Abstract
Stellera chamaejasme L. (Thymelaeaceae), a perennial weed, distributes widely in the grasslands of Russia, Mongolia and China. The plant synthesizes various secondary metabolites including a group of flavonoids. To our knowledge, flavonoids play important roles in the interactions between plants and the environment. So, what are the benefits to S. chamaejasme from producing these flavonoids? Here, we discuss the potential ecological role of flavonoids from S. chamaejasme in protecting the plant from insects and other herbivores, as well as pathogens and competing plant species, and new data are provided on the phytotoxicity of flavonoids from S. chamaejasme toward Poa annua L.
Flavonoids are small molecular secondary metabolites synthesized only by plants.Citation1 Due to the strong antioxidative properties, flavonoids display various biological activities. Also, the flavonoids are beneficial to plants themselves from participating in plants’ interactions with other organisms and their reactions to environmental stresses.Citation2 S. chamaejasme synthesizes a group of flavonoids, which gained much attention due to their biological activities to cancer cells and virus.Citation3,4 Additionally, their relevance to the plant itself also deserve extensive investigations. Several reports demonstrated that flavonoids from S. chamaejasme had insecticidal effects or showed contact toxicity to pathogenic nematodes. The antifungal and antibacterial effects of S. chamaejasme were well studied. Flavonoids from S. chamaejasme displayed relatively high level of phytotoxicity, indicating that allelopathy could be a beneficial function of the flavonoids to the producing plant.
The populations of S. chamaejasme have been increasing constantly in recent years.Citation5 As one of the most toxic weeds in the grassland, S. chamaejasme can poison or even kill the cattle if they eat the leaves or flowers of the weed by mistake.Citation6 Total flavonoid obtained from S. chamaejsme showed strong toxicity to mice and rabbit.Citation7 Chamaechromone and isochamaejasmin isolated from S. chamaejasme displayed cytotoxicity to canine kidney MDCK cells.Citation8 Therefore, the flavonoids may be a benefit to S. chamaejsme for deterring grazing of vertebrate herbivores. Additionally, S. chameajasme showed antifeeding activity to plant pathogenic insects. Ethanol extract of S. chameajasme strongly inhabited the growth of insect pests Pieris rapae, Myzus persicae and Ostrina furnacalis,Citation9 and displayed contact and oral toxicities against Sesamia inferens and Chilo suppressalis walker.Citation10 Furthermore, (+)-epiafzelechin had strong acaricidal activity against Tetranychus cinnabarinus B, while, quercetin displayed relatively weaker activity.Citation11 Chamechromone possessed strong effect of antifeedant and stomach poison on the 5th instar larvae of P. rapae.Citation12 Ethanol extract of S. chamaejasme showed significantly nematicidal activity against pine parasitic nematodes Bursaphelenchus xylophilus and Bursaphelenchus mucronatus. Ten nematicidal compounds were isolated by a bioassay guided fractionation. Among them, chamaejasmenin C induced the highest mortality against B. xylophilus, and chamaechromone exhibited strongly nematicidal activity against B. mucronatus. Biflavonoids ruixianglangdusu B, 7-methoxyneochaejasmin A, (+)-chamaejasmine, isosikokianin A, isoneochamaejasmin A and neochamaejasmin B also exhibited significantly nematicidal activities.Citation13,14 These results indicated that S. chamaejasme has strong antifeeding effects to phytophagous animals such as livestock, pests, acarid and nematodes, and the antifeeding effects are at least partially dependent on the flavonoids synthesized by the weed.
Ethanol extract, ethyl acetate and methanol fractions of S. chamaejasme showed antimicrobial activity against 14 phytopathogens.Citation15 Bioassay guided fraction of S. chamaejasme led to the isolation of neochamaejasmine B and chamaechromone, and both compounds could effectively inhibit the growth of Bakeri rehm, Fusarium graminearum, Alternaria solani, Fusarium bulbigenum, Exserohilum turcicum, Alternaria alternata and Phytophthora capsici.Citation16 Moreover, chamaechromone had antifungal activities on Botrytis cinerea, Colletotrichum acutatum and Monilinia fructicola.Citation17 Biflavanones chamaejasmenin A, chamaejasmenin D and isochamaejasmenin B displayed potent antimitotic and antifungal activity against Pyricularia oryzae, which cause rice blast to affect rice production grown in upland and wet-land.Citation18 Another biflavanone 4′, 4, 5, 5″, 7, 7″-hexahydroxy-3, 3″-biflavone also exhibited antibacterial activity.Citation19 Further study of the relationship between structure and antibacterial activity of neochamaejasmine B indicated that 7-OH and 7″-OH were the main active groups.Citation20 These results indicated that flavones maybe the main antimicrobial compounds of S. chamaejasme.
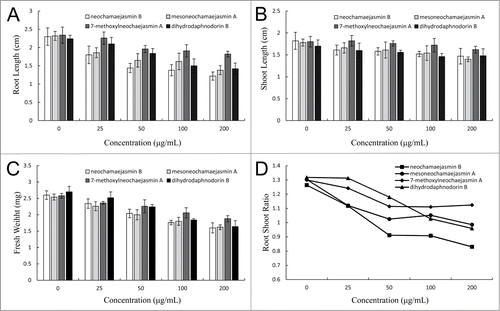
Water and ethanol extracts of S. chamaejasme inhibited seed germination and/or seedling growth of 13 plant species,Citation21-23 and the phytotoxic effects were more stronger on dicotyledonous plants than monocotyledonous plants. As a result of decomposition in the soil, the tissues of S. chamaejasme reduced seedling growth of Melilotus suaveolens Ledeb, Lolium perenne L., Psathyrostachys juncea, Onobrychis viciifolia, Bromus inermis and alfalfa,Citation24-27 and dicotyledonous plants were more sensitive to the decomposed tissues than monocotyledonous plants. In our previous research, a bioassay guided fractionation of root extracts of S. chamaejasme led to the isolation of 8 flavonoids. Most of these compounds displayed strong phytotoxic effects on seedling growth of Arabidopsis. Among them, neochamaejasmin B, mesoneochamaejasmin A, 7-methoxylneochaejasmin A and dihydrodaphnodorin B were found in soils associated with S. chamaejasme, and these potential allelochemicals also showed phytotoxicity to Clinelymus nutans L., an associated grass of S. chamaejasme.Citation28 Another grass (P. annua) was also used as a receptor plant to evaluate the phytotoxicity of the allelochemicals. Results showed that the allelochemicals could inhibit shoot and root elongation, and seedling growth of P. annua (). In consistent with bioassay on C. nutans, all compounds displayed stronger inhibitory effects on root than shoot because the root shoot ratio of P. annua dropped after treatments (). A similar result was also found in the phytotoxic effects of water extract of S. chamaejasme, which strongly reduced the root length of rapeseed, sesame, wheat and maize, while slightly inhabited the shoot elongation. Moreover, dicotyledonous plant (Arabidopsis) was more sensitive to the flavonoids than monocotyledonous plants (C. nutans and P. annua). These results indicated that S. chamaejasme is more phytoxtoxic to dicotyledonous plants than monocotyledonous plants, and showed tissue-specific phytotoxicity on root growth. Flavonoids are at least partly responsible for the strong phytotoxic effects of S. chamaejasme. The potential allelopathic behavior may facilitate this weed to become a good competitor against other plant species in the environment.
Figure 1. Phytotoxic effects of potential flavonoid allelochemicals from S. chamaejasme on P. annua seedlings. Five-day-old seedlings were treated with neochamaejasmin B, mesoneochamaejasmin A, 7-methoxylneochaejasmin A and dihydrodaphnodorin B at concentrations of 25, 50, 100 and 200 μg/mL for 3 days, respectively. Root length (A), shoot length (B), and fresh weight (C) of the seedlings were collected, and root shoot ratio (D) was calculated. Values in the table are presented as percentage of the mean compared to the control. SE is one standard error of the mean, n = 12.
In conclusion, the multifunctional flavonoids synthesized by S. chamaejasme may play important ecological roles in protecting the plant from vertebrate herbivores, insects, pathogenic microorganisms, as well as competitive plant species. These benefits may contribute to the competitive behavior of S. chamaejasme, one of the most ecologically-threatening weed in the grassland.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
We would like to thank the National Natural Science Foundation of China (31070386 and 31300290), around five top priorities program of “One-Three-Five” Strategic Planning of Lanzhou Institute of Chemical Physics, CAS, the Open Project of Key Laboratory of Tobacco Diseases and Insect Pests Monitoring Controlling and Integrated Management, Institute of Tobacco Research, Chinese Academy of Agricultural Sciences (IPM201404) and the Province-academy Cooperation Program of Henan Province of China (102106000021) for the funding that made this work possible.
References
- Buer CS, Imin N, Djordjevic MA. Flavonoids: new roles for old molecules. J Integr Plant Biol 2010; 52:98-111; PMID:20074144; http://dx.doi.org/10.1111/j.1744-7909.2010.00905.x
- Mierziak J, Kostyn K, Kulma A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014; 19:16240-65; PMID:25310150; http://dx.doi.org/10.3390/molecules191016240
- Liu W, Cheung FWK, Liu BPL, Li C, Ye W, Che CT. Involvement of p21 and FasL in induction of cell cycle arrest and apoptosis by neochamaejasmin A in human prostate LNCaP cancer cells. J Nat Prod 2008; 71:842-6; PMID:18380477; http://dx.doi.org/10.1021/np8001223;
- Asada Y, Sukemori A, Watanabe T, Malla KJ, Yoshikawa T, Li W, Kuang X, Koike K, Chen CH, Akiyama T, et al. Isolation, structure determination, and anti-HIV evaluation of tigliane-type diterpenes and biflavonoid from Stellera chamaejasme. J Nat Prod 2013; 76:852-7; PMID:23611151; http://dx.doi.org/10.1021/np300815t;
- Xing F, Song R. Population distribution pattern and dynamic of Stellera chamaejasme, a poisonous grassland plant. Pratacul Sci 2002; 19:16-9.
- Liu Y, Long R, Yao T. Research progress on Stellera chamaejasme L. in grassland. Pratacul Sci 2004; 21:55-61.
- Song XP, Cao GR, Li SJ. Abstraction and toxicity of total-flavonoid in Stellera chamaejsme L. Acta Univ Agric Boreali-occidentalis 1996; 24:35-8.
- Wang YH, Yang X, Sun LJ, Yang LM. Studies on the cytotoxicity of Stellera chamaejasme L. in vitro. Chin Med Biotechnol 2012; 7:9-13; http://dx.doi.org/10.1186/1749-8546-7-9
- Wang YW, Zhang GZ, Xu HH, Chiu SF. Biological activity of extract of Stellera chamaejasme against five pest insects. Acta Entomol Sinica 2002; 9:17-22
- He Y, Zhao X, Deng J, Wu H. Study on the killing activity for bioactive compound from Stellera chamrrejasme against Sesamia inferens and Chilo suppressalis Walker. Food Ferment Technol 2012; 48:43-6.
- Wang XG, Shi GL, Wang YN, Cheng J. The study on chemical structure of flavonoids from Stellera chamaejasme L. and the acaricidal activity against Tetranychus cinnabarinus B. Chin Agri Sci Bull 2013; 29:154-8.
- Zhang GZ, Xiu HH, Wang YW. Effects of antifeedant and stomach poison of daphnoritin and chamechromone to the insect. J Qinghai Univ 2001; 19:1-3.
- Cui HY, Jin H, Liu Q, Yan ZQ, Ding L, Qin B. Nematicidal metabolites from roots of Stellera chamaejasme against Bursaphelenchus xylophilus and Bursaphelenchus mucronatus. Pest Manag Sci 2014; 70:827-35; PMID:23934879; http://dx.doi.org/10.1002/ps.3625
- Cui HY, Jin H, Wang DD, Liu Q, Qin B. Nematicidal activities of isoneochamaejasmin A and neochamaejasmin B from the roots of Stellera chamaejasme L. against Bursaphelenchus xylophilus and Bursaphelenchus mucronatus. Nat Prod Res Dev 2014; 26: 639-44.
- Ma L, Kang X, Huang Y, Hou D, Hou T. Antimicrobial activity of root extracts of Stellera chamaejasme L. from China. World Appl Sci J 2009; 6:664-8.
- Zhou L, Yuan C, Qin BF, Miao F, Liu LP. Study on the antifungal components in the root of Stellera chamaejasme. Acta Bot Boreal-Occident Sin 2004; 24:2346-9.
- Bu CY, Cheng J, Wang YN, Gong YW, Wang CQ, Shi GL. Studying on antifungal compounds from root extracts of Stellera chamaejasme against plant pathogenic fungi. North Hortic 2013; 19:120-3.
- Yang GH, Liao ZX, Xu ZY, Zhang HP, Chen DF. Antimitotic and antifungal C-3/C-3″-biflavanones from Stellera chamaejasme. Chem Pharm Bull 2005; 53:776-9; PMID:15997133; http://dx.doi.org/10.1248/cpb.53.776
- Xu ZH, Qin GW, Li XY, Xu RS. New biflavanones and bioactive compounds from Stellera chamaejasme L. Acta Pharm Sin 2001; 36:668-71; PMID:12580104
- Gao Y, Liu GD, Yang J, Zhou L, Sun W. Further study on relationship between structure and antibacterial activity of neochamaejasmine B. J Northwest A-F Univ (Nat Sci) 2008; 36:179-84.
- Ma L, Wu H, Bai R, Zhou L, Yuan X, Hou D. Phytotoxic effects of Stellera chamaejasme L. root extract. Afr J Agr Res 2011; 6:1170-6; http://dx.doi.org/10.5897/AJAR10.732
- Cao CY, Fu Y, Wang WX, Gao FF. Inhibition influence of extraction liquids from Stellera chamaejasme root on seed germination. J Northeast Univ (Nat Sci) 2007; 28:729-32.
- Bi L, Liao R. Study on allelopathic effects of aqueous extracts from Stellera chamaejasme L. and Cynanchum komarovii. J Anhui Agri Sci 2010; 38:3294-97.
- Zhou SQ, Wang H, Huang ZJ, Liu YL, Hu HF. Research on allelopathy of Stellera chamaejasme in decompose process in the soil on Alfalfa. Chin J Grassl 2008; 30:78-32.
- Wang H, Zhou SQ, Huang ZJ. A study on allelopathic effect of Stellera chamaejasme L. on Melilotus suaveolens Ledeb and Lolium perenne L. Acta Agrectir> Sinica 2009; 17:826-9.
- Zhou SQ, Huang ZJ, Wang H, Liu YL, Hu HF. Research on allelopathy of Stellera chamaejasme on Psathyrostachys juncea. Chin J Grassl 2009; 31:94-7.
- Zhou SQ, Huang ZJ, Wang H, Liu YL, Hu HF. Allelopathic effect of Stellera chamaejasme decomposing in soil on Onobrychis viciifolia. Pratacul Sci 2009; 26:91-4.
- Yan ZQ, Guo HR, Yang JY, Liu Q, Jin H, Xu R, Cui HY, Qin B. Phytotoxic flavonoids from roots of Stellera chamaejasme L. (Thymelaeaceae). Phytochemistry 2014; 106:61-8; PMID:25096753; http://dx.doi.org/10.1016/j.phytochem.2014.07.013
