Abstract
The role of abscisic acid (ABA) during early development was investigated in tomato seedlings. The endogenous content of ABA in particular organs was analyzed in seedlings grown in the dark and under blue light. Our results showed that in dark-grown seedlings, the ABA accumulation was maximal in the cotyledons and elongation zone of hypocotyl, whereas under blue-light, the ABA content was distinctly reduced. Our data are consistent with the conclusion that ABA promotes the growth of etiolated seedlings and the results suggest that ABA plays an inhibitory role in de-etiolation and photomorphogenesis in tomato.
Light is an important signal regulating plant development. After germination in the dark (D), the seedling undergoes etiolated growth referred as skotomorphogenesis. This developmental step is characterized by rapid elongation of the hypocotyl topped by a hook with underdeveloped cotyledons. This is to ensure the seedling reaches a light environment before exhausting its reserves. As soon as the seedling perceives the light, photomorphogenesis starts. The transition from skoto- to photomorphogenesis, called de-etiolation, represents the switch from heterotrophy to autotrophy.Citation1 On a cellular level, light is absorbed by photoreceptors specific to particular monochromatic light: phytochromes for red (RL) and far-red wavelengths, cryptochromes/phototropins for blue light (BL). Although other receptors have been identified, these 3 classes of photoreceptors represent the major light sensors. Even though all are implicated in the inhibition of hypocotyl growth,Citation2 at the same intensity, BL is a more efficient inhibitor of elongation than RL.Citation3,4 The phytohormones gibberellins, auxin, brassinosteroids, ethylene and cytokinin, regulate Arabidopsis hypocotyl growth both during skoto- and photomorphogenesis.Citation1 The establishment of skotomorphogenesis depends on interactions between hormones. Despite the fact abscisic acid (ABA) contributes to these complex hormonal networks in different physiological responses, its particular role during skoto- and photomorphogenesis has not been thoroughly investigated.
We recently studied the role of ABA in the growth of etiolated tomato hypocotyls using ABA-deficient sitiens and notabilis mutants.Citation5 Physiological experiments showed a significant reduction in hypocotyl growth in the mutants compared to wild-types (WTs). Low concentrations of ABA (nM) stimulated the mutant growth but not the WTs. Higher concentration of ABA led to growth inhibition in all genotypes. Our results suggested that in plants with physiological ABA concentrations (i.e. WTs), exogenous ABA leads to an “over-dose” effect responsible for the observed growth inhibition. In contrast, in the ABA-deficient plants that are “starving” for ABA, the response to exogenous ABA can be considered as closer to the “real physiological situation” of the plant. In summary, it appears that a fine-tuned regulation of endogenous ABA content is required to support hypocotyl growth. At the same time, we analyzed the endogenous ABA content in WT tomato plants (cv. Rutgers) during germination and seedling development. If the hypothesis that ABA inhibits plant growth in the light is true,Citation6,7 then ABA should be synthesized and accumulate during photomorphogenesis. Interestingly, the quantitative analysis of ABA content in whole D-grown and de-etiolated seedlings revealed ABA accumulated to a greater extent in D-grown seedlings. These physiological data are supported by the expression analysis of LeNCED1 gene, encoding an enzyme involved in ABA synthesis. Indeed, LeNCED1 transcripts accumulated significantly in the D-grown seedlings than in de-etiolated seedlings.Citation5 Altogether, our previously published results demonstrated ABA accumulation is required to stimulate the fast growth of tomato hypocotyl during skotomorphogenesis.
We further investigated the effect of BL on the spatio-temporal distribution of free ABA content in different organs of tomato (cv Rheinlands Ruhm) seedlings: radicles, cotyledons with endosperm, roots, whole hypocotyls or elongating zone of hypocotyl,Citation8 and cotyledons (). The extraction and quantification of ABA was performed as described by Turečková et al.Citation9 At the onset of germination, ABA accumulated more in the emerging radicle than in the rest of the seed. During the development of the seedling, ABA concentration increased in both hypocotyl and in cotyledons to a similar extent after 96 h of cultivation. One day later (120h), a significant increase in ABA content was observed in cotyledons but not in the intact hypocotyl. This result is in agreement with data from the Arabidopsis abi3 (ABA insensitive 3) mutant. Indeed, ABI3, a component of the ABA signaling pathway, is required to maintain unfolded, yellowish cotyledons containing undifferentiated plastids.Citation10 ABA also controls the entry into stomatal-lineage development in Arabidopsis leaves.Citation11 Interestingly, there was marked great accumulation of free ABA in the elongating zone of hypocotyl, further supporting our published data.Citation5 Finally, the low concentration of free ABA in the root throughout the experimental period indicated that ABA probably has no or only a limited role during early root development in tomato seedling. However, a potential effect of artificial in vitro cultivation especially in root development cannot be excluded. When seedlings were grown under BL, ABA accumulation was significantly reduced in the hypocotyl and cotyledons () showing similar proportional distribution in particular organs as in the D ().
Figure 1. Spatiotemporal changes in ABA content presented in particular organs of tomato seedlings. ABA concentrations during germination in the D (72h) and etiolated growth in the D and during de-etiolation and photomorphogensis in BL (96 h-120 h) are visualized as a “heat-map” by color range. The colors represent median values of absolute ABA concentrations in given part of the seedling that were calculated from 3 biological repeats and correspond to the scale (0 – 80 pmol/g of fresh weight - FW).
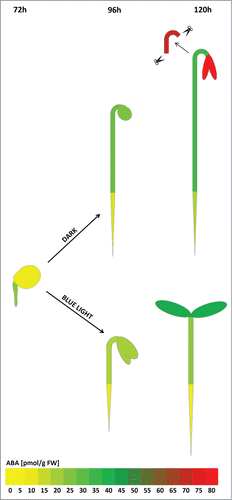
Table 1. Quantification of free ABA in particular plant organs of tomato seedlings grown in the dark and under blue light. The data are presented as medians of 3 biological experiments, statistical significance was performed using Mann-Whitney test.
The regulation of endogenous ABA by light was described more than 20 y ago. In pea, deficiency in R/FR light perception resulted in accumulation of ABA in seeds and leaves.Citation12 RL also reduced ABA accumulation in etiolated Lemna gibba and D-treatment of previously light-grown Arabidopsis and Lemna gibba plants led to a significant accumulation of ABA.Citation13 The treatment of photoblastic lettuce seeds by RL decreased ABA content and down-regulated the ABA biosynthetic genes LsNCED2 and LsNCED4.Citation14 All these data were obtained from plants grown in RL. As the same responses were observed in tomato seedlings grown in BL, we hypothesize that the different light signaling pathways converge to induce hormonal-regulated plant growth and development. Nevertheless, in barley grain, exposure to light induced expression of the HvNCED gene and accumulation of ABA.Citation15 The discrepancy between our data and the published literature can be explained by the fact that barley is differently regulated by light than other species. For example, whereas light induces germination of lettuce seeds,Citation14 it strengthens the dormancy of barley grains, i.e., inhibit their germination.Citation16
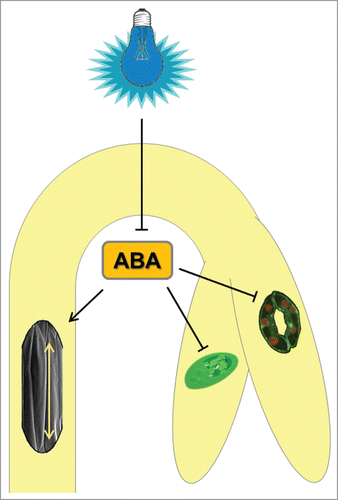
To summarize, in tomato seedlings, ABA accumulates in elongation zone of hypocotyl to participate in cell expansion during skotomorphogenesis. Light reduces the ABA content to a minimum required to maintain the steady-state growth rate characteristic of photomorphogenesis. Moreover, ABA markedly accumulates in cotyledons during skotomorphogenesis. ABA might thus contribute to the inhibition of cotyledon development in particular, by maintaining unfolded cotyledons to protect the apical meristem from damage by soil particles, and restrict stomata initiation and maturation of plastids ().
Figure 2. Hypothetical model of ABA action in etiolated growth of tomato seedlings. The abscisic acid content is reduced by BL exposure to promote de-etiolation. When the seedlings are kept in the D, ABA inhibits maturation of chloroplasts and development of stomata, but supports the cell elongation to maintain hypocotyl growth. (The part of the figure was adopted from Wikipedia free illustrations under CC license).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Authors thank Alex Outlon for proofreading of the manuscript.
Funding
This work was supported by the Ministry of Education, Youth and Sports of the Czech Republic grant no. LO1204 (National Program of Sustainability) and internal grant from Palacký University IGA_PrF_2015_021. V. Bergougnoux and M. Fellner were supported by the Operational Programs Education for Competitiveness - European Social Fund, project no. CZ.1.07/2.3.00/20.0165 and project no. CZ.1.07/2.3.00/30.0004, respectively.
References
- Arsovski AA, Galstyan A, Guseman JM, Nemhauser JL. Photomorphogenesis. Arabidopsis Book 2012; 10:e0147; PMID:22582028; http://dx.doi.org/10.1199/tab.0147
- Casal JJ. Phytochromes, cryptochromes, phototropin: photoreceptor interactions in plants. Photochem Photobiol 2000; 71:1-11; PMID:10649883; http://dx.doi.org/10.1562/0031-8655(2000)071%3c0001:PCPPII%3e2.0.CO;2
- Parks BM, Cho MH, Spalding EP. Two genetically separable phases of growth inhibition induced by blue light in Arabidopsis seedlings. Plant Physiol 1998; 118: 609-15; PMID:9765547; http://dx.doi.org/10.1104/pp.118.2.609
- Parks BM, Spalding EP. Sequential and coordinated action of phytochromes A and B during Arabidopsis stem growth revealed by kinetic analysis. Proc Natl Acad Sci USA 1999; 96: 14142-6; PMID:10570212; http://dx.doi.org/10.1073/pnas.96.24.14142
- Humplík JF, Bergougnoux V, Jandová M, Šimura J, Pěnčík A, Tomanec O, Rolčík J, Novák O, Fellner, M. Endogenous abscisic acid promotes hypocotyl growth and affects endoreduplication during dark-induced growth in tomato (Solanum lycopersicum L.). PloS One 2015; 10:e0117793; http://dx.doi.org/10.1371/journal.pone.0117793
- Fellner M, Zhang R, Pharis RP, Sawhney VK. Reduced de‐etiolation of hypocotyl growth in a tomato mutant is associated with hypersensitivity to, and high endogenous levels of, abscisic acid. J Exp Bot 2001; 52:725-38; PMID:11413209
- Chen H, Zhang J, Neff MM, Hong S-W, Zhang H, Deng XW, Xiong L. Integration of light and abscisic acid signaling during seed germination and early seedling development. Proc Natl Acad Sci USA 2008; 105:4495-500; PMID:18332440; http://dx.doi.org/10.1073/pnas.0710778105
- Bergougnoux V, Zalabák D, Jandová M, Novák O, Wiese-Klinkenberg A, Fellner M. Effect of blue light on endogenous isopentenyladenine and endoreduplication during photomorphogenesis and de-etiolation of tomato (Solanum lycopersicum L.) seedlings. PLoS One 2012; 7:e45255; http://dx.doi.org/10.1371/journal.pone.0045255
- Turečková V, Novák O, Strnad M. Profiling ABA metabolites in Nicotiana tabacum L. leaves by ultra-performance liquid chromatography–electrospray tandem mass spectrometry. Talanta 2009; 80:390-9; http://dx.doi.org/10.1016/j.talanta.2009.06.027
- Rohde A, De Rycke R, Beeckman T, Engler G, Van Montagu M, Boerjan W. ABI3 affects plastid differentiation in dark-grown Arabidopsis seedling. Plant Cell 2000; 12: 35-52; PMID:10634906; http://dx.doi.org/10.1105/tpc.12.1.35
- Tanaka Y, Nose T, Jikumaru Y, Kamiya Y. 2013. ABA inhibits entry into stomatal-lineage development in Arabidopsis leaves. Plant J 2013; 74: 448-57; PMID:23373882; http://dx.doi.org/10.1111/tpj.12136
- Kraepiel Y, Rousselin P, Sotta B, Kerhoas L, Einhorn J, Caboche M, Miginiac E. Analysis of phytochrome- and ABA-deficient mutants suggests that ABA degradation is controlled by light in Nicotiana plumbaginifolia. 1994; 6:665-72
- Weatherwax SC, Ong MS, Degenhardt J, Bray EA, Tobin EM. The interaction of light and abscisic acid in the regulation of plant gene expression. Plant Physiol 1996; 111:363-70; PMID:8787022; http://dx.doi.org/10.1104/pp.111.2.363
- Sawada Y, Aoki M, Nakaminami K, Mitsuhashi W, Tatematsu K, Kushiro T, Koshiba T, Kamiya Y, Inoue Y, Nambara E, et al. Phytochrome- and gibberellin-mediated regulation of abscisic acid metabolism during germination of photoblastic lettuce seeds. Plant Physiol 2008; 146:1386-96; PMID:18184730; http://dx.doi.org/10.1104/pp.107.115162
- Gubler F, Hughes T, Waterhouse P, Jacobsen J. Regulation of dormancy in barley by blue light and after-ripening: effects on abscisic acid and gibberellin metabolism. Plant Physiol 2008; 147:886-96; PMID:18408047; http://dx.doi.org/10.1104/pp.107.115469
- Bethke PC, Gubler F, Jacobsen JV, Jones RL. Dormancy of Arabidopsis seeds and barley grains can be broken by nitric oxide. Planta 2004; 219:847-55; PMID:15133666; http://dx.doi.org/10.1007/s00425-004-1282-x
