ABSTRACT
The mechanisms by which the ageing process is associated to an unhealthy lifestyle and how they play an essential role in the aetiology of systemic arterial hypertension have not yet been completely elucidated. Our objective is to investigate the influence of NOS3 polymorphisms [−786T > C and (Glu298Asp)] on systolic blood pressure (SBP) and diastolic blood pressure (DBP) response, differentially methylated regions (DMRs), and physical fitness of adult and older women after a 14-week combined training intervention. The combined training was carried out for 14 weeks, performed 3 times a week, totalling 180 minutes weekly. The genotyping experiment used Illumina Infinium Global Screening Array version 2.0 (GSA V2.0) and Illumina’s EPIC Infinium Methylation BeadChip. The participants were separated into SNP rs2070744 in TT (59.7 ± 6.2 years) and TC + CC (60.0 ± 5.2 years), and SNP rs17999 in GluGlu (58.8 ± 5.7 years) and GluAsp + AspAsp (61.6 ± 4.9 years). We observed an effect of time for variables BP, physical capacities, and cholesterol. DMRs related to SBP and DBP were identified for the rs2070744 and rs17999 groups pre- and decreased numbers of DMRs post-training. When we analysed the effect of exercise training in pre- and post-comparisons, the GluGlu SNP (rs17999) showed 10 DMRs, and after enrichment, we identified several biological biases. The combined training improved the SBP and DBP values of the participants regardless of the SNPs. In addition, exercise training affected DNA methylation differently between the groups of NOS3 polymorphisms.
Introduction
Population ageing is growing all over the world, and it is estimated that the older population will represent one-fifth of the world population by 2050 (1 United Nations, 2019). Ageing is a process characterized by the accumulation of deleterious changes in cells and tissues, which results in the decline of the full functionality of physiological systems and causes important losses in functional capacity and physical ability, and increases the risk of developing diseases, such as systemic arterial hypertension [Citation1].
High blood pressure is one of the main modifiable risk factors for cardiovascular morbidity and mortality [Citation2,Citation3], and it is estimated that 90% of individuals who did not develop hypertension by the age of 55 will develop it over the next few years of life [Citation4]. In addition to ageing, the aetiology of hypertension is closely related to an unhealthy lifestyle (smoking, alcohol abuse, low levels of physical activity, sedentary behaviour, and poor eating habits) [Citation5].
The mechanisms by which the ageing process and an unhealthy lifestyle play an important role in the aetiology of hypertension have not yet been completely elucidated. Studies indicated that arterial stiffness is related to elevated systemic blood pressure (BP), increasing the risk of developing hypertension and cardiovascular diseases [Citation6]. The increase in endothelial stiffness and dysfunction occurs in response to the decrease in vasodilatory molecules produced by endothelial cells, mainly nitric oxide (NO), a soluble gas produced by nitric oxide synthase (NO3) [Citation7].
It is known that the chronic practice of exercise training can promote various health benefits [Citation8], including an increase in NO synthesis [Citation9–11]. However, individuals may exhibit different responses in BP after a period of exercise training practice [Citation12–15]. Genetic variables, such as NOS3 polymorphisms, may be related to differences in responses to exercise training [Citation12,Citation13,Citation16,Citation17].
In a study conducted by our research group [Citation17], we showed that specific single nucleotide polymorphisms (SNPs) of NOS3 (−786T > C, 894 G > T (Glu298Asp)) influenced responsiveness to multicomponent exercise training regarding BP and nitrite concentration in physically inactive pre-hypertensive or hypertensive middle-aged and older women. The decreases in systolic and diastolic BP and increases in nitrite expected from exercise training were smaller in the variant genotype (−786T > C – TC + CC, Glu298Asp: GluAsp + AspAsp) than ancestral genotype (−786T > C – TT, and Glu298Asp: GluGlu). Similar studies using only aerobic exercise training intervention showed that NOS3 polymorphisms may or may not influence responses to exercise training [Citation12–15,Citation18]. Thus, there is still no high level of evidence indicating whether genetic polymorphisms are determinants for responses in blood pressure and NO synthesis after exercise training practice, particularly in relation to combined exercise training. This is a type of exercise training in which aerobic exercises targeting greater cardiorespiratory work (aerobic exercises) and exercises aiming for greater strength work (resistance or strength exercises) are performed in the same session [Citation9–11].
Additionally, studies have shown that changes in epigenetic mechanisms are associated with an increased or decreased risk of developing various diseases [Citation19,Citation20]. These mechanisms are modified by lifestyle factors (external or environmental factors), such as exercise training and dietary habits. These factors interact with the genome and affect the phenotype through modifications in epigenetic mechanisms, which can suppress or increase gene expression and protein expression [Citation21]. Potentially, DNA methylation is the epigenetic mechanism that has the greatest impact on gene expression regulation [Citation19,Citation20,Citation22]. It is proposed that methylation acts as a ‘barrier’ to reprogramming [Citation23,Citation24] and the binding of transcription factors to previously methylated sites [Citation25] or the removal of the methyl group from methylated cytosine [Citation26] is involved in cell differentiation or reprogramming.
Studies have demonstrated that exercise training can affect DNA methylation in muscle tissue, causing changes in the expression of specific genes in physically inactive healthy men and women [Citation27]. Changes in leukocyte methylation associated with increased cardiorespiratory ability, running economy, and decreased LDL-c concentrations have also been observed after exercise training [Citation28,Citation29], but to date there is no evidence of how exercise training will influence DNA methylation when comparing different SNPs, analysing how these SNP mutations will respond to the combined exercise training protocol and what is the epigenetic impact on DNA methylation in women carrying NOS3 polymorphisms.
To date, there is no evidence regarding the changes in DNA methylation that exercise training provides when comparing NOS3 polymorphisms and how these polymorphisms will respond to combined exercise training when analysing older women.
The literature shows that combined exercise training presents significant clinical changes for adult women when NOS3 polymorphisms are not used as a comparison [Citation8,Citation30,Citation31], but there remains a need to understand whether these clinical changes are in sync when we analyse DNA methylation when genetics can be a limiting factor for the best clinical response to physical exercise.
Aiming to advance the study on the different responses in blood pressure after combined exercise training, the objective is to investigate the influence of NOS3 polymorphisms [−786T > C e (Glu298Asp)] [Citation17] on blood pressure response, identify differentially methylated regions in the various polymorphisms using exploratory analysis, and analyse the physical fitness of adult and older women after a 14-week intervention of combined exercise training.
Methods
This is a prospective single-arm longitudinal clinical trial study. The study was submitted and approved by the Research Ethics Committee for Human Subjects at the School of Physical Education and Sports of Ribeirão Preto, University of São Paulo (CAAE: 79582817.0.0000.5659), based on Resolution 466/12 of the Ministry of Health, regulated by the National Health Council. It is registered in the Brazilian Registry of Clinical Trials with the registration number RBR-3g38dx. Women aged 50 and 70 years who had been physically inactive for at least six months were included. Exclusion criteria included participants with physical limitations to perform exercises, current smokers or those who had smoked in the last six months, and those who consumed caffeine 24 hours before any blood collection. Women who agreed to participate in the study had to read and sign the informed consent form before the start of activities.
Combined exercise training protocol
The protocol performed was combined exercise training, in which 60 min- sessions were performed three times a week for 14 weeks. The combined exercise training sessions consisted of 30 minutes of cardiorespiratory training (treadmill or bicycle) and 30 minutes of muscular strength exercises [Citation8].
We used 8 exercises for strength training, including 3 for the lower limbs and 5 for the upper limbs. The training protocol included leg curls, leg presses 45°, leg extensions, triceps with a neutral grip, bicep curls with dumbbells, lat pulldowns on the high pulley, seated rows, and inclined bench press exercises [Citation30].
Exercise training protocol
The training protocol was periodized into the first two weeks consisting of 2 sets of 15 to 17 maximal repetitions for strength exercises and 30 minutes on a treadmill or stationary bike using 50% of the heart rate reserve. In the subsequent 12 weeks, totalling 14 weeks, 2 sets of 10 to 12 maximal repetitions and 30 minutes on a treadmill or stationary bike with 70% of the heart rate reserve were performed [Citation30]. Training intensities were measured using the Borg Rating of Perceived Exertion Scale [Citation32], maintained between values 3 and 6 on a scale of zero to 10, representing moderate exercise intensity. The Polar Team2, Finland, was also utilized to enhance the accuracy of the perceived exertion scale [Citation33].
Assessments
Assessments were conducted before (pre) and after (post) 14 weeks of combined exercise training. Anthropometric evaluations included height (cm), weight (kg), body mass index (kg/m2), and waist circumference. Motor variables assessed encompassed elbow flexion and extension, sit-to-stand, six-minute walk, dynamic agility, maximum upper limb strength measured in the inclined bench press, and maximum lower limb strength assessed in the leg press 45° apparatus [Citation34].
Blood pressure assessment
The same investigator performed blood pressure measurements in the morning, which were the pre- and post-intervention evaluations. The measurements were conducted in a quiet place, with light and temperature (22ºC) controlled. The participants must not drink alcoholic beverages, smoke, or consume tobacco, nor eat and/or drink any food/drink containing caffeine and/or polyphenols at least 24 hours before blood pressure assessments [Citation35].
Blood pressure was assessed using an upper arm blood pressure, employing the oscillometric method (OMRON, model HEM-7113). Initially, blood pressure was measured in both arms, starting with the left arm and, subsequently, the right. Following these initial measurements, two more readings were taken exclusively in the right arm, with a two-minute interval between each measurement. The measurements were conducted after the participant rested for five minutes in a seated position, per the Brazilian Guidelines for Arterial Hypertension [Citation36].
DNA extraction, genotyping, and methylation analysis
Peripheral blood samples were collected in EDTA tubes before and after 14 weeks of training. Genomic DNA was isolated from peripheral blood using the salting-out method [Citation37]. DNA quality and integrity were assessed by spectrophotometry [Citation38]. Participants were genotyped using the Illumina Infinium Global Screening Array version 2.0 (GSA V2.0), following the manufacturer’s instructions. DNA methylation assays were conducted using the EPIC Infinium Methylation BeadChip from Illumina, and fluorescence signals were detected by the Illumina iScan system [Citation39].
Bioinformatics analysis
For genotyping beadchips, the two-colour channels *.idats were processed in Genome Studio v 2.0 to perform SNP calling. All samples in this study had a call rate > 0.90 [Citation40]. For DNA methylation data, we converted intensities into methylation Beta values and exported them as raw data. Data processing was conducted using Rstudio software (R version 3.6.3) [Citation39], analysing methylation patterns using the chAMP Bioconductor package recommendations. To identify differentially methylated regions (DMRs), we altered the command to pheno = myLoad$pd$Sample_Group and performed compare.group (POST INTERVENTION vs. PRE-INTERVENTION) with p < 0.05.
After analysing DMRs for groups with different single nucleotide polymorphisms (SNPs), we modified the pheno command to identify DMRs associated with blood pressure values in pre- and post-intervention groups, calculating regressions to identify DMRs related to SNPs pre and post-intervention. Another analysis conducted was singular value decomposition analysis (SVD), which is a powerful tool to assess the number and nature of significant components of variation in a dataset. The result is a heat map (saved as SVDsummary.pdf) of the main components correlated with the covariate information provided [Citation39].
Enrichment analysis (string)
We enriched our gene list using STRING software. Notably, the desired specificity cut-off for functional associations in STRING corresponds approximately to the granularity of annotation on KEGG pathway maps [Citation41].
Sample size calculation
We utilized GPower software to determine the necessary sample size for the study. Independent sample testing was employed to analyse results and assess post-intervention changes. By setting the alpha error probability at 0.05 and adjusting the power to 0.8%, we calculated that a minimum of 18 participants would be required for the study to detect a difference between the means.
Statistical analysis
Motor and anthropometric data were entered into an Excel file by two researchers and subsequently cross-referenced to eliminate possible errors. GraphPad Prism 8 software was adopted for statistical analyses. Normality was tested using Shapiro-Wilk, and Levene’s test was employed to assess the equality of variances between groups. As the analysis showed that the data met assumptions, repeated-measures ANOVA was utilized, considering p < 0.05. He chosen p-value threshold of < 0.05 for two-sided tests in our study. It is widely accepted in biological research as a criterion for declaring statistical significance. This threshold was selected to balance the risks of Type I and Type II errors within our study’s design and objectives.In our study, ‘Group’ refers to the distinct experimental or control groups, while ‘Time’ represents the two-time points at which data were collected (pre- and post-intervention). The ‘Group*Time’ interaction term investigates whether the change over time differs between groups.Significant group-time interactions indicate that the intervention’s impact varies by group across different time points. This understanding is critical for comprehending the dynamics and effects of interventions within different groups over time.
Results
Variables such as age, education, number of former smokers, prevalence of obesity, hypertension, and use of anti-hypertensive drugs from all participants are showed in .
Table 1. Variables: age, education, number of former smokers, prevalence of obesity, hypertension, and use of anti-hypertensive drugs from all participants.
Combined exercise training for 14 weeks promoted significant improvements in 50 women (60.0 ± 4.7 years). There were reductions in systolic blood pressure values (p = 0.001), diastolic blood pressure (p = 0.039), and values from the agility test (p < 0.001). Furthermore, the 14-week training programme resulted in increased functional strength of the upper limbs, as measured by the Biceps curl test (p < 0.001), and enhanced strength of the lower limbs demonstrated through the chair stand test (p < 0.001) ().
Table 2. Anthropometric measurement, blood pressure, physical capacities, and biochemical variables from all participants.
There was also a notable increase in maximum strength for both the upper limbs, as assessed by the MP upper limbs test (p < 0.001), and the lower limbs, evaluated using the MP lower limbs test (p < 0.001). Additionally, the training led to improved aerobic fitness among the participants, as indicated by the results of the 6-minute walk test (6MWT) (p = 0.001) ().
Fifty women aged 50 to 70 years were divided into groups considering the NOS3 polymorphisms rs2070744 (TT and TC+CC) and rs1799983 (GluGlu and GluAsp+AspAsp) to understand the influences on the blood pressure response, identifying the regions differentially methylated in the polymorphisms (). The presence of the two variant genotypes [−786T > C: TC + CC, and 894 G > T (Glu298Asp): GluAsp + AspAsp].
Clinical variables and blood pressure divided by SNPs
Variables such as age, education, number of former smokers, prevalence of obesity, hypertension, and use of anti-hypertensive drugs from TT and TC+CC groups were showed in .
Table 3. Variables: age, education, number of former smokers, prevalence of obesity, hypertension, and use of anti-hypertensive drugs from TT and TT+TC groups.
The groups were for the rs2070744 SNP into TT (59.7 ± 6.2 years) and TC + CC (60.0 ± 5.2 years). Upon analysing the variables (), no significant differences were observed in the Time x group interaction. However, significant differences over time were observed for the blood pressure, physical capacities, and cholesterol variables.
Table 4. Anthropometric measurement, blood pressure, physical capacities and biochemical variables from TT and TT+TC groups.
In the distribution of participants into groups for the rs17999 SNP, they were subdivided into GluGlu (58.8 ± 5.7 years) and GluAsp + AspAsp (61.6 ± 4.9 years). Variables such as age, education, number of former smokers, prevalence of obesity, hypertension, and use of anti-hypertensive drugs from GluGlu and GluAsp + AspAsp groups are showed in .
Table 5. Variables: age, education, number of former smokers, prevalence of obesity, hypertension, and use of anti-hypertensive drugs from GluGlu and GluAsp+AspAsp groups.
No differences were observed in the time x group (). However, significant differences were observed over time for the blood pressure, physical capacities, and cholesterol variables.
Table 6. Anthropometric measurement, blood pressure, physical capacities and biochemical variables from GluGlu and GluAsp+AspAsp groups.
Individual changes in systolic and diastolic blood pressure divided by SNPs
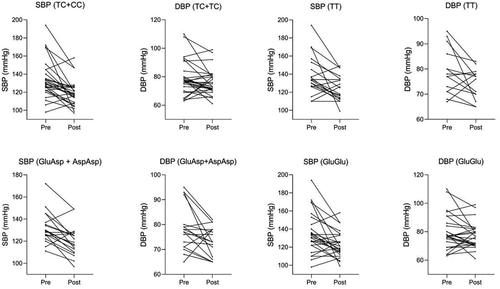
In the blood pressure variables (), we observed a significant time difference for systolic and diastolic pressures, indicating a clinical improvement in the values post-intervention for the TC + CC and GluAsp + AspAsp groups. To better understand the individual response of each participant to systolic and diastolic blood pressure variables, we present the pre- and post-intervention variations for each individual, detailing the changes after 14 weeks of intervention ().
Regressions to examine the impact of SNPs on differentially methylated regions
We performed Singular Value Decomposition (SVD) to analyse methylation components with systolic and diastolic blood pressures. Following the processing, we identified regions that were differentially methylated before and after 14 weeks of exercise training in our study groups. We used ‘pheno = myLoad$pd$DBP’ and ‘pheno = myLoad$pd$SBP’ to identify DMRs in both pre- and post-intervention. For the TC+CC group and systolic blood pressure, we observed 51 DMRs at the pre-intervention stage, and no significant regions were identified post-intervention (). In contrast, for diastolic blood pressure, there was a reduction in one region after training () – three in the pre-training and two in the post-training. The TT genotype initially presented 3 regions for systolic after 14 weeks of training, there was an increase in regions for systolic 10 regions (), and 4 for diastolic blood pressure after 14 weeks of training, there was an increase in regions for systolic 28 regions (). Notably, the AL68 region appeared as a stimulated region both before and after training for systolic blood pressure ().
Figure 3. Differentially methylated regions associated with systolic and diastolic blood pressure for the SNPs TC+CC and TT.
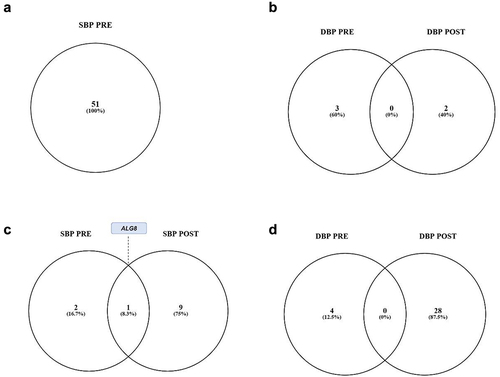
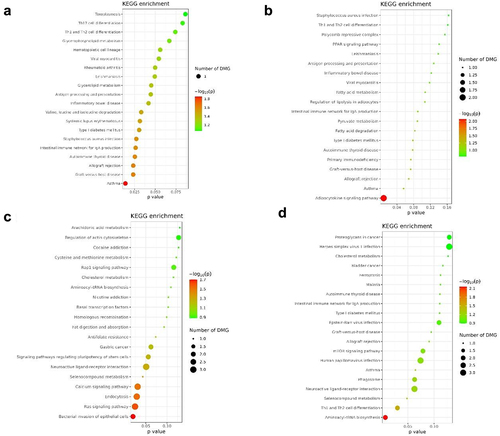
Figure 4. KEEG enrichment of DMR sites for TC+CC and TT SNPs.
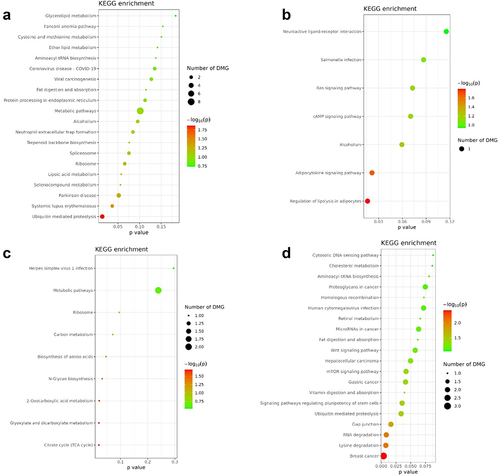
There was a reduction in differentially methylated regions for the GluAsp+AspAsp polymorphism, decreasing from 9 in the pre-evaluation to 3 regions in the post-evaluation (). When considering diastolic blood pressure, there were 21 regions in the pre-evaluation, which decreased to 7 regions after 14 weeks, with the regions HLA-DPB1, MIR596, MIR886, and NPY common to both time points ().
Figure 5. Differentially methylated regions related to systolic and diastolic blood pressure for the SNPs GluAsp+AspAsp and GluGlu.
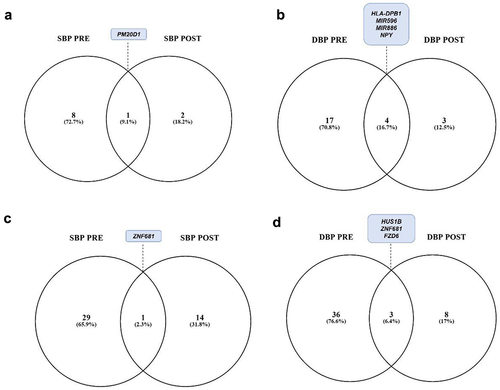
We identified 30 differentially methylated regions associated with systolic blood pressure (SBP) in the pre-intervention period, decreasing to 14 regions post-intervention (). Notably, the DMR ZNF681 was stimulated in both pre and post comparisons ().
For diastolic blood pressure (DBP), there were 40 DMRs in the pre-intervention period, decreasing to 12 DMRs post-intervention. The common DMRs in both pre and post-comparisons were HUS1B, ZNF681, and FZD6 ().
Differentially methylated regions in the pre- versus post-training comparison for the GluGlu SNP
Following the comparisons in the diagrams, we conducted analyses of differentially methylated regions by comparing pre- and post-intervention in our study groups. We observed that after 14 weeks of training, the only group that exhibited differentially methylated regions was the GluGlu SNP rs17999 group (n = 29), and we identified 10 DMRs (). The majority of these regions were located in promoter zones, with two found in intergenic regions ().
Table 7. Results of differentially methylated regions after 14 weeks of training for the GluGlu SNP.
Enrichment of pre- and post-training DMRs for the GluGlu SNP
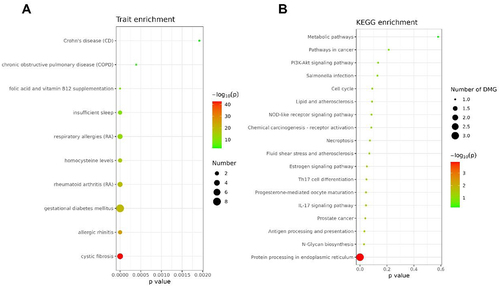
To gain a better understanding of the differentially methylated regions (DMRs) identified in participants with the GluGlu SNP after 14 weeks, we performed site enrichment analyzes to identify key related pathways. We identified trait enrichment associated with Crohn’s disease, insufficient sleep, among other processes (). When we observed KEEG, we identified pathways related to Metabolic pathways, pathways in cancer, PI3K-Akt signalling pathway, among others ().
Figure 7. Enrichment of DMRs for the GluGlu SNP after 14 weeks of combined training.
Discussion
The outcomes of this investigation underscore the efficacy of exercise training in significantly decrease diastolic and systolic blood pressure in individuals, irrespective of the presence of genetic polymorphisms linked to arterial hypertension. These results align with prior research emphasizing the positive impact of exercise training on blood pressure regulation, regardless of genetic predisposition. Embracing healthier habits has been associated with a substantial decrease of up to 80% in the occurrence of chronic diseases, including hypertension, as observed in previous studies [Citation42].
The study conducted by the GBD 2017 Risk Factor Collaborators [Citation43] analysed an extensive dataset comprising over 100,000 individuals from 18 countries. The findings indicate that lifestyle modifications, such as adopting a healthy diet, engaging in regular physical activity, and refraining from smoking, can potentially reduce mortality from heart diseases, strokes, and cancer by up to 80%. Furthermore, another study involving over 1 million participants globally demonstrated that individuals adhering to a healthy diet, engaging in regular physical activity, and abstaining from smoking experience a 30% lower risk of mortality from any cause [Citation44].
Specific studies have also investigated how exercise training can impact blood pressure in individuals with specific genetic polymorphisms. One study revealed that moderate-intensity exercise performed for 30 minutes, five days a week, over 12 weeks reduced blood pressure in individuals with the ApoE4 genetic polymorphism [Citation45]. Another comparative study found that high-intensity exercise performed for 20 minutes, three days a week, over 12 weeks lowered blood pressure in individuals with the ACE genetic polymorphism [Citation46]. These findings underscore the effectiveness of physical exercise as a relevant intervention in reducing blood pressure, even in individuals with a genetic predisposition to hypertension.
Our results demonstrated that the benefits of a combined exercise training intervention do not appear to be genotype-dependent, as combined exercise training improved systolic and diastolic values in participants irrespective of SNPs. Regarding the investigated NOS3 polymorphisms (−786T > C and 894 G > T), the studies provide inconclusive findings [Citation12–15]. However, in a study conducted by our group [Citation17], a distinct response to multicomponent training, lasting 90 minutes per session, was observed among groups of older women with pre-hypertension (pre-HAs) and hypertension (HAS). It was evident that improvement and response were associated with genotype, with the presence of the three variant genotypes [−786T > C: TC + CC, 894 G > T (Glu298Asp): GluAsp + AspAsp] negatively affecting the reduction of diastolic blood pressure and the increase in nitrite concentration responses, particularly in the group of older women with HAS. Such differences were not identified in our study. However, it is noteworthy that the evaluated sample consisted of older women who had been inactive for a minimum of six months and had no diagnosis of pre-hypertension or hypertension. A combined training protocol of 60 minutes per session was employed. Thus, our results underscore the beneficial role of physical exercise in blood pressure control, regardless of genetic predisposition.
We observed an increase in the number of DMRs after exercise in the TT group (3b was the only one that showed an increase in DMRs), while in all other groups, the quantities of DMRs decreased after exercise (). This indicates that even with polymorphism, DMRs were modulated after exercise. In our analysis, we conducted regressions to assess the impact of SNPs on blood pressure variables. The results in indicate that the exercise regimen reshaped regions related to blood pressure. In the forthcoming discussion, we plan to delve into these changes and highlight specific regions, such as PM20D1, miR-596, NPY, ZNF681, HU1D, ZNF681, and FZD6, which emerged as noteworthy in the pre-and post-analysis. We aim to investigate whether any of these genes exhibit associations with blood pressure, suggesting their potential as novel markers for this field. This succinctly summarizes the key findings of our study.
PM20D1 is a coding gene for the M20 metalloprotease family and a peptidase M20 domain-containing protein 1. Identified as a circulating biomarker, PM20D1 is associated with obesity, insulin resistance, and metabolic syndrome, indicating its potential significance in these health conditions [Citation47]. The gene’s promoter region displays significant hypermethylation in individuals diagnosed with stroke. The study further highlights that the methylation levels of KCNQ1 in white blood cells are influenced by both obesity and ischaemic stroke, indicating a potential epigenetic link between DNA methylation patterns and the pathophysiology of stroke, particularly concerning PM20D1 and KCNQ1 [Citation48].
Recently, a link between DNA methylation and arterial stiffness (AS) and pulsatile haemodynamics (PH) has been described. The authors highlight that HLA-DPB1 has emerged as a potential biomarker, shedding light on its role in understanding the pathophysiology of AS/PH, and the epigenetic regulation offers potential targets for modulation in cardiovascular health [Citation49].
One study by Yang Huang et al. showed the correlation between blood pressure evaluation and DNA methylation, with a focus on IPAH, unlike previous research on DNA methylation in gastric and oral cancer. The study aims to determine if plasma exosomal microRNAs (miRNAs) like miR-596 can be prognostic indicators in IPAH patients [Citation50].
The study found elevated levels of miR-596 in nonsurvivors compared to survivors, with a significant correlation observed between miR-596 levels and different variables associated with blood pressure, such as vascular pulmonary resistance, survival time (ST), and cardiac index. The findings suggest that heightened levels of plasma exosomal miR-596 are significantly associated with disease severity and an unfavourable prognosis for individuals with IPAH, contributing novel insights into the prognostic potential of miR-596 in the context of IPAH and expanding our understanding of biomarkers in cardiovascular health [Citation50].
The investigation of Neuropeptide Y (NPY) as a crucial target in metabolic diseases revealed limited literature specifically linking NPY to blood pressure and DNA methylation. However, noteworthy findings emerged concerning NPY and blood pressure in human subjects of Finnish and Dutch ancestry. The rs16139 variation (T > C) demonstrated that the minor C-allele, associated with higher NPY release, correlated with an accelerated increase in typical carotid artery intima-media thickness, elevated systolic and diastolic blood pressure, increased serum cholesterol and LDL-cholesterol levels, and poorer glycaemic control. Contrarily, our study, spanning adolescence to young adulthood, identified associations between all four NPY SNPs and blood pressure, with CC-homozygotes for rs5574 and TT-homozygotes for rs16147 exhibiting a higher increase in diastolic blood pressure. Intriguingly, in this younger age group, heterozygotes for rs16139 and rs17149106 displayed lower systolic and diastolic blood pressure than TT and GG homozygotes. While our results align with some prior findings, they also highlight an age-related complexity in the impact of NPY variants on blood pressure, urging further longitudinal studies across a broad age range. Our findings underscore the potential role of NPY in glucose and lipid metabolism, emphasizing the need for comprehensive investigations that consider age as a modifying factor [Citation51].
Regrettably, our literature review did not yield sufficient information to discuss the identified targets comprehensively, namely ALG8, miR-886, ZNF681, HUS1D, and FZD6. Despite diligent efforts in exploring existing literature, limited data and studies were available regarding these specific targets. The scarcity of information on ALG8, miR-886, ZNF681, HUS1D, and FZD6 in the current body of literature underscores the need for further research and investigation to elucidate their roles and significance in relevant biological contexts. This paucity of literature highlights the potential gaps in our understanding of these targets, emphasizing the importance of future studies to unravel their functions and possible implications in various pathways or diseases.
These data showed DMR enriched mainly in pathways related to protein processing in endoplasmic reticulum and DMR related with disease cystic fibrosis. Cystic fibrosis (CF) is a chronic infection and excessive inflammation in lung, and alterations in endoplasmic reticulum (ER) (stress) is involved with pathophysiology mechanisms of many inflammatory diseases [Citation52].
Some studies demonstrated that a form of ER stress is activated in airway epithelia by bacterial infection-induced airway inflammation [Citation53]. Pulmonary hypertension is common found in CF, and overall survival is lower in CF patients with pulmonary hypertension than those without [Citation54].
However, regular exercise is able to help to lives of CF patients [Citation55]. A review related the benefits of including physical activity in people with CF [Citation56]. In our cohort, the patients with hypertension before practice exercise had DMR-related CF compared to the group after practical exercise.
This study’s limitations include the absence of a sedentary control group and groups exclusively focused on aerobic or strength training exercises. Although the research presents new compelling data on combined exercise training and its impact on blood pressure, further studies are needed to investigate discrepancies within the groups of interest.
Conclusion
Combined exercise training yielded positive outcomes in women with the NOS3 polymorphism in motor variables, cholesterol, and systolic and diastolic blood pressure. This justifies that regardless of the polymorphism for this gene, the combination of strength and aerobic exercise in the same session for 14 weeks was adequate to promote benefits in this population group.
Analysing the effects of DNA methylation allowed us to identify new genes related to blood pressure not presented in the literature. These genes can be examined in future studies on hypertensive population groups to better elucidate their genuine relationships. When comparing the clinical changes observed in systolic and diastolic blood pressure values, we see coherence with the presented DMRs.
This indicates that the exercise training modulates blood pressure clinically and epigenetically in women with the NOS3 polymorphism. Our findings also identified that the DMRs were related to blood pressure, providing a deeper understanding of the relationship.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The DNA methylation data used in these studies is available at: 10.6084/m9.figshare.21763535
Additional information
Funding
References
- Colón CJP, Molina-Vicenty IL, Frontera-Rodríguez M, et al. Muscle and bone mass loss in the elderly population: Advances in diagnosis and treatment. J Biomed. 2018;3:40–18. doi: 10.7150/jbm.23390
- Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020 Feb;75(2):285–292. doi: 10.1161/HYPERTENSIONAHA.119.14240
- Wang MC, Lloyd-Jones DM. Cardiovascular risk assessment in hypertensive patients. Am J Hypertens. [2021 Jun 22];34(6):569–577. doi: 10.1093/ajh/hpab021
- Vasan RS, Beiser A, Seshadri S, et al. Residual lifetime risk for developing hypertension in middle-aged women and men: the Framingham heart study. JAMA. [2002 Feb 27];287(8):1003–1010. doi: 10.1001/jama.287.8.1003
- Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardiometabolic risk factors from 1980 to 2010: a comparative risk assessment. Lancet Diabetes Endocrinol. 2014 Aug;2(8):634–647. doi: 10.1016/S2213-8587(14)70102-0
- Zaydun G, Tomiyama H, Hashimoto H, et al. Menopause is an independent factor augmenting the age-related increase in arterial stiffness in the early postmenopausal phase. Atherosclerosis. 2006 Jan;184(1):137–142. doi: 10.1016/j.atherosclerosis.2005.03.043
- Herrera MD, Mingorance C, Rodríguez-Rodríguez R, et al. Endothelial dysfunction and aging: an update. Ageing Res Rev. 2010 Apr;9(2):142–152. doi: 10.1016/j.arr.2009.07.002
- da Sda S RG, Rodrigues KP, de Almeida ML, et al. Comparing fourteen weeks of multicomponent training versus combined training in physically inactive older women: a randomized trial. Int J Environ Res Public Health. [2023 Feb 2];20(3):2699. doi: 10.3390/ijerph20032699
- Son WM, Sung KD, Cho JM, et al. Combined exercise reduces arterial stiffness, blood pressure, and blood markers for cardiovascular risk in postmenopausal women with hypertension. Menopause. 2017 Mar;24(3):262–268. doi: 10.1097/GME.0000000000000765
- Ruangthai R, Phoemsapthawee J. Combined exercise training improves blood pressure and antioxidant capacity in elderly individuals with hypertension. J Exerc Sci Fit. 2019 May;17(2):67–76. doi: 10.1016/j.jesf.2019.03.001
- Ozaki H, Loenneke JP, Thiebaud RS, et al. Resistance training induced increase in VO2max in young and older subjects. Eur Rev Aging Phys Act. 2013 Oct;10(2):107–116. doi: 10.1007/s11556-013-0120-1
- Sponton CHG, Rezende TM, Mallagrino PA, et al. Women with TT genotype for eNOS gene are more responsive in lowering blood pressure in response to exercise. Eur J Cardiovasc Prev Rehabil. 2010 Dec;17(6):676–681. doi: 10.1097/HJR.0b013e32833a1301
- Sponton CH, Esposti R, Rodovalho CM, et al. The presence of the NOS3 gene polymorphism for intron 4 mitigates the beneficial effects of exercise training on ambulatory blood pressure monitoring in adults. Am J Physiol Heart Circ Physiol. [2014 Jun 15];306(12):H1679–91. doi: 10.1152/ajpheart.00844.2013
- Zago AS, Reis Silveira L, Kokubun E. Effects of aerobic exercise on the blood pressure, oxidative stress and eNOS gene polymorphism in pre-hypertensive older people. Eur J Appl Physiol. 2010 Nov;110(4):825–832. doi: 10.1007/s00421-010-1568-6
- Esposti RD, Sponton CHG, Malagrino PA, et al. Influence of eNOS gene polymorphism on cardiometabolic parameters in response to physical training in postmenopausal women. Braz J Med Biol Res. 2011 Sep;44(9):855–863. doi: 10.1590/S0100-879X2011007500106
- Bouchard C, Antunes-Correa LM, Ashley EA, et al. Personalized preventive medicine: genetics and the response to regular exercise in preventive interventions. Prog Cardiovasc Dis. 2015 Jan;57(4):337–346. doi: 10.1016/j.pcad.2014.08.005
- Trapé ÁA, Rodrigues JAL, Ferezin LP, et al. NOS3 polymorphisms can influence the effect of multicomponent training on blood pressure, nitrite concentration and physical fitness in prehypertensive and hypertensive older adult women. Front Physiol. [2021 Mar 10];12:566023. doi: 10.3389/fphys.2021.566023
- Rezende TM, Sponton CHG, Malagrino PA, et al. Effect of exercise training on the cardiovascular and biochemical parameters in women with eNOS gene polymorphism. Arch Physiol Biochem. 2011 Dec;117(5):265–269. doi: 10.3109/13813455.2011.596548
- Zoghbi HY, Beaudet AL. Epigenetics and human disease. Cold Spring Harb Perspect Biol. 2016 Feb;8(2):a019497. doi: 10.1101/cshperspect.a019497
- Barrón-Cabrera E, Ramos-Lopez O, González-Becerra K, et al. Epigenetic modifications as outcomes of exercise interventions related to specific metabolic alterations: a systematic review. Lifestyle Genom. 2019;12(1–6):25–44. doi: 10.1159/000503289
- Alegría-Torres JA, Baccarelli A, Bollati V. Epigenetics and lifestyle. Epigenomics. 2011 Jun;3(3):267–277. doi: 10.2217/epi.11.22
- Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14(10):R115. doi: 10.1186/gb-2013-14-10-r115
- Polo JM, Anderssen E, Walsh RM, et al. A molecular roadmap of reprogramming somatic cells into ips cells. Cell. 2012 Dec;151(7):1617–1632. doi: 10.1016/j.cell.2012.11.039
- Looney TJ, Zhang L, Chen CH, et al. Systematic mapping of occluded genes by cell fusion reveals prevalence and stability of cis -mediated silencing in somatic cells. Genome Res. 2014 Feb;24(2):267–280. doi: 10.1101/gr.143891.112
- Rishi V, Bhattacharya P, Chatterjee R, et al. CpG methylation of half-CRE sequences creates C/EBPα binding sites that activate some tissue-specific genes. Proc Natl Acad Sci. [2010 Nov 23];107(47):20311–20316. doi: 10.1073/pnas.1008688107
- Schübeler D. Function and information content of DNA methylation. Nature. [2015 Jan 15];517(7534):321–326. doi: 10.1038/nature14192
- Barrès R, Yan J, Egan B, et al. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab. 2012 Mar;15(3):405–411. doi: 10.1016/j.cmet.2012.01.001
- Pedersen BK, Saltin B. Exercise as medicine – evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sport. 2015 Dec;25(S3):1–72. doi: 10.1111/sms.12581
- Febbraio MA. Health benefits of exercise — more than meets the eye! Nat Rev Endocrinol. 2017 Feb;13(2):72–74. doi: 10.1038/nrendo.2016.218
- da Silva Rodrigues G, Noronha NY, Almeida ML, et al. Exercise training modifies the whole blood DNA methylation profile in middle-aged and older women. J Appl Physiol. [2023 Mar 1];134(3):610–621. doi: 10.1152/japplphysiol.00237.2022
- da Silva Rodrigues G, Noma IHY, Noronha NY, et al. Eight weeks of physical training decreases 2 years of DNA methylation age of sedentary women. Res Q Exerc Sport. [2023 Jul 19];95(2):405–415. doi: 10.1080/02701367.2023.2228388
- Milot MH, Léonard G, Corriveau H, et al. Using the Borg rating of perceived exertion scale to grade the intensity of a functional training program of the affected upper limb after a stroke: a feasibility study. Clin Interv Aging. 2018 Dec;14:9–16. doi: 10.2147/CIA.S179691
- Perrotta AS, Koehle MS, White MD, et al. Consecutive non-training days over a weekend for assessing cardiac parasympathetic variation in response to accumulated exercise stress. Eur J Sport Sci. [2020 Sep 13];20(8):1072–1082. doi: 10.1080/17461391.2019.1688397
- Rikli RE, Jones CJ. Development and validation of a functional fitness test for community-residing older adults. J Aging Phys Act. 1999 Apr;7(2):129–161. doi: 10.1123/japa.7.2.129
- Peace A, Pinna V, Timmen F, et al. Role of blood pressure in mediating carotid artery dilation in response to sympathetic stimulation in healthy, middle-aged individuals. Am J Hypertens. [2020 Feb 22];33(2):146–153. doi: 10.1093/ajh/hpz159
- Barroso WKS, Rodrigues CIS, Bortolotto LA, et al. Diretrizes Brasileiras de Hipertensão Arterial – 2020. Arq Bras Cardiol. [2021 Mar 3];116(3):516–658. doi: 10.36660/abc.20201238
- Kalousová M, Levová K, Kuběna AA, et al. Comparison of DNA isolation using salting-out procedure and automated isolation (MagNA system). Prep Biochem Biotechnol. [2017 Aug 9];47(7):703–708. doi: 10.1080/10826068.2017.1303613
- Chandra BS, Bhogela SS, Shaik M, et al. Simple and sensitive spectrophotometric methods for the analysis of mesalamine in bulk and tablet dosage forms. Quím Nova. 2011;34(6):1068–1073. doi: 10.1590/S0100-40422011000600026
- Noronha NY, da Silva Rodrigues G, de Souza Pinhel MA, et al. Sample preparation to bioinformatics analysis of DNA methylation: association strategy for obesity and related trait studies. J Vis Exp. [2022 May 6];(183):62598. doi: 10.3791/62598
- Texis T, Luis Cruz-Jaramilllo J, García-Muñoz W, et al. COVID-19 relevant genetic variants confirmed in an admixed population [Internet]. Genetic Evi Med. 2022 Apr [cited 2023 Dec 16]. doi: 10.1101/2022.04.15.22273925
- Kanehisa M, Furumichi M, Tanabe M, et al. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. [2017 Jan 4];45(D1):D353–61. doi: 10.1093/nar/gkw1092
- Virolainen SJ, VonHandorf A, Kcmf V, et al. Gene–environment interactions and their impact on human health. Genes Immun. [2022 Dec 30];24(1):1–11. doi: 10.1038/s41435-022-00192-6
- Stanaway JD, Afshin A, Gakidou E, et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018 Nov;392(10159):1923–1994.
- Troeschel AN, Byrd DA, Judd S, et al. Associations of dietary and lifestyle inflammation scores with mortality due to CVD, cancer, and all causes among Black and White American men and women. Br J Nutr. [2023 Feb 14];129(3):523–534. doi: 10.1017/S0007114522001349
- Kaufman CS, Honea RA, Pleen J, et al. Aerobic exercise improves hippocampal blood flow for hypertensive apolipoprotein E4 carriers. J Cereb Blood Flow Metab. 2021 Aug;41(8):2026–2037. doi: 10.1177/0271678X21990342
- Clark T, Morey R, Jones MD, et al. High-intensity interval training for reducing blood pressure: a randomized trial vs. moderate-intensity continuous training in males with overweight or obesity. Hypertens Res. 2020 May;43(5):396–403. doi: 10.1038/s41440-019-0392-6
- Yang R, Hu Y, Lee CH, et al. PM20D1 is a circulating biomarker closely associated with obesity, insulin resistance and metabolic syndrome. Eur J Endocrinol. [2022 Feb 1];186(2):151–161. doi: 10.1530/EJE-21-0847
- Gómez-Úriz AM, Milagro FI, Mansego ML, et al. Obesity and ischemic stroke modulate the methylation levels of KCNQ1 in white blood cells. Hum Mol Genet. [2015 Mar 1];24(5):1432–1440. doi: 10.1093/hmg/ddu559
- Sharma K, Singh P, Amjad Beg M, et al. Revealing new therapeutic opportunities in hypertension through network-driven integrative genetic analysis and drug target prediction approach. Gene. 2021 Oct;801:145856. doi: 10.1016/j.gene.2021.145856
- Huang Y, Wang ZG, Tang L, et al. Plasma exosomal miR-596: a novel biomarker predicts survival in patients with idiopathic pulmonary artery hypertension. J Int Med Res. 2021 Mar;49(3):030006052110023. doi: 10.1177/03000605211002379
- Katus U, Villa I, Ringmets I, et al. Neuropeptide Y gene variants in obesity, dietary intake, blood pressure, lipid and glucose metabolism: a longitudinal birth cohort study. Peptides. 2021 May;139:170524. doi: 10.1016/j.peptides.2021.170524
- Tang AC, Saferali A, He G, et al. Endoplasmic reticulum stress regulates chemokine production in cystic fibrosis airway cells through STAT3 modulation. J Infect Dis. [2016 Oct 31]:jiw516. doi: 10.1093/infdis/jiw516
- Ribeiro CMP, Boucher RC. Role of endoplasmic reticulum stress in cystic fibrosis-related airway inflammatory responses. Proc Am Thorac Soc. [2010 Nov 1];7(6):387–394. doi: 10.1513/pats.201001-017AW
- Li D, Wang B, Wang H, et al. Prognostic significance of pulmonary hypertension in patients with cystic fibrosis: a systematic review and meta-analysis. Medicine (Baltimore). 2018 Feb;97(7):e9708. doi: 10.1097/MD.0000000000009708
- Ding S, Zhong C. Exercise and cystic fibrosis. Adv Exp Med Biol. 2020;1228:381–391.
- Radtke T, Smith S, Nevitt SJ, et al. Physical activity and exercise training in cystic fibrosis. Cochrane Database Syst Rev. [2022 Aug 9];8(8): CD002768. doi: 10.1002/14651858.CD002768.pub5