Abstract
Cold shock domain (CSD) proteins are RNA chaperones that destabilize RNA secondary structures. Arabidopsis Cold Shock Domain Protein 2 (AtCSP2), one of the 4 CSD proteins (AtCSP1-AtCSP4) in Arabidopsis, is induced during cold acclimation but negatively regulates freezing tolerance. Here, we analyzed the function of AtCSP2 in salt stress tolerance. A double mutant, with reduced AtCSP2 and no AtCSP4 expression (atcsp2–3 atcsp4–1), displayed higher survival rates after salt stress. In addition, overexpression of AtCSP2 resulted in reduced salt stress tolerance. These data demonstrate that AtCSP2 acts as a negative regulator of salt stress tolerance in Arabidopsis.
Abbreviations
| CSD | = | cold shock domain |
| CSP | = | cold shock protein |
The cold shock domain (CSD) contains about 70 amino acid residues including 2 consensus RNA binding motifs (RNP-1and RNP-2).Citation1,2 CSD is a nucleic acid-binding domain that is highly conserved among bacteria, animals, and plants.Citation3,4 There is a large superfamily of CSD proteins in these organisms. The bacterial cold shock protein (CSP) is a small protein composed of only one CSD.Citation4 Escherichia coli contains 9 CSP members (cspA to cspI) and 4 of them (cspA, cspB, cspG, and cspI) are induced in response to cold shock.Citation5 It has been demonstrated that a quadruple mutant of cspA, cspB, cspG, and cspE results in growth defects at low temperature.Citation6 CSPs can bind RNA and melt RNA secondary structures in vitro and in vivo.Citation7,8 Therefore, it has been suggested that bacterial CSPs act as RNA chaperones that destabilize RNA secondary structures formed under low temperatures and thereby promote efficient transcription and translation.
CSD proteins have also been extensively studied in animals. Animal CSD proteins contain various C-terminal auxiliary domains in addition to CSD. Y-box binding protein 1 (YB-1) is the most extensively studied CSD protein in vertebrates. YB-1 consists of 3 domains: the N-terminal alanine/proline rich domain, the CSD, and the C-terminal domain with alternating clusters of positively and negatively charged amino acid residues.Citation9,10 YB-1 is a multifunctional DNA/RNA binding protein that is involved in DNA repair, pre-mRNA splicing, transcription, and translation of several genes whose products are involved in cell division, stress response, immune response, drug resistance, etc.Citation10 It has been demonstrated that disruption of YB-1 results in embryonic or perinatal lethality in mice.Citation11,12 In addition, mouse embryonic fibroblasts (MEFs) from YB-1-deficient embryos show increased sensitivity to oxidative, genotoxic, and oncogene-induced stresses.Citation11 However, YB-1 deficiency causes no global changes in the transcriptome and proteome in freshly isolated MEFs.Citation11 YB-1 may be involved in the selective expression of genes whose proteins are essential for cell proliferation and stress response.Citation13
Another class of widely studied animal CSD proteins is LIN28. LIN28 was first identified as a key regulator of developmental timing in Caenorhabditis elegans.Citation14 LIN28 is an evolutionarily conserved RNA binding protein that contains N-terminal CSD and C-terminal retroviral-type Cys-Cys-His-Cys (CCHC) zinc fingers.Citation14 LIN28 is highly expressed in embryonic stem (ES) cells and decreases during ES cell differentiation.Citation15,16 Recently, LIN28, together with 3 transcriptional factors has been used to reprogram somatic cells into induced pluripotent stem cells.Citation17 At the molecular level, LIN28 suppresses the maturation of let-7 microRNAs which are absent in ES cells, but accumulate during ES cell differentiation.Citation18 Therefore, it was suggested that LIN28 plays an important role in blocking cell differentiation. In addition to microRNA regulation, it has been reported that LIN28 acts as a translational activator or repressor in ES and differentiated cells, respectively.Citation19-21
Plant CSD proteins typically contain a large glycine-rich region interspersed with CCHC zinc fingers as a C-terminal auxiliary domain.Citation22 The first characterized plant CSD protein was wheat cold shock protein 1 (WCSP1) which was identified as a cold-induced gene in winter wheat.Citation23 WCSP1 can melt double-stranded RNA/DNA and can complement a cold sensitive phenotype of an E. coli csp quadruple mutant.Citation24 Therefore, it was suggested that WCSP1 shares a function with E. coli CSPs for cold adaptation. Arabidopsis thaliana has 4 genes that encode CSD proteins (AtCSP1 to AtCSP4).Citation22,25 Among them, AtCSP3 is the most extensively studied and its function in acquiring abiotic stress tolerance has been demonstrated. Compared with wild type, a knockout mutant of AtCSP3 is more sensitive to freezing, drought, and salt stresses.Citation26,27 Conversely, overexpression of AtCSP3 confers enhanced stress tolerance.Citation26,27 AtCSP3 regulates the expression of abiotic stress responsive genes, but the functions of these genes are mostly unknown.Citation26,27 AtCSP3 interacts with different classes of proteins associated with RNA, including decapping protein 5, poly(A)-binding proteins, and RNA helicases.Citation28 During abiotic stress, AtCSP3 may regulate gene expression through mRNA stability, splicing, and translation.
AtCSP2 shows the highest level of expression among the AtCSPs.Citation29 Although AtCSP2 is up-regulated during cold acclimation,Citation30,31 a double mutant, with reduced levels of AtCSP2 and no expression of AtCSP4 (the closest paralog of AtCSP2), shows higher freezing tolerance than wild-type after cold acclimation.Citation32 By contrast, overexpression of AtCSP2 results in decreased freezing tolerance under cold-acclimated condition.Citation32 Interestingly, both double mutants and AtCSP2-overexpressors show no difference in freezing tolerance under the non-acclimated condition.Citation32 It is known that C-repeat binding factors (CBFs) confer freezing tolerance through activation of the expression of cold-regulated (COR) genes.Citation33,34 AtCSP2 negatively regulates the expression of CBFs and COR genes during cold acclimation.Citation32 AtCSP2 is a negative regulator of the CBF-dependent pathway for cold acclimation. AtCSP2 is also a negative regulator of seed germination.Citation35
AtCSP2-overexpressing lines show retarded germination as compared with the wild type. Overexpression of AtCSP2 results in reduced expression of an ABA catabolic gene (CYP707A2) and gibberellin biosynthesis genes (GA20ox and GA3ox).35 The ABA levels in AtCSP2-overexpressing seeds are higher than those in the wild type.35 Therefore, it is proposed that AtCSP2 negatively regulates seed germination by controlling ABA and GA levels.
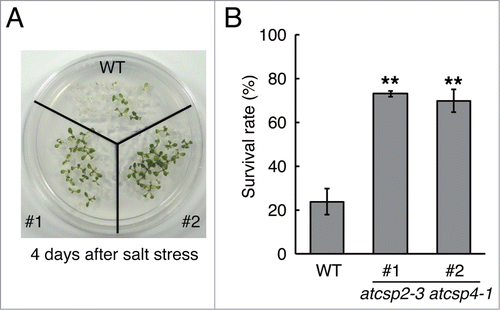
It has been reported that expression of AtCSP2 is also upregulated by salt treatment.Citation36 In this paper, we examined whether AtCSP2 is associated with salt stress tolerance. Since AtCSP2 and AtCSP4 redundantly regulate freezing tolerance in Arabidopsis,Citation32 we utilized the previously-isolated atcsp2–3 atcsp4–1 mutant lines (#1 and #2) for the analysis of salt stress tolerance. For the salt tolerance test, 7-day-old wild type (Col-0) and atcsp2–3 atcsp4–1 seedlings were transferred to MS medium supplemented with 185 mM NaCl and grown for 3 d. Survival rates were determined 4 d after transfer back to MS medium. Compared to wild type, atcsp2–3 atcsp4–1 showed significantly increased survival rates after salt stress (). The atcsp2–3 atcsp4–1 plants had survival rates of 73% and 69.8% (#1 and #2, respectively), while the survival rate of wild type plants was 23.8% (). These data indicate that AtCSP2 has negative effects on salt stress tolerance in Arabidopsis.
Figure 1. Salt tolerance of the atcsp2–3 atcsp4–1 double mutant. (A) Phenotype of atcsp2–3 atcsp4–1 plants after recovery from salt treatment (185 mM NaCl, 3 days). The photograph was taken 4 d after recovery growth on MS medium without salt. (B) Survival rates were calculated from 3 independent experiments (n = 21). Data represent the means ±SD.
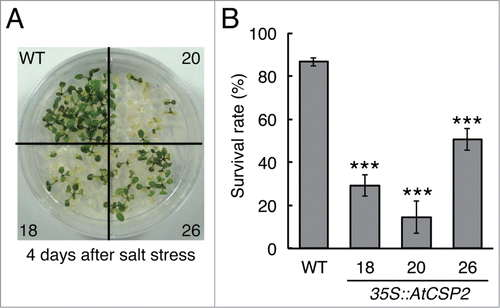
To further examine the effect of AtCSP2 on salt stress tolerance, AtCSP2-overexpressing lines (35S::AtCSP2–18, 20, and 26) Citation32 were used for analysis. Eight-day-old seedlings were transferred to MS medium supplemented with 185 mM NaCl and grown for 2.5 d The survival rate was calculated 4 d after transfer back to MS medium. All 35S::AtCSP2 lines exhibited substantially lower survival rates than wild-type plants after salt stress (). The survival rate of wild type plants was 86.7%, while 3 35S::AtCSP2 lines showed survival rates of 29.3%, 14.7%, and 50.7% (for lines 18, 20, and 26, respectively) (). Taken together, these data indicate that, in addition to freezing tolerance, AtCSP2 acts as a negative regulator of salt tolerance in Arabidopsis.
Figure 2. Salt tolerance of AtCSP2-overexpressing lines. (A) Phenotype of AtCSP2-overexpressing lines after recovery from salt treatment (185 mM NaCl, 2.5 days). The photograph was taken 4 d after recovery growth on MS medium without salt. (B) Survival rates were calculated from 3 independent experiments (n = 25). Data represent the means ±SD.
Our data suggest that AtCSP2 is involved in attenuating salt stress signaling. It has been reported that expression of CBF3 and RD29A (a CBF regulon gene) is significantly up-regulated by salt stress.Citation37,38 In addition, according to data from the public microarray database (BAR; http://www.bar.utronto.ca/), CBF3 is transiently upregulated in both shoots and roots during a 24-h salt treatment. Following the initiation of salt treatment, the CBF3 transcript reaches maximum levels at hour 3 in shoots (6.5-fold up-regulated) and at hour 6 in roots (33.3-fold upregulated). Furthermore, overexpression of CBF3 confers salt stress tolerance in Arabidopsis.Citation39 Our previous research showed that AtCSP2 negatively regulates expression of CBF3 and RD29A during cold acclimation.Citation32 From these data, it is reasonable to speculate that AtCSP2 also attenuates the CBF pathway under salt stress conditions.
Attenuation of stress-signaling pathway by negative regulators is an important process for the proper management of abiotic stress responses. Under cold stress, the expression of CBF genes peaks within several hours and decreases quickly thereafter.Citation40,41 It has been demonstrated that expression of CBF3 is tightly regulated by negative regulators HOS1 (RING E3 ligase) and MYB15 (R2R3 type MYB transcriptional factor) during cold acclimation.Citation42,43 Indeed, constitutive overexpression of one of the CBF genes resulted in severe growth retardation.Citation44,45 Therefore, it is thought that negative regulators are necessary for preventing detrimental effects caused by over-activation of stress responses during the process of plant growth and development.
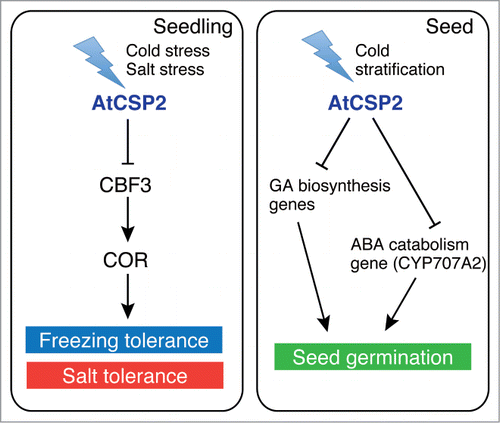
Our current and previous studies have shown that AtCSP2 negatively regulates abiotic stress tolerance and seed germination by altering expression of genes critical for these responses (). However, the mechanisms by which AtCSP2 regulates gene expression is still unclear. As an RNA chaperone, AtCSP2 may regulate processing and stability of mRNA and/or translation to attenuate signaling during abiotic stress and germination. Consistent with this hypothesis, our preliminary data using yeast 2-hybrid system suggested that AtCSP2 interacts with the nuclear poly(A)-binding proteins PABN2 and PABN3 (data not shown). In order to further characterize the function of AtCSP2, it will be necessary to identify functional complex involving AtCSP2 and target mRNAs.
Figure 3. A model for the negative regulation of abiotic stress and seed germination by AtCSP2. AtCSP2 is induced by cold or salt stress and represses CBF3 expression, which results in attenuation of abiotic stress tolerance. On the other hand, AtCSP2 is up-regulated during stratification and represses the expression of gibberellin (GA) biosynthesis and abscisic acid (ABA) catabolism genes for negative regulation of seed germination.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grants from Japan Society for the Promotion of Science (KAKENHI Scientific Research B nos. 22380063 and 19380063) and National Agriculture and Food Research Organization (NARO) (Development of Innovative Crops through the Molecular Analysis of Useful Genes, No. 3204) (to R. I. and K. S., respectively).
References
- Graumann PL, Marahiel MA. A superfamily of proteins that contain the cold-shock domain. Trends Biochem Sci 1998; 23:286-90; PMID:9757828; http://dx.doi.org/10.1016/S0968-0004(98)01255-9
- Yamanaka K, Fang L, Inouye M. The CspA family in Escherichia coli: multiple gene duplication for stress adaptation. Mol Microbiol 1998; 27:247-55; PMID:9484881; http://dx.doi.org/10.1046/j.1365-2958.1998.00683.x
- Matsumoto K, Wolffe AP. Gene regulation by Y-box proteins: coupling control of transcription and translation. Trends Cell Biol 1998; 8:318-23; PMID:9704408; http://dx.doi.org/10.1016/S0962-8924(98)01300-2
- Sommerville J. Activities of cold-shock domain proteins in translation control. Bioessays 1999; 21:319-25; PMID:10377894; http://dx.doi.org/10.1002/(SICI)1521-1878(199904)21:4%3c319::AID-BIES8%3e3.0.CO;2-3
- Phadtare S. Recent developments in bacterial cold-shock response. Curr Issues Mol Biol 2004; 6:125-36; PMID:15119823
- Xia B, Ke H, Inouye M. Acquirement of cold sensitivity by quadruple deletion of the cspA family and its suppression by PNPase S1 domain in Escherichia coli. Mol Microbiol 2001; 40:179-88; PMID:11298285; http://dx.doi.org/10.1046/j.1365-2958.2001.02372.x
- Bae W, Xia B, Inouye M, Severinov K. Escherichia coli CspA-family RNA chaperones are transcription antiterminators. Proc Natl Acad Sci USA 2000; 97:7784-9; PMID:10884409; http://dx.doi.org/10.1073/pnas.97.14.7784
- Phadtare S, Inouye M, Severinov K. The nucleic acid melting activity of Escherichia coli CspE is critical for transcription antitermination and cold acclimation of cells. J Biol Chem 2002; 277:7239-45; PMID:11756430; http://dx.doi.org/10.1074/jbc.M111496200
- Wolffe AP. Structural and functional properties of the evolutionarily ancient Y-box family of nucleic acid binding proteins. Bioessays 1994; 16:245-51; PMID:8031301; http://dx.doi.org/10.1002/bies.950160407
- Kohno K, Izumi H, Uchiumi T, Ashizuka M, Kuwano M. The pleiotropic functions of the Y-box-binding protein, YB-1. Bioessays 2003; 25:691-8; PMID:12815724; http://dx.doi.org/10.1002/bies.10300
- Lu ZH, Books JT, Ley TJ. YB-1 is important for late-stage embryonic development, optimal cellular stress responses, and the prevention of premature senescence. Mol Cell Biol 2005; 25:4625-37; PMID:15899865; http://dx.doi.org/10.1128/MCB.25.11.4625-4637.2005
- Uchiumi T, Fotovati A, Sasaguri T, Shibahara K, Shimada T, Fukuda T, Nakamura T, Izumi H, Tsuzuki T, Kuwano M, et al. YB-1 is important for an early stage embryonic development: neural tube formation and cell proliferation. J Biol Chem 2006; 281:40440-9; PMID:17082189; http://dx.doi.org/10.1074/jbc.M605948200
- Lyabin DN, Eliseeva IA, Ovchinnikov LP. YB-1 protein: functions and regulation. Wiley Interdiscip Rev RNA 2013; 5:95-110; PMID:24217978; http://dx.doi.org/10.1002/wrna.1200
- Moss EG, Lee RC, Ambros V. The cold shock domain protein LIN-28 controls developmental timing in C. elegans and is regulated by the lin-4 RNA. Cell 1997; 88:637-46; PMID:9054503; http://dx.doi.org/10.1016/S0092-8674(00)81906-6
- Richards M, Tan S-P, Tan J-H, Chan W-K, Bongso A. The transcriptome profile of human embryonic stem cells as defined by SAGE. Stem Cells 2004; 22:51-64; PMID:14688391; http://dx.doi.org/10.1634/stemcells.22-1-51
- Darr H, Benvenisty N. Genetic analysis of the role of the reprogramming gene LIN-28 in human embryonic stem cells. Stem Cells 2009; 27:352-62; PMID:19038789; http://dx.doi.org/10.1634/stemcells.2008-0720
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007; 318:1917-20; PMID:18029452
- Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by Lin28. Science 2008; 320:97-100; PMID:18292307
- Polesskaya A, Cuvellier S, Naguibneva I, Duquet A, Moss EG, Harel-Bellan A. Lin-28 binds IGF-2 mRNA and participates in skeletal myogenesis by increasing translation efficiency. Genes Dev 2007; 21:1125-38; PMID:17473174
- Xu B, Huang Y. Histone H2a mRNA interacts with Lin28 and contains a Lin28-dependent posttranscriptional regulatory element. Nucl Acids Res 2009; 37:4256-63; PMID:19443445; http://dx.doi.org/10.1093/nar/gkp372
- Qiu C, Ma Y, Wang J, Peng S, Huang Y. Lin28-mediated post-transcriptional regulation of Oct4 expression in human embryonic stem cells. Nucl Acids Res 2010; 38:1240-8; PMID:19966271; http://dx.doi.org/10.1093/nar/gkp1071
- Sasaki K, Imai R. Pleiotropic roles of cold shock domain proteins in plants. Front Plant Sci 2012; 2:116; PMID:22639630
- Karlson D, Nakaminami K, Toyomasu T, Imai R. A cold-regulated nucleic acid-binding protein of winter wheat shares a domain with bacterial cold shock proteins. J Biol Chem 2002; 277:35248-56; PMID:12122010
- Nakaminami K, Karlson DT, Imai R. Functional conservation of cold shock domains in bacteria and higher plants. Proc Natl Acad Sci USA 2006; 103:10122-7; PMID:16788067
- Karlson D, Imai R. Conservation of the cold shock domain protein family in plants. Plant Physiol 2003; 131:12-5; PMID:12529510
- Kim M-H, Sasaki K, Imai R. Cold shock domain protein 3 regulates freezing tolerance in Arabidopsis thaliana. J Biol Chem 2009; 284:23454-60; PMID:19556243
- Kim M-H, Sato S, Sasaki K, Saburi W, Matsui H, Imai R. COLD SHOCK DOMAIN PROTEIN 3 is involved in salt and drought stress tolerance in Arabidopsis. FEBS Open Bio 2013; 3:438-42; PMID:24251108
- Kim M-H, Sonoda Y, Sasaki K, Kaminaka H, Imai R. Interactome analysis reveals versatile functions of Arabidopsis COLD SHOCK DOMAIN PROTEIN 3 in RNA processing within the nucleus and cytoplasm. Cell Stress Chaperon 2013; 18:517-25
- Nakaminami K, Hill K, Perry SE, Sentoku N, Long JA, Karlson DT. Arabidopsis cold shock domain proteins: relationships to floral and silique development. J Exp Bot 2009; 60:1047-62; PMID:19269998; http://dx.doi.org/10.1093/jxb/ern351
- Sasaki K, Kim MH, Imai R. Arabidopsis COLD SHOCK DOMAIN PROTEIN2 is a RNA chaperone that is regulated by cold and developmental signals. Biochem Biophys Res Commun 2007; 364:633-8
- Fusaro AF, Bocca SN, Ramos RLB, Barrôco RM, Magioli C, Jorge VC, Coutinho TC, Rangel-Lima CM, De Rycke R, Inzé D, et al. AtGRP2, a cold-induced nucleo-cytoplasmic RNA-binding protein, has a role in flower and seed development. Planta 2007; 225:1339-51; PMID:17123099
- Sasaki K, Kim M-H, Imai R. Arabidopsis COLD SHOCK DOMAIN PROTEIN 2 is a negative regulator of cold acclimation. New Phytol 2013; 198:95-102; PMID:23323758
- Jaglo-Ottosen KR. Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science 1998; 280:104-6; PMID:9525853
- Gilmour SJ, Sebolt AM, Salazar MP, Everard JD, Thomashow MF. Overexpression of the Arabidopsis CBF3 transcriptional activator mimics multiple biochemical changes associated with cold acclimation. Plant Physiol 2000; 124:1854-65; PMID:11115899
- Sasaki K, Kim M-H, Kanno Y, Seo M, Kamiya Y, Imai R. Arabidopsis COLD SHOCK DOMAIN PROTEIN 2 influences ABA accumulation in seed and negatively regulates germination. Biochem Biophys Res Commun 2015; 456:380-4; PMID:25475723; http://dx.doi.org/10.1016/j.bbrc.2014.11.092
- Park SJ, Kwak KJ, Oh TR, Kim YO, Kang H. Cold shock domain proteins affect seed germination and growth of Arabidopsis thaliana under abiotic stress conditions. Plant Cell Physiol 2009; 50:869-78; PMID:19258348
- Kant P, Kant S, Gordon M, Shaked R, Barak S. STRESS RESPONSE SUPPRESSOR1 and STRESS RESPONSE SUPPRESSOR2, two DEAD-box RNA helicases that attenuate Arabidopsis responses to multiple abiotic stresses. Plant Physiol 2007; 145:814-30; PMID:17556511
- He Y, Li W, Lv J, Jia Y, Wang M, Xia G. Ectopic expression of a wheat MYB transcription factor gene, TaMYB73, improves salinity stress tolerance in Arabidopsis thaliana. J Exp Bot 2012; 63:1511-22; PMID:22140235; http://dx.doi.org/10.1093/jxb/err389
- Kasuga M, Liu Q, Miura S, Yamaguchi-Shinozaki K, Shinozaki K. Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat Biotechnol 1999; 17:287-91; PMID:10096298
- Shi Y, Tian S, Hou L, Huang X, Zhang X, Guo H, Yang S. Ethylene signaling negatively regulates freezing tolerance by repressing expression of CBF and type-A ARR genes in Arabidopsis. Plant Cell 2012; 24:2578-95; PMID:22706288; http://dx.doi.org/10.1105/tpc.112.098640
- Hu Y, Jiang L, Wang F, Yu D. Jasmonate regulates the inducer of CBF expression-C-repeat binding factor/DRE binding factor1 cascade and freezing tolerance in Arabidopsis. Plant Cell 2013; 25:2907-24; PMID:23933884; http://dx.doi.org/10.1105/tpc.113.112631
- Dong C-H, Agarwal M, Zhang Y, Xie Q, Zhu J-K. The negative regulator of plant cold responses, HOS1, is a RING E3 ligase that mediates the ubiquitination and degradation of ICE1. Proc Natl Acad Sci USA 2006; 103:8281-6; PMID:16702557
- Agarwal M, Hao Y, Kapoor A, Dong C-H, Fujii H, Zheng X, Zhu J-K. A R2R3 type MYB transcription factor is involved in the cold regulation of CBF genes and in acquired freezing tolerance. J Biol Chem 2006; 281:37636-45; PMID:17015446
- Gilmour SJ, Fowler SG, Thomashow MF. Arabidopsis transcriptional activators CBF1, CBF2, and CBF3 have matching functional activities. Plant Mol Biol 2004; 54:767-81; PMID:15356394
- Achard P, Gong F, Cheminant S, Alioua M, Hedden P, Genschik P. The cold-inducible CBF1 factor-dependent signaling pathway modulates the accumulation of the growth-repressing DELLA proteins via its effect on gibberellin metabolism. Plant Cell 2008; 20:2117-29; PMID:18757556; http://dx.doi.org/10.1105/tpc.108.058941
