Abstract
Cell elongation requires directional deposition of cellulose microfibrils regulated by transverse cortical microtubules. Microtubules respond differentially to suppression of cell elongation along the developmental zones of Arabidopsis thaliana root apex. Cortical microtubule orientation is particularly affected in the fast elongation zone but not in the meristematic or transition zones of thanatos and pom2–4 cellulose-deficient mutants of Arabidopsis thaliana. Here, we report that a uniform phenotype is established among the primary cell wall mutants, as cortical microtubules of root epidermal cells of rsw1 and prc1 mutants exhibit the same pattern described in thanatos and pom2–4. Whether cortical microtubules assume transverse orientation or not is determined by the demand for cellulose synthesis, according to each root zone's expansion rate. It is suggested that cessation of cell expansion may provide a biophysical signal resulting in microtubule reorientation.
Cortical Microtubule Patterning is Aberrant in the Fast Elongation Zone of Primary Cell Wall Mutants
Anisotropic cell expansion relies on wall reinforcement due to deposition of cellulose microfibrils transversely to the growth axis. In primary walls of expanding cells, cellulose is produced by cellulose synthase complexes, consisting mainly of Cellulose SynthaseA1 (CesA1), CesA3 and CesA6,Citation1 which are connected to cortical microtubules by Cellulose Synthase Interacting1 protein (CSI1).Citation2,3 Cortical microtubules guide the movement of cellulose synthase complexes and, ultimately, regulate microfibril orientation.Citation4 Besides the role of cortical microtubules in cell wall structure, cell wall itself influences microtubule organization.Citation5 Hence, defects in cellulose synthesis have been reported to be related with alterations of microtubule orientation.Citation6-Citation9 We recently showed that in Arabidopsis thaliana cellulose-deficient thanatos and pom2–4 mutants, of AtCesA3 and AtCSI1 respectively, suppression of cell expansion results in cortical microtubule reorientation in the fast elongation zone.Citation10 As both genes are involved in cellulose synthesis in primary cell walls, we examined the orientation of microtubules in 2 additional primary wall-related mutants, radially swollen1 (rsw1)Citation11,12 and procuste1 (prc1)Citation13 of AtCesA1 and AtCesA6, respectively.
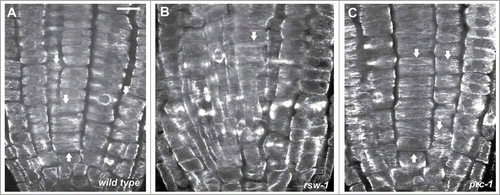
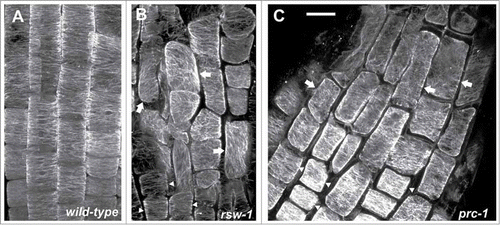
The orientation of cortical microtubules in the fast elongation zone of 5 days-old wild-type roots, kept at either 22°C or 31°C for 24 h, was transverse (). On the contrary, in the fast elongation zone of rsw1 roots, temperature-induced suppression of cell expansion resulted in alteration of cortical microtubule orientation from a transverse to a random pattern (). Likewise, cortical microtubules were not transverse in the fast elongation zone of prc1 roots (). These findings, in agreement with the recently reported results in than (AtCesA3) and pom2–4 (AtCSI1) roots,Citation10 indicate that in fast elongating cells of primary cell wall mutants the orientation of microtubules is aberrant. Nevertheless, in the cells of the meristematic and transition zones of the deficient in primary wall cellulose synthase complex mutants rsw1, than and prc1 or in the cellulose-deficient pom2–4 mutant, cortical microtubules were transverse as in wild-type roots (; ref. 10). Taken together, cortical microtubule patterning is consistent throughout the developmental zones of primary cell wall mutant roots.
Figure 1. Maximum projections of consecutive CLSM sections after α-tubulin immunostaining, as described in ref. 10, through the transition and elongation zones of wild-type (A) and rsw1 roots kept for 24 h at 31°C (B), or prc1 (C) roots at 22°C. Cortical microtubules are transversely oriented in transition zone cells (arrowheads) in all roots. In cells of the elongation zone (arrows), microtubule orientation is not transverse in the mutants, compared to the wild-type (B, C; cf. A). All plant materials were purchased from NASC (http://arabidopsis.info/). Scale bar: 20μm.
Microtubule Orientation and Cell Expansion: It Takes Two to Tango
The term “microtubule-microfibril syndrome”Citation14 refers to a dynamic seque2ce of events driven by the cooperation between microtubules and cell wall elements, to accomplish growth and morphogenesis of plant organs. Any malfunction of either partner, microtubules or cell wall elements, inevitably affects the other and ultimately the development of the whole plant. As root growth is guided by gravity, anisotropy establishes an inherent bias toward transverse orientation of cortical microtubules.Citation15 Cell expansion, slow or fast, requires deposition of new cellulose microfibrils, as the preexisting microfibrils separate.Citation16 Concomitantly, new microtubules are recruited under the expanding plasma membrane, parallel to these that already exist, aiming to maintain the microtubule density of the cortical array. While cellulose synthase complexes “glide” on these microtubules, they deposit new transverse microfibrils in the expanding wall. Cell expansion commences at low rate in the meristematic zone and remains moderate in the transition zone, whereas in the fast elongation zone it accelerates.Citation17 As cells acquire their final length in growth terminating zone, the newly deposited microfibrils are no more strictly transverse and thereby cortical microtubules are reoriented.Citation18
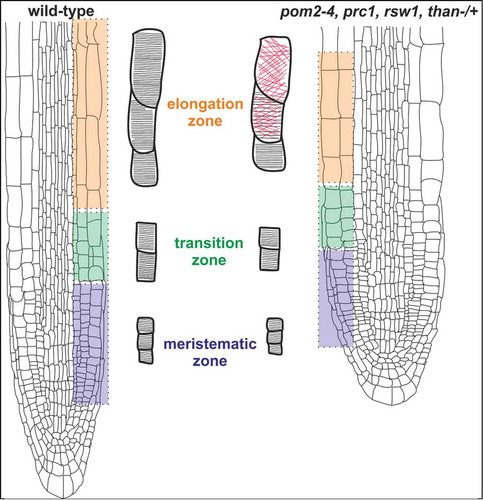
The supply of newly synthesized microfibrils has to keep pace with wall expansion throughout cell elongation. It has been suggested that cellulose deficiency in primary wall mutants is probably not severe enough in slowly expanding cells of the meristematic and transition zones.Citation10 Thus, as the cells within these zones keep elongating, the orientation of microtubules remains transverse (). On the contrary, cellulose shortage restricts cell expansion in the fast elongation zone, as the newly synthesized microfibrils deposited in the expanding cell wall may not be enough for cell elongation. Cessation of expansion at this stage is similar to that of fully expanded wild-type cells and results in premature microtubule reorientation ().
Figure 3. A graphical model illustrating the effect of cellulose-deficient primary cell wall mutants on cortical microtubule orientation in Arabidopsis thaliana root. The short primary root phenotype of pom2–4, prc1, rsw1 and than mutants is caused by reduction of anisotropic cell expansion, originating from decreased cellulose microfibril deposition. Suppression of cell expansion results in alteration of cortical microtubule orientation from a transverse (black lines) to a random (red lines) pattern in the fast elongation zone. On the contrary, in the meristematic and transition zones cortical microtubules exhibit transverse orientation (black lines) reminiscent of the pattern in wild-type roots. The mutants are: pom2–4 of Cellulose Synthase Interacting1 protein (CSI1),Citation3 prc1 (procuste1 of CesA6),Citation13 rsw1 (radially swollen1 of CesA1),Citation11,12 and than (thanatos of CesA3).Citation19
As recently reported,Citation10 inhibition of cell expansion, irrespective of the cause, induces the reorientation of cortical microtubules, since there is no demand for deposition of more transversely oriented microfibrils in the cell wall. Therefore, it seems plausible that a signal of biophysical nature is transduced from the cell wall to the protoplast, triggering the shift of microtubule orientation from transverse to random in cells ceasing elongation. The components involved in this process remain still unknown and further research is required to determine these factors. Likewise, the cues involved in the initial establishment of transverse microtubule orientation in the meristematic root cells need to be elucidated.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Prof. Polydefkis Hatzopoulos for critical reading and suggestions on the manuscript.
Funding
This work was supported by the Research Committee of Aristotle University of Thessaloniki (grant no. 89348). Gerasimos Daras acknowledges support from IKY fellowships of excellence for postgraduate studies in Greece-Siemens program.
References
- Persson S, Paredez A, Carroll A, Palsdottir H, Doblin M, Poindexter P, Khitrov N, Auer M, Somerville CR. Genetic evidence for three unique components in primary cell-wall cellulose synthase complexes in Arabidopsis. Proc Natl Acad Sci USA 2007; 104:15566-71; PMID:17878302; http://dx.doi.org/10.1073/pnas.0706592104
- Gu Y, Kaplinsky N, Bringmann M, Cobb A, Carroll A, Sampathkumar A, Baskin TI, Persson S, Somerville CR. Identification of a cellulose synthase-associated protein required for cellulose biosynthesis. Proc Natl Acad Sci USA 2010; 107:12866-71; PMID:20616083; http://dx.doi.org/10.1073/pnas.1007092107
- Bringmann M, Li E, Sampathkumar A, Kocabek T, Hauser M-T, Persson S. POM-POM2/CELLULOSE SYNTHASE INTERACTING1 is essential for the functional association of cellulose synthase and microtubules in Arabidopsis. Plant Cell 2012; 24:163-77; PMID:22294619; http://dx.doi.org/10.1105/tpc.111.093575
- Paredez AR, Somerville CR, Ehrhardt DW. Visualization of cellulose synthase demonstrates functional association with microtubules. Science 2006; 312:1491-95; PMID:16627697; http://dx.doi.org/10.1126/science.1126551
- Fisher DD, Cyr RJ. Extending the microtubule/microfibril paradigm: cellulose synthesis is required for normal cortical microtubule alignment in elongating cells. Plant Physiol 1998; 116:1043-51; PMID:9501137; http://dx.doi.org/10.1104/pp.116.3.1043
- Himmelspach R, Williamson RE, Wasteneys GO. Cellulose microfibril alignment recovers from DCB-induced disruption despite microtubule disorganization. Plant J 2003; 36:565-75; PMID:14617086; http://dx.doi.org/10.1046/j.1365-313X.2003.01906.x
- Chu Z, Chen H, Zhang Y, Zhang Z, Zheng N, Yin B, Yan H, Zhu L, Zhao X, Yuan M, et al. Knockout of the AtCESA2 gene affects microtubule orientation and causes abnormal cell expansion in Arabidopsis. Plant Physiol 2007; 143:213-24; PMID:17085513; http://dx.doi.org/10.1104/pp.106.088393
- Paredez AR, Persson S, Ehrhardt DW, Somerville CR. Genetic evidence that cellulose synthase activity influences microtubule cortical array organization. Plant Physiol 2008; 147:1723-34; PMID:18583534; http://dx.doi.org/10.1104/pp.108.120196
- Peng L, Zhang L, Cheng X, Fan L-S, Hao H-Q. Disruption of cellulose synthesis by 2,7dichlorobenzonitrile affects the strucrure of the cytoskeleton and cell wall construction in Arabidopsis. Plant Biol 2013; 15:405-14; PMID:22759307; http://dx.doi.org/10.1111/j.1438-8677.2012.00630.x
- Panteris E, Adamakis I-DS, Daras G, Hatzopoulos P, Rigas S. Differential responsiveness of cortical microtubule orientation to suppression of cell expansion among the developmental zones of Arabidopsis thaliana root. PLoS One 2013; 8:e82442; PMID:24324790; http://dx.doi.org/10.1371/journal.pone.0082442
- Arioli T, Peng L, Betzner AS, Bum J, Wittke W, Herth W, Camilleri C, Höfte H, Plazinski J, Birch R, et al. Molecular analysis of cellulose biosynthesis in Arabidopsis. Science 1998; 279:717-20; PMID:9445479; http://dx.doi.org/10.1126/science.279.5351.717
- Sugimoto K, Williamson RE, Wasteneys GO. Wall architecture in the cellulose-defficient rsw1 mutant of Arabidopsis thaliana: microfibrils but not microtubules lose their transverse alignment before microfibrils become unrecognizable in the mitotic and elongation zones of roots. Protoplasma 2001; 215:172-83; PMID:11732056; http://dx.doi.org/10.1007/BF01280312
- Fagard M, Desnos T, Desprez T, Goubet F, Refregier G, Mouille G, McCann M, Rayon C, Vernhettes S, Höfte H. PROCUSTE1 encodes a cellulose synthase required for normal cell elongation specifically in roots and dark-grown hypocotyls of Arabidopsis. Plant Cell 2000; 12:2409-23; PMID:11148287; http://dx.doi.org/10.1105/tpc.12.12.2409
- Robinson DG, Quader H. The microtubule-microfibril syndrome. The Cytoskeleton in Plant Growth and Development The Cytoskeleton in Plant Growth and Development. London: Academic Press; 1982; pp. 109-26.
- Ambrose C, Allard JF, Cytrynbaum EN, Wasteneys GO. A CLASP-modulated cell edge barrier mechanism drives cell-wide cortical microtubule organization in Arabidopsis. Nat Commun 2011; 2:430; PMID:21847104; http://dx.doi.org/10.1038/ncomms1444
- Marga F, Grandbois M, Cosgrove DJ, Baskin TI. Cell wall extension results in the coordinate separation of parallel microfibrils: evidence from scanning electron microscopy and atomic force microscopy. Plant J 2005; 43:181-90; PMID:15998305; http://dx.doi.org/10.1111/j.1365-313X.2005.02447.x
- Verbelen J-P, De Cnodder T, Le J, Vissenberg K, Baluška F. The root apex of Arabidopsis thaliana consists of four distinct zones of growth activities. Plant Signal Behav 2006; 1:296-04; PMID:19517000; http://dx.doi.org/10.4161/psb.1.6.3511
- Sugimoto K, Williamson RE, Wasteneys GO. New techniques enable comparative analysis of microtubule orientation, wall texture, and growth rate in intact roots of Arabidopsis. Plant Physiol 2000; 124:1493-06; PMID:11115865; http://dx.doi.org/10.1104/pp.124.4.1493
- Daras G, Rigas S, Penning B, Milioni D, McCann MC, Carpita NC, Fasseas C, Hatzopoulos P. The thanatos mutation in Arabidopsis thaliana cellulose synthase 3 (AtCESA3) has a dominant-negative effect on cellulose synthesis and plant growth. New Phytol 2009; 184:114-26; PMID:19645738; http://dx.doi.org/10.1111/j.1469-8137.2009.02960.x