic alcohol use. They observed that alcohol upregulates long terminal repeat (LTR) transposons through a decrease in methylation. They also discovered that alcohol decreases the activity of DNA methyltransferase 1 (DNMT1), which may explain a mechanism for this hypomethylation. Another study found that F0 female rats exposed to alcohol during gestation produced F1 males with decreased expression and increased methylation of hypothalamic Pomc. This effect was also found to be transmitted to the F2 and F3 male progeny though the male germline. Notably, the hypermethylation of the Pomc gene in the hypothalamus was accompanied by a similar hypermethylation of Pomc in sperm cells of F1-F3 [Citation20]. In cattle, maternal nutrition during the periconceptual period programmes pregnancy establishment, foetal and placental development, and postnatal development and growth [Citation21]. In sheep, increased late pregnancy nutrition upregulates genes regulating adipogenesis and lipogenesis in foetal perirenal adipose tissue, which may predispose the mature animal to adiposity [Citation22]. Furthermore, a restricted supply of methyl donors around conception is associated with epigenetic alterations, increased adiposity, insulin resistance, and hypertension in adult sheep [Citation23].
To investigate whether TEI occurs in birds, Leroux et al. [Citation24] injected genistein, a plant-derived phytoestrogen and putative methylation modifier, into Japanese quail eggs and monitored the effects of genistein on the F3 generation. They observed significant effects of genistein egg treatment on sexual maturity, adult body weight, and behavioural traits across generations. However, there was no evidence of epigenetic mechanisms underlying these traits, as no significant DNA methylation differences were detected between the genistein-injected and control birds [Citation24]. These and numerous other studies on epigenetic inheritance have established that parental exposure to diverse environmental factors can alter epigenetic modifications, leading to phenotypic changes in the offspring.
Challenges of observing transgenerational epigenetic inheritance in mammals
The concept of TEI in mammals faces several challenges, some of which are biological and others of which are due to a lack of consensus on the definition of TEI. A major challenge to the existence of TEI in mammals are the two distinct epigenetic reprogramming events: in primordial germ cells and in the early developing embryo. Due to these two rounds of epigenetic reprogramming, Heard and Martienssen [Citation25] concluded that it is unlikely for environmentally induced or programmed epigenetic marks to be inherited. Several studies [Citation26,Citation27] concluded that many reported TEI effects were actually intergenerational effects. A further challenge facing true observation of TEI in mammals is the potential confounding effect of intrauterine environment and genetic factors in the transmission of epigenetic marks and their subsequent phenotypic effects [Citation6,Citation8,Citation28]. Epigenetic changes can be caused by DNA mutations, as well as by environmental exposures. Therefore, disentangling genetic from epigenetic effects in TEI studies is challenging [Citation28].
The reproducibility of TEI evidence across studies has also been challenged by several scientists [Citation29]. For example, Anway et al. [Citation30] exposed rats in utero to vinclozolin, an endocrine-disrupting fungicide used in crops, during the period of gonadal sex determination and monitored developmental abnormalities in the F1 and subsequent generations of males. The authors reported transgenerational disease effects such as immune system and testicular abnormalities, prostate disease, and tumorigenesis up to the F4 generation [Citation30]. However, in a subsequent study from the same research group, Guerrero-Bosagna et al. [Citation31] found that vinclozolin exposure was associated with transgenerational disease phenotypes in outbred CD-1 mice, but not in inbred 129 mice. Additionally, analysis of the F3 generation sperm epigenome identified DNA methylation regions altered by grandparental vinclozolin exposure. In contrast, Iqbal et al. [Citation32] found that DNA methylation changes induced by vinclozolin in gestating female inbred mice were not passed to the F2 generation. The authors concluded that epigenetic changes induced by endocrine disruptors could be reversed in subsequent generations, which contradicts the TEI results of Guerrero-Bosagna et al. [Citation31]. However, the use of different mouse or rat strains and the lack of similar phenotypes across these studies make it difficult to draw definitive conclusions on the existence of TEI in mammals. Consequently, Nadeau [Citation33] concluded that these studies do not represent true TEI.
Defining and substantiating transgenerational epigenetic inheritance
Notably, many studies have described the effects of environmental factors on subsequent generations using the term ‘transgenerational epigenetic inheritance.’ While this has raised public awareness of gene-environment interactions and stimulated significant private and public funding for epigenetic research, as well as unprecedented media attention, it has also generated considerable confusion about the definition of TEI. Consequently, many scientists have called for a clear definition of TEI, and some have even expressed scepticism about its existence in mammals [Citation25].
Given the aforementioned challenges in TEI studies, numerous literature reviews have discussed definitions of mammalian TEI. There is a broad consensus that TEI in mammals requires the persistence of phenotypic changes induced by environmental stimuli across successive generations unexposed to the initial environmental stimulus [Citation7,Citation8,Citation26,Citation27,Citation34]. However, it is important to note that the mere transgenerational inheritance of phenotypic changes does not definitively demonstrate TEI, and may erroneously classify intergenerational epigenetic effects as TEI, as evidenced by several studies [Citation26]. Therefore, it is essential that environmentally induced epimutations in germ cells [Citation35] evade epigenetic reprogramming and are subsequently transmitted to generations unexposed to the initial environmental stimulus [Citation8,Citation26,Citation28,Citation34,Citation36]. Van Otterdijk and Michels [Citation8] have opined that, in mammals, TEI due to environmental exposure during gestation requires that epigenetic marks be maintained for at least four generations. When a gestating female (F0) is exposed to an environmental insult, the embryo (F1) and its germline (F2) are also exposed. Therefore, the F3 generation is required to validate TEI. To assess TEI effects in males, at least the third-generation offspring (F2) must be investigated [Citation8]. However, data supporting this phenomenon in mammals remain scarce and controversial.
Blanco Rodríguez and Camprubí Sánchez [Citation36] have outlined several prerequisites for TEI substantiation in mammals, including the identification of epimutations in germ cells altered by the environment, the transmission of these epimutations to unexposed generations, the association of phenotypic consequences with these epimutations, and the persistent presence of the same mutations in both sperm and somatic tissues across successive generations. Other scientists have defined TEI as changes in chromatin state, gene expression, or phenotypes inherited by subsequent generations independent of DNA sequence alterations [Citation37]. The lack of consensus among the scientific community on the requirements for TEI has led to the conclusion that many reported TEI observations are actually intergenerational epigenetic inheritance effects [Citation26] or are due to intrauterine or genetic factors [Citation8]. Therefore, the objectives of this study are to establish consensus criteria for TEI and conduct a critical evaluation of existing epigenetic inheritance studies to determine their adherence to these criteria.
Methods
In this study, we identified five essential elements of TEI and assessed whether published research papers cited as TEI studies met these criteria. The selected TEI criteria include:
Inheritance of phenotypes by the first unexposed generation induced by environmental factor(s) applied to the F0 generation.
Inheritance of epigenetic marks by the first unexposed generation induced by environmental factor(s) applied to the F0 generation.
Inheritance of the same epimutation(s) across generations.
Gene expression changes in subsequent generations.
Germ cells tested in each generation.
Obviously, fulfilling the TEI requirement requires other criteria, such as the transmission of epigenetic variations from germ cells to somatic tissues [Citation36], the challenge of disentangling genetic vs. epigenetic effects [Citation28], and the demonstration of causative effects of epigenetic variations [Citation7,Citation28]. We selected 80 original research papers investigating TEI effects in response to various environmental stimuli in a wide range of species (Supplementary Table 1). Papers were considered eligible for review if they claimed to demonstrate TEI or if the paper had been cited as a transgenerational study. These studies include parental nutrition (n = 20), vinclozolin (n = 20), bisphenol A (n = 20), and other environmental stressors such as social stress (n = 8), smoking (n = 8), and heat stress (n = 4). We assessed each of the 80 studies against the five TEI criteria above to determine whether it met those criteria.
Results and discussion
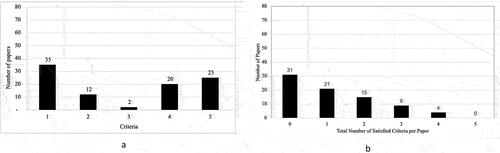
shows the total number of studies that met each TEI criterion and the total number of criteria that were met per paper. Out of the 80 studies evaluated, 35 reported the inheritance of phenotypes in the first unexposed generation following environmental exposure (). Notably, only 12 studies documented the inheritance of epigenetic marks in the first unexposed generation, with two studies reporting the transmission of the same epimutation across generations (). Furthermore, 20 studies observed gene expression changes spanning multiple generations following environmental triggers (). Recognizing the role of germ cells in transferring environmentally induced epigenetic alterations to offspring, 25 studies examined sperm or oocytes (). illustrates that among the 80 studies evaluated, 31 did not meet any TEI criteria, while 21 met only one criterion. Additionally, 15 studies met two criteria, nine met three criteria, and four studies met four criteria. Notably, none of the studies met all five TEI criteria. The following sections present specific examples of investigations examining environmental exposure effects on subsequent generations and assess whether they meet the criteria for studies of TEI.
Figure 1. (a) Summation of the number of papers reviewed that met each of the different specified criteria necessary for TEI qualification. A total of 80 papers were analysed for each TEI criteria category. Criteria on the x-axis include 1 = inheritance of phenotypes; 2 = inheritance of epigenetic marks; 3 = inheritance of the same epimutations; 4 = gene expression changes across generations; and 5 = germ cell tested in each generation. The numbers above each column depict the total number of papers that met the qualifications for that criteria category. (b) Summation of the number of total TEI criteria that were met per paper reviewed. The x-axis indicates the total number of criteria that were satisfied per paper, ranging from 0 (no criteria met) to 5 (all criteria met). The numbers above each column depict the total number of papers that met that number of criteria.
Parental nutrition studies
To assess whether parental nutrition studies meet the transgenerational epigenetic inheritance criteria, we selected 20 research papers representing various species, including mouse, rat, sheep, human, pig, and guinea pig (Supplementary Table 1). Nine studies demonstrated inheritance of phenotypes by the first generation not exposed to the diet, and five studies reported inheritance of epigenetic marks across multiple generations. However, only one study reported the inheritance of methylated cytosines by the F2 generation in response to the paternal diet [Citation38]. Gene expression changes in subsequent generations were reported in four studies, and only three studies examined germ cells in each generation (Supplementary Table 1).
As an example study, in the one by Carone et al. [Citation15], male mice were fed a low-protein diet from weaning to sexual maturity and bred to control females to produce the F1 generation. Upon evaluating the phenotypes, the livers of F1 animals born to low-protein fathers exhibited depleted cholesterol and cholesterol esters, accompanied by an increase in saturated free fatty acids and monosaturated triacylglycerides. However, these phenotypic changes do not align with our definition of TEI, as only the F1 generation was assessed. To elucidate how the diet influenced the germ cells of the F0 generation, a global DNA methylation analysis was conducted on the sperm of two control mice, one low-protein diet mouse, and one calorie-restricted mouse using MeDIP-Seq. This analysis revealed no obvious differences between the treatment and control groups, and F1 sperm were not evaluated for methylation alterations. Instead, Reduced Representation Bisulphite Sequencing (RRBS) was performed on the livers of one control and one low-protein offspring (F1) mouse, yielding a single gene of interest: PPARA. Using bisulphite sequencing, 17 additional offspring livers were analysed, revealing an 8% methylation difference at multiple CpGs within the PPARA locus. The F0 sperm was examined for methylation changes at the PPARA locus; however, no differences were detected between the treatment and control groups. These analyses of epigenetic marks also do not meet our TEI definition, as germ cells were only evaluated in F0, followed by somatic cell evaluation in F1 without including an F2 generation. Similarly, differentially expressed liver miRNAs were identified between the treatment and control offspring, but only for a single generation. Therefore, despite being one of the most frequently cited papers on transgenerational effects, this study does not fulfil the TEI criteria because the measured effects are constrained to only one generation.
While the majority of nutritional epigenetic studies have focused on mice and rats, livestock species have largely been overlooked. This prompted our laboratory to utilize Polypay sheep as a model for TEI [Citation38]. This study randomly assigned 10 male twin pairs to two groups. One animal from each pair received the control diet, while the other twin received an additional top dress of rumen-protected methionine from weaning until puberty. Five rams from each group were then bred to untreated females to generate the F1 generation. Subsequently, to generate the F2 generation, 10 F1 rams (one from each F0 sire) were bred to untreated females. Across each generation, phenotypes were collected, revealing a significant association between testicular size in males from both the F1 and F2 generations and the F0 diet. This finding provides evidence of a transgenerationally inherited phenotype. To investigate the influence of the methionine diet on DNA methylation, whole-genome bisulphite sequencing was conducted on sperm cells from the F0, F1, and F2 generations. Notably, 107 differentially methylated cytosines (DMCs) were found to overlap across all three generations, with 96 of these DMCs exhibiting the same trend of hypermethylation or hypomethylation across all generations. This observation fulfils our criteria for the inheritance of the same DMCs through the germ cells of multiple generations. Finally, RNA-Seq analysis of sperm samples from the F2 generation identified 12 genes with expression levels correlated to the methylation levels of the TEI DMCs. This study fulfilled four TEI criteria: the inheritance of phenotypes and epigenetic marks induced by the paternal diet to the first untreated generation, the assessment of germ cells in each generation, and the transmission of environmentally altered sperm DMCs to the F1 and F2 generations. However, gene expression changes were evaluated only in sperm samples from the F2 generations.
Vinclozolin studies
Vinclozolin, a commonly used fungicide to protect fruits and vegetables from fungal damage, has been subjected to extensive scrutiny in questionable TEI studies. Anway et al. [Citation13] reported that exposure of pregnant rats to the endocrine disruptor vinclozolin during sex determination resulted in reduced male fertility and a decrease in gene expression of DNA methyltransferases. However, other studies have not confirmed these findings [Citation39,Citation40]. In our analysis, we examined 20 original research papers on the effects of maternal exposure to vinclozolin in mice and rats to evaluate whether these studies align with the TEI criteria. The inheritance of phenotypes across multiple generations in response to vinclozolin exposure was reported in five studies (Supplementary Table 1). The inheritance of epigenetic marks to the first unexposed generation was reported in two studies, whereas only ones study reported the inheritance of the same epimutation (Supplementary Table 1). However, many of the vinclozolin studies examined germ cells in each generation (n = 14), and gene expression changes were reported in seven studies (Supplementary Table 1).
Nilsson et al. [Citation41] assessed the effect of several environmental toxicants, including pesticides, plastics, dioxin, jet fuel, and vinclozolin on promoting transgenerational inheritance of ovarian disease in females. They administered 100 mg/kg BW/d vinclozolin to pregnant rats during gestation on days 8–14. The authors reported a statistically significant decrease in ovarian primordial follicle pool size and an increase in ovarian cysts compared to the control in F1 and F3 generations in response to vinclozolin exposure. The MeDIP-Chip analysis of F3 generation granulosa cells revealed 43 DMRs between the control and vinclozolin lineages. However, F1 and F2 generations were not tested for differential methylation analysis. Interestingly, 523 differentially expressed genes were identified between the vinclozolin and the control groups, of which 30 were related to ovarian disease. In conclusion, the inheritance of phenotypes and DMRs was observed in the F3 generation, and there was no evidence of inheritance in the F2 generation. Also, there was no evidence of inheritance of specific epimutations altered by vinclozolin across generations.
In a separate study, Brieño-Enríquez et al. [Citation42] examined vinclozolin’s potential to induce transgenerational inheritance in mice. Pregnant mothers were given vinclozolin orally at two different doses: low dose (V1 = 1 mg/kg bw/d) and high dose (V2 = 100 mg/kg bw/d), from 0.5 to 13.5 days post coitum (dpc). The authors found evidence of phenotype transmission across F1 and F3 generations. Male mice from each generation in both V1 and V2 groups exhibited an elevated rate of apoptotic cells in the seminiferous tubules and a reduced fertility rate. However, no statistically significant DNA methylation changes were found in sperm or primordial germ cells between the treated and control generations. Furthermore, the researchers did not report the inheritance of specific DNA methylation changes across generations. However, they did find evidence of gene expression alterations. They observed deregulation and alternative gene expression of the microRNA Lin28/let-7/Blimp1 pathway in primordial germ cells in all three generations of male experimental mice. In conclusion, although the researchers observed the inheritance of phenotypes and changes in gene expression and met the TEI requirement of germ cell testing and gene expression, there is no evidence supporting the inheritance of specific epigenetic marks. This indicates a lack of evidence that vinclozolin is a causal factor in TEI.
Bisphenol a studies
Bisphenol A (BPA) is a chemical used to make polycarbonate plastics, which are used in various products, including beverage containers, compact disks, plastic dinnerware, automobile parts, and toys. BPA has been shown to affect the reproductive systems of laboratory animals, and there is some concern that it may also harm human health. Some of the potential health effects of BPA include aggressive behaviour and hyperactivity in children; reduced sperm production, sperm count, and motility in men; increased risk of cardiovascular disease; diabetes; liver enzyme abnormalities; and decreased bone strength and bone mineral content [Citation43]. We assessed the TEI criteria in 20 original research papers investigating the transgenerational effects of paternal and maternal exposure to BPA in rats and mice. Fourteen studies reported the inheritance of phenotypes across multiple generations, whereas only four reported the inheritance of epigenetic marks (Supplementary Table 1). Interestingly, these studies have not reported the inheritance of specific epimutations altered by BPA but provided evidence for gene expression changes and the testing of germ cells across generations in six studies each (Supplementary Table 1).
For example, the study by Rahman et al. (2020)[Citation44] aimed to investigate the impact of paternal BPA exposure on male fertility in multiple generations of mice. They exposed 10 male mice to two different levels of BPA, classified as the no observed adverse effect level (NOAEL) and the lowest observed adverse effect level (LOAEL), corresponding to doses of 5 and 50 mg/kg·bw/day, respectively. Using these 10 exposed mice, each male was bred with an unexposed female to generate the first F1 generation. This process was repeated to produce subsequent generations up to the F3 generation, utilizing male offspring from each new generation. Transgenerational phenotypic analysis revealed a significant decrease in the size of testicular seminiferous tubules in both the F1 and F2 generations descended from BPA-exposed mice. The number of stage 8 testicular seminiferous epithelial cells was also decreased in the F0-F2 BPA mice but slightly increased again in the F3 generation. However, all BPA-exposed mice still exhibited a significantly smaller amount compared to the control group. Interestingly, global sperm DNA methylation analysis demonstrated significantly higher levels of 5-mC in the F0-F3 generations of BPA mice, with more prominent changes observed in the LOAEL group compared to the NOAEL group. Moreover, 5-mC levels were notably higher in F1-F3 generation males compared to F0 males. Western blot analysis indicated a significant decrease in protein kinase-A and tyrosine phosphorylation in the spermatozoa of the F0 BPA mice compared to the control group, potentially linked to sperm motility impairment due to BPA exposure. However, protein analysis was not conducted on the spermatozoa of the F1-F3 generations.
Although this study is novel in investigating the multigenerational effects of BPA on male fertility following paternal exposure, it only fulfils partial TEI criteria. The study demonstrated the inheritance of phenotypes and epigenetic marks by at least the first nonexposed generation. Additionally, the criterion of testing germs cells was met by analysing sperm motility, sperm concentration, sperm viability, and sperm hyperactivated motility for all generations. However, gene expression was conducted in only the F0 generation, and there was no evidence of the inheritance of specific epimutations altered by the BPA exposure.
In a different study, Wolstenholme et al. [Citation45] examined the transgenerational effects of prenatal BPA exposure on social recognition using different phenotypic assessments that indicate changes in social behaviour and recognition within juvenile C57BL/6J mice. The authors exposed female mice to 20 μg/day of BPA, or 5 mg/kg, over the course of gestation. The F1 offspring were separated and placed with a foster mom to reduce post-gestational BPA exposure. Afterwards, brother-sister pairs from each generation were used to produce the subsequent F2 and F3 offspring.
Transgenerational phenotypic analysis revealed an increase in social investigation in both male and female F1 generation mice exposed to BPA. When all juvenile social recognition test trials were combined, BPA-exposed F1 mice spent significantly more time investigating both the known and unknown stimuli compared to the control group, with no differences observed between sexes. In the F3 generation, male and female mice from the BPA lineage exhibited greater interaction with the known stimulus than the control group, while the control group showed higher interaction time with the unknown stimulus compared to the BPA group. Subsequently, an open field locomotor activity test was conducted on the F1 and F3 generations. No significant differences were observed between the BPA and control groups in the F1 generation. However, in the F3 generation, mice from the BPA lineage displayed higher activity levels compared to their control counterparts, with significantly more crossings through the centre and outer edge of the testing arena, as well as a higher overall number of crossings. Both the F3 control and BPA mice exhibited similar anxiety-related behaviours, spending comparable amounts of time in the arena centre, corners, and along the arena wall. Thus, it was suggested that F3 BPA mice exhibit anxiety behaviour similar to the control group but are more active overall. Additionally, an olfactory discrimination test was conducted on adult F3 mice, revealing similar investigation times for olfactory stimuli between the control and BPA groups. It is important to note that all results focused on and were limited to phenotypic observations.
This study utilizes unique investigative tools to measure and assess differences in mouse behaviour. However, it only fulfils one out of five criteria for TEI. The criterion of inheriting phenotypes was demonstrated by testing through the first unexposed generation. Despite evaluating the appropriate number of generations to investigate TEI effects, there is a lack of evidence regarding the assessment of gene expression, inheritance of specific epimutations, testing of germ cells, and inheritance of epigenetic marks. As a result, this study does not meet the requirements of the TEI criteria.
Stress and smoking studies
While specific exposures to known compounds can cause epigenetic changes, it is also important to consider exposures that may occur in the environment or during different phases of life. Examples of such instances include heat stress, social stress, and smoking. Both heat stress and social stress represent periods of duress for an animal, which can be observed in their epigenome. Similarly, exposure to smoke is sometimes an unavoidable and significant environmental insult. Although we know that these instances can have an impact on the epigenome, ongoing research aims to define the transgenerational inheritance of these effects. It is possible that these exposures could lead to epigenetic changes that are passed down to future generations. To assess whether these exposures can induce phenotypic and epigenetic changes evident in subsequent generations, we analysed eight social stress studies, eight smoking studies, and four heat stress studies in various species, including human, cattle, mice, rats, swine, and wild guinea pigs (Supplementary Table 1). The transgenerational inheritance of phenotypes was reported in four social stress studies, two smoking studies, and only one heat stress study, with only one study reporting the inheritance of epigenetic marks (Supplementary Table 1). However, the inheritance of the same epimutation was not reported in any of these studies. Gene expression changes across multiple generations were examined in two social stress studies and one smoking study, and germ cells testing in each generation was performed in only two social stress studies (Supplementary Table 1).
The study conducted by Franklin et al. [Citation46] examined the transmission of epigenetic effects of early stress through the F3 generation. A group of mice underwent unpredictable maternal separation and maternal stress (MSUS). In each generation, a male mouse from the exposed line was bred with a control female until reaching the F3 generation. Maternal care scoring and behaviour testing were carried out in each generation and compared to control groups. The study found that MSUS induced depressive-like behaviours in F3 MSUS males, supporting the epigenetic inheritance of this phenotype. The observed phenotypic changes were further supported by molecular evidence. Epigenetic marks, specifically differential methylation of cytosines, were quantified using bisulphite-converted DNA through pyrosequencing. The study revealed a decrease in methylation of the CRFR2 CpG island in the germline of F2 mice compared to F2 MSUS males. However, the observed DNA methylation differences between the groups were relatively small (<10%), and their biological functional relevance is questionable. DNA methylation in sperm was also examined in both F1 and F2 MSUS mice, showing altered DNA methylation patterns across multiple generations. In summary, this study partially met four of the TEI criteria.
In the study by Dias and Ressler [Citation47], male mice were subjected to odour fear conditioning by acetophenone or propanol and then bred to unexposed females to produce the F1 generation. Unexposed F1 animals were then mated to produce the F2 generation. In the F0 generation, olfactory fear conditioning adult males to acetophenone was found to increase the fear potentiated startle when the pain stimulus was paired with the acetophenone scent. Though the F1 generation was never conditioned to fear the scent, the offspring of fathers conditioned to acetophenone showed an enhanced odour potentiated startle when compared to controls. This startle response to acetophenone was also found to persist into the F2 generation. In addition, M71-specific glomeruli in the olfactory bulb of F1 and F2 offspring of acetophenone-trained F0 males were found to be significantly increased in size compared to control and propanol animals, thus meeting our phenotype criterion for TEI. In order to explain the enhanced representation for the M71 receptor, bisulphite sequencing of sperm cells was performed around the Olfr151 (M71) locus as well as the non-acetophenone responsive Olfr6 locus. The Olfr151 locus was found to be significantly less methylated in the sperm produced from F0 acetophenone exposed animals when compared to propanol animals, with this trend also persisting into the F1 sperm. There was one overlapping significant CpG observed between the F0 and F1 sperm. However, sperm from the F2 generation was not evaluated, therefore we are unable to conclude if epigenetic marks are truly transgenerationally inherited in this study.
In summary, this comprehensive study scrutinized the literature on TEI across diverse species and environmental factors to evaluate whether studies claiming or citing TEI adhere to fundamental TEI criteria. These criteria were established based on recent calls for a unified TEI definition. Notably, fewer than half (45%) of the studies reported phenotypic inheritance in the first unexposed generation, while only 14% documented epigenetic mark inheritance in the first unexposed generation. Surprisingly, only three TEI studies reported the inheritance of the same epimutation triggered by the environmental factor. Indeed, establishing a link between epigenetic modifications and phenotypic outcomes poses a significant challenge in TEI studies [Citation6,Citation28]. Therefore, the inheritance of the same epimutation across generations is critical to rule out confounding factors such as DNA sequence changes and parental effects that could alter phenotypic outcomes [Citation26]. Approximately 28% of the studies examined gene expression changes across generations in response to environmental triggers. These findings underscore the need for the epigenetics community to adopt consensus criteria for determining TEI in mammals. Elucidating the existence of TEI in mammals holds profound implications for understanding the potential inheritance of acquired traits. Additionally, it could shed light on epigenetic reprogramming in mammals and the intricate interplay between genetic and epigenetic variation.
Author contributions
HK conceived the study and wrote the manuscript. GC, JAH, MAK, and JT collected data and contributed to the manuscript writing. All authors discussed the results and commented on the manuscript.
Supplementary Table 1.docx
Download MS Word (100.3 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15592294.2024.2333586
Additional information
Funding
References
- Radford EJ. Exploring the extent and scope of epigenetic inheritance. Nat Rev Endocrinol. 2018;14(6):345–12. doi: 10.1038/s41574-018-0005-5
- Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293(5532):1089–1093. doi: 10.1126/science.1063443
- Rotondo JC, Lanzillotti C, Mazziotta C, et al. Epigenetics of male infertility: the role of DNA methylation. Front Cell Dev Biol. 2021;9:689624. doi: 10.3389/fcell.2021.689624
- Tahmasbpour Marzouni E, Ilkhani H, Beigi Harchegani A, et al. Epigenetic modifications, a new approach to male infertility etiology: a review. Int J Fertil Steril. 2022;16(1):1–9. doi: 10.22074/IJFS.2021.138499.1032
- Slyvka Y, Zhang Y, Nowak FV. Epigenetic effects of paternal diet on offspring: emphasis on obesity. Endocrine. 2015;48(1):36–46. doi: 10.1007/s12020-014-0328-5
- Fallet M, Blanc M, Di Criscio M, et al. Present and future challenges for the investigation of transgenerational epigenetic inheritance. Environ Int. 2023;172:107776. doi: 10.1016/j.envint.2023.107776
- Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8(4):253–262. doi: 10.1038/nrg2045
- van Otterdijk SD, Michels KB. Transgenerational epigenetic inheritance in mammals: how good is the evidence? FASEB J Off Publ Fed Am Soc Exp Biol. 2016;30(7):2457–2465. doi: 10.1096/fj.201500083
- Heijmans BT, Tobi EW, Stein AD, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci USA. 2008;105(44):17046–17049. doi: 10.1073/pnas.0806560105
- Blewitt ME, Vickaryous NK, Paldi A, et al. Dynamic reprogramming of DNA methylation at an epigenetically sensitive allele in mice. PLoS Genet. 2006a;2(4):e49. doi: 10.1371/journal.pgen.0020049
- Dolinoy DC, Weidman JR, Waterland RA, et al. Maternal genistein alters coat color and protects A vy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect. 2006;114(4):567–572. doi: 10.1289/ehp.8700
- Morgan HD, Sutherland HG, Martin DI, et al. Epigenetic inheritance at the agouti locus in the mouse. Nat Genet. 1999;23(3):314–318. doi: 10.1038/15490
- Anway MD, Cupp AS, Uzumcu M, et al. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308(5727):1466–1469. doi: 10.1126/science.1108190
- Roseboom TJ, Painter RC, van Abeelen AFM, et al. Hungry in the womb: what are the consequences? Lessons from the Dutch famine. Maturitas. 2011;70(2):141–145. doi: 10.1016/j.maturitas.2011.06.017
- Carone BR, Fauquier L, Habib N, et al. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell. 2010;143(7):1084–1096. doi: 10.1016/j.cell.2010.12.008
- Terashima M, Barbour S, Ren J, et al. Effect of high fat diet on paternal sperm histone distribution and male offspring liver gene expression. Epigenetics. 2015;10(9):861–871. doi: 10.1080/15592294.2015.1075691
- Ryan DP, Henzel KS, Pearson BL, et al. A paternal methyl donor-rich diet altered cognitive and neural functions in offspring mice. Mol Psychiatry. 2018;23(5):1345–1355. doi: 10.1038/mp.2017.53
- Wen J, Hong Q, Wang X, et al. The effect of maternal vitamin D deficiency during pregnancy on body fat and adipogenesis in rat offspring. Sci Rep. 2018;8(1):365. doi: 10.1038/s41598-017-18770-4
- Ponomarev I, Wang S, Zhang L, et al. Gene coexpression networks in human brain identify epigenetic modifications in alcohol dependence. J Neurosci Off J Soc Neurosci. 2012;32(5):1884–1897. doi: 10.1523/JNEUROSCI.3136-11.2012
- Govorko D, Bekdash RA, Zhang C, et al. Male germline transmits fetal alcohol adverse effect on hypothalamic proopiomelanocortin gene across generations. Biol Psychiatry. 2012;72(5):378–388. doi: 10.1016/j.biopsych.2012.04.006
- Caton JS, Crouse MS, McLean KJ, et al. Maternal periconceptual nutrition, early pregnancy, and developmental outcomes in beef cattle. J Anim Sci. 2020;98(12):skaa358. doi: 10.1093/jas/skaa358
- Muhlhausler BS, Duffield JA, McMillen IC. Increased maternal nutrition stimulates peroxisome proliferator activated receptor-γ, Adiponectin, and leptin messenger ribonucleic acid expression in adipose tissue before birth. Endocrinology. 2007;148(2):878–885. doi: 10.1210/en.2006-1115
- Sinclair KD, Allegrucci C, Singh R, et al. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc Natl Acad Sci. 2007;104(49):19351–19356. doi: 10.1073/pnas.0707258104
- Leroux S, Gourichon D, Leterrier C, et al. Embryonic environment and transgenerational effects in quail. Genet Sel Evol. 2017;49(1):14. doi: 10.1186/s12711-017-0292-7
- Heard E, Martienssen RA. Transgenerational epigenetic inheritance: myths and mechanisms. Cell. 2014;157(1):95–109. doi: 10.1016/j.cell.2014.02.045
- Martos SN, Tang W, Wang Z. Elusive inheritance: transgenerational effects and epigenetic inheritance in human environmental disease. Prog Biophys Mol Biol. 2015;118(1–2):44–54. doi: 10.1016/j.pbiomolbio.2015.02.011
- Santilli F, Boskovic A. Mechanisms of transgenerational epigenetic inheritance: lessons from animal model organisms. Curr Opin Genet Dev. 2023;79:102024. doi: 10.1016/j.gde.2023.102024
- Tuscher JJ, Day JJ. Multigenerational epigenetic inheritance: one step forward, two generations back. Neurobiol Dis. 2019;132:104591. doi: 10.1016/j.nbd.2019.104591
- Horsthemke B. A critical view on transgenerational epigenetic inheritance in humans. Nat Commun. 2018;9(1):2973. doi: 10.1038/s41467-018-05445-5
- Anway MD, Leathers C, Skinner MK. Endocrine Disruptor Vinclozolin Induced Epigenetic Transgenerational Adult-Onset Disease. Endocrinology. 2006;147(12):5515–5523. doi: 10.1210/en.2006-0640
- Guerrero-Bosagna C, Covert TR, Haque MM, et al. Epigenetic transgenerational inheritance of vinclozolin induced mouse adult onset disease and associated sperm epigenome biomarkers. Reprod Toxicol. 2012;34(4):694–707. doi: 10.1016/j.reprotox.2012.09.005
- Iqbal K, Tran DA, Li AX, et al. Deleterious effects of endocrine disruptors are corrected in the mammalian germline by epigenome reprogramming. Genome Biol. 2015;16(1):59. doi: 10.1186/s13059-015-0619-z
- Nadeau JH. The nature of evidence for and against epigenetic inheritance. Genome Biol. 2015;16(1):137. doi: 10.1186/s13059-015-0709-y
- Blake GE, Watson ED. Unravelling the complex mechanisms of transgenerational epigenetic inheritance. Curr Opin Chem Biol. 2016;33:101–107. doi: 10.1016/j.cbpa.2016.06.008
- Nilsson EE, Sadler-Riggleman I, Skinner MK, et al. Environmentally induced epigenetic transgenerational inheritance of disease. Environ Epigenetics. 2018;4(2). doi: 10.1093/eep/dvy016
- Blanco Rodríguez J, Camprubí Sánchez C. Epigenetic Transgenerational Inheritance. Adv Exp Med Biol. 2019;1166:57–74. doi: 10.1007/978-3-030-21664-1_4
- Wang HD, Allard P. Challenging dogmas: how transgenerational epigenetics reshapes our views on life. J Exp Zool Part Ecol Integr Physiol. 2022;337(1):70–74. doi: 10.1002/jez.2465
- Braz CU, Taylor T, Namous H, et al. Paternal diet induces transgenerational epigenetic inheritance of DNA methylation signatures and phenotypes in sheep model. PNAS Nexus. 2022;1(2):pgac040. doi: 10.1093/pnasnexus/pgac040
- Inawaka K, Kawabe M, Takahashi S, et al. Maternal exposure to anti-androgenic compounds, vinclozolin, flutamide and procymidone, has no effects on spermatogenesis and DNA methylation in male rats of subsequent generations. Toxicol Appl Pharmacol. 2009;237(2):178–187. doi: 10.1016/j.taap.2009.03.004
- Schneider S, Kaufmann W, Buesen R, et al. Vinclozolin—the lack of a transgenerational effect after oral maternal exposure during organogenesis. Reprod Toxicol. 2008;25(3):352–360. doi: 10.1016/j.reprotox.2008.04.001
- Nilsson E, Larsen G, Manikkam M, et al. Environmentally induced epigenetic transgenerational inheritance of ovarian disease. PLoS One. 2012;7(5):e36129. doi: 10.1371/journal.pone.0036129
- Brieño-Enríquez MA, García-López J, Cárdenas DB, et al. Exposure to endocrine disruptor induces transgenerational epigenetic deregulation of MicroRNAs in primordial germ cells. PLoS One. 2015;10(4):e0124296. doi: 10.1371/journal.pone.0124296
- Abraham A, Chakraborty P. A review on sources and health impacts of bisphenol a. Rev Environ Health. 2020;35(2):201–210. doi: 10.1515/reveh-2019-0034
- Rahman MS, Pang WK, Ryu DY, et al. Multigenerational and transgenerational impact of paternal bisphenol a exposure on male fertility in a mouse model. Hum Reprod. 2020;35(8):1740–1752. doi: 10.1093/humrep/deaa139.
- Wolstenholme JT, Goldsby JA, Rissman EF. Transgenerational effects of prenatal bisphenol a on social recognition. Horm Behav. 2013;64(5):833–839. doi: 10.1016/j.yhbeh.2013.09.007
- Franklin TB, Russig H, Weiss IC, et al. Epigenetic transmission of the impact of early stress across generations. Biol Psychiatry. 2010;68(5):408–415. doi: 10.1016/j.biopsych.2010.05.036
- Dias BG, Ressler KJ. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat Neurosci. 2014;17(1):89–96. doi: 10.1038/nn.3594
