Abstract
Tissue-specific functions of the circadian clock in Arabidopsis have recently been revealed. The vasculature clock shows distinctive gene expression profiles compared to the clock in other tissues under light-dark cycles. However, it has not yet been established whether the vasculature clock also shows unique gene expression patterns that correlate with temperature cycles, another important environmental cue. Here, we detected diel phase of TIMING OF CAB EXPRESSION 1 (TOC1) expression in the vasculature and whole leaf under long-day light-dark cycles and temperature cycles. We found that the vasculature clock had advanced TOC1 phase under light-dark cycles but not under temperature cycles, suggesting that the vasculature clock has lower sensitivity against temperature signals. Furthermore, the phase advancement of TOC1 was seen only under long-day condition but not under short-day condition. These results support our previous conclusion that the circadian clock in vasculature preferentially senses photoperiodic signals.
Abbreviations
| TOC1 | = | TIMING OF CAB EXPRESSION 1 |
| CAB2 | = | CHLOROPHYLL A/B BINDING PROTEIN 2 |
| CAT3 | = | CATALASE 3 |
In many organisms, circadian clock systems are used for processing environmental stimuli and predicting daily and seasonal changes. The plant circadian clock system had long been assumed to process these signals cell-autonomously. Recently, we have revealed that there are tissue-specific functions of the circadian clock system in Arabidopsis, and demonstrated that vasculature has a unique clock system compared to other tissues.Citation1 We also uncovered a role of the vasculature clock in photoperiodic flowering. A previous study demonstrated that CHLOROPHYLL A/B BINDING PROTEIN 2 (CAB2) and CATALASE 3 (CAT3) have differential sensitivities to light and temperature signals.Citation2 The diel oscillation of CAB2 expression was entrainable by light-dark cycles but not temperature cycles. By contrast, the CAT3 expression pattern was entrained by temperature cycles but not light-dark cycles. Since CAB2 is known to be a mesophyll-specific gene, we hypothesized that dedicated tissue-specific circadian clock systems incorporate these different sensitivities of CAB2 and CAT3 against photoperiod and temperature cycles. However, little is known how photoperiod and temperature signals affect clock gene expression in vasculature.
To study the effects of photoperiod and temperature signals on circadian clock gene expression in the vasculature and whole leaf, plants were grown under long-day (16:8 light-dark cycles or temperature cycles) and short-day (8:16 light-dark cycles) conditions for 10 days. Then, the vasculature and whole leaf samples were collected every 2 to 4 hours and TOC1 gene expression was monitored by quantitative real-time PCR.Citation1 In the whole leaf samples, the diel phase of TOC1 expression showed almost the same phase both under long-day light-dark cycles and long-day temperature cycles. By contrast, the TOC1 phase in the vasculature was slightly but significantly advanced compared to the whole leaf under long-day light-dark cycles, whereas no comparative phase advancement was observed under long-day temperature cycles (). These results suggested that photoperiodic signals and temperature signals do not have equal effects on the vasculature clock entrainment. A previous study demonstrated that the diel phase of TOC1 expression was sensitive to the day length in the whole leaf context.Citation3 Therefore, we tested whether the phase advancement of vasculature TOC1 was detectable under short-day light-dark cycles (). Interestingly, the phase advancement was not observed in that condition, indicating that the phase advancement of TOC1 operates in a long-day light-dark specific manner.
Figure 1. TOC1 expression in the whole leaf and the vasculature under long-day light-dark cycles, temperature cycles and short-day light-dark cycles. Plants were grown under long-day light-dark cycles (16 hour light / 8 hour dark at 22°C) (A), temperature cycles (16 hour 22°C / 8 hour 12°C in continuous light) (B) and short-day light-dark cycles (8 hour light / 16 hour dark at 22°C) (C) for 10 days. ZT, zeitgeber time. Mean ± s.e.m. (long day, temperature cycles, n=3; and short day, n=2). Geometric mean of IPP2 and APA1 were used as internal controls.1
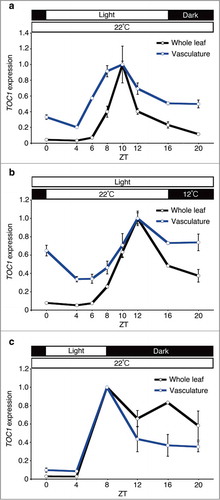
Despite the subtle phase advancement under long-day condition, the vasculature clock is rather insensitive to photoperiodic changes. The diel phase of TOC1 expression in the whole leaf was drastically changed between long-day and short-day conditions. Since about 80% of mRNA in the whole leaf came from mesophyll cells1; therefore most of TOC1 mRNA in whole leaves is expected to come from mesophyll cells. By contrast, the diel phase of TOC1 expression was rather phase-locked. This feature of TOC1 expression in vasculature might be beneficial for plants to register environmental light conditions, according to the external coincidence model,Citation4 since the internal clock gene expression pattern is less affected by photoperiodic changes. These results support our view that the vasculature clock is important for photoperiodic flowering.Citation5 More interestingly, we could not detect any phase advancement in the long-day temperature cycles. This result implies that there is another tissue in which temperature signals are processed. Since CAT3 is expressed in both mesophyll and epidermis,2 epidermis might be a candidate tissue for temperature sensing.
Cytosolic calcium oscillation is also reported as cell and stimulus-type specific.6 Thus, as we demonstrated here, a tissue-specific approach in circadian clock studies will provide new insights into environmental signal sensing and processing by the circadian clock.
References
- Endo M, Shimizu H, Nohales MA, Araki T, Kay SA. Tissue-specific clocks in arabidopsis show asymmetric coupling. Nature 2014; 515: 419–22; PMID: 25363766; http://dx.doi.org/10.1038/nature13919
- Michael TP, Salome PA, McClung CR. Two arabidopsis circadian oscillators can be distinguished by differential temperature sensitivity. Proc Natl Acad Sci U S A 2003; 100: 6878–83; PMID: 12736379; http://dx.doi.org/10.1073/pnas.1131995100
- Perales M, Más P. A functional link between rhythmic changes in chromatin structure and the arabidopsis biological clock. Plant Cell 2007; 19: 2111–23; PMID: 17616736; http://dx.doi.org/10.1105/tpc.107.050807
- Sawa M, Nisinow DA, Kay SA, Imaizumi T. FKF1 and GIGANTEA complex formation is required for day length measurement in arabidopsis. Science 2007; 318: 261–65; PMID: 17872410; http://dx.doi.org/10.1126/science.1146994
- Endo M, Mochizuki N, Suzuki T, Nagatani A. CRYPTOCHROME2 in vascular bundles regulates flowering in arabidopsis. Plant Cell 2007; 19: 84–93; PMID: 17259260; http://dx.doi.org/10.1105/tpc.106.048157
- Cell-and stimulus type-specific intracellular free Ca2+ signals in Arabidopsis. Martí MC, stancombe MA, Webb AA. Plant Physiol 2013; 163: 625–34; PMID: 24027243; http://dx.doi.org/10.1104/pp.113.222901
